
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ramendra Pati Pandey | -- | 3020 | 2023-04-24 11:48:29 | | | |
| 2 | Rita Xu | Meta information modification | 3020 | 2023-04-25 04:29:56 | | |
Video Upload Options
Autophagy is a lysosomal protein degradation system that eliminates cytoplasmic components such as protein aggregates, damaged organelles, and even invading pathogens. Autophagy is an evolutionarily conserved homoeostatic strategy for cell survival in stressful conditions and has been linked to a variety of biological processes and disorders. It is vital for the homeostasis and survival of renal cells such as podocytes and tubular epithelial cells, as well as immune cells in the healthy kidney. Autophagy activation protects renal cells under stressed conditions, whereas autophagy deficiency increases the vulnerability of the kidney to injury, resulting in several aberrant processes that ultimately lead to renal failure.
1. Introduction
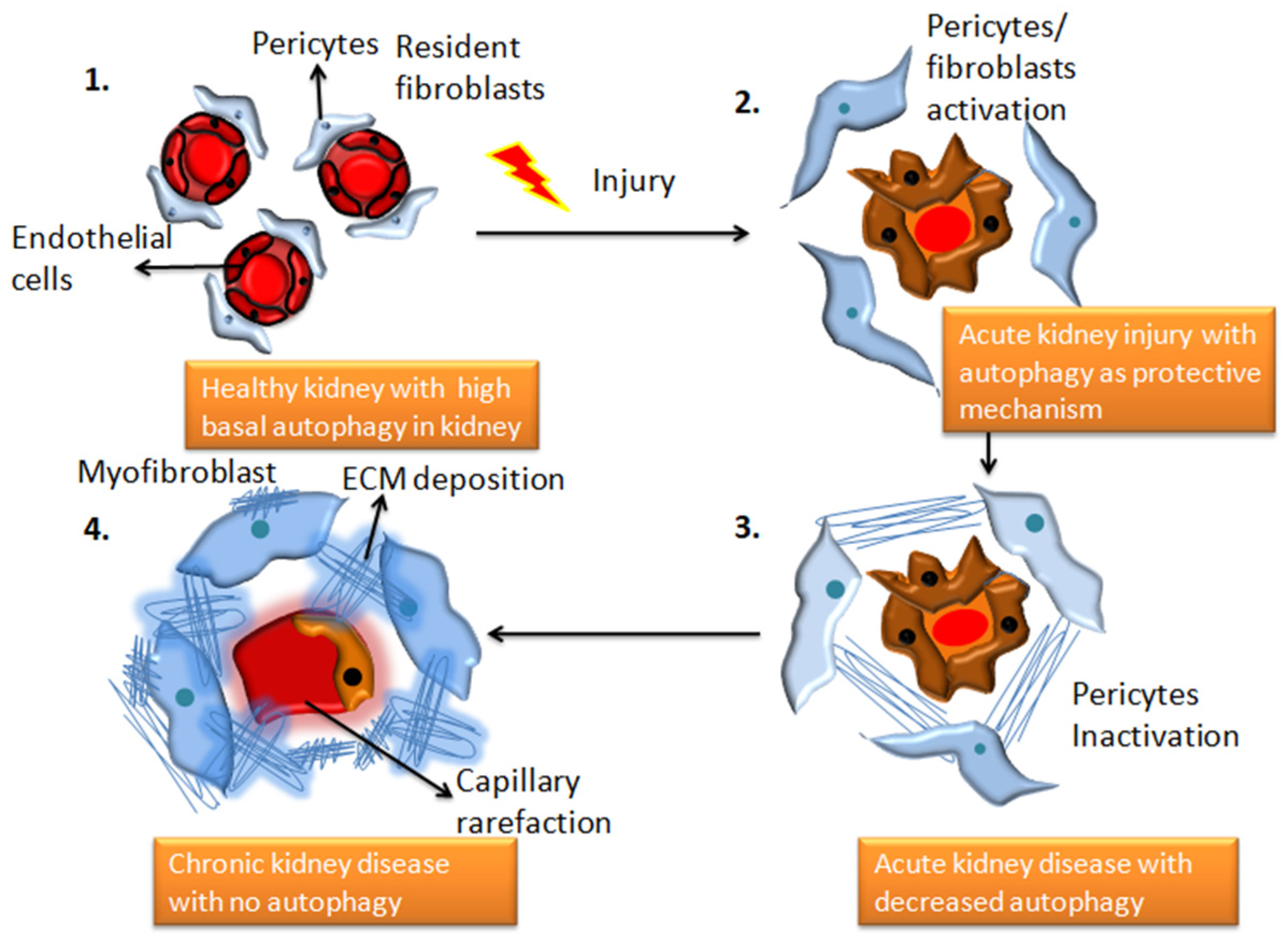
2. Autophagy and Chronic Kidney Disease
2.1. Fibrosis in Chronic Kidney Disease
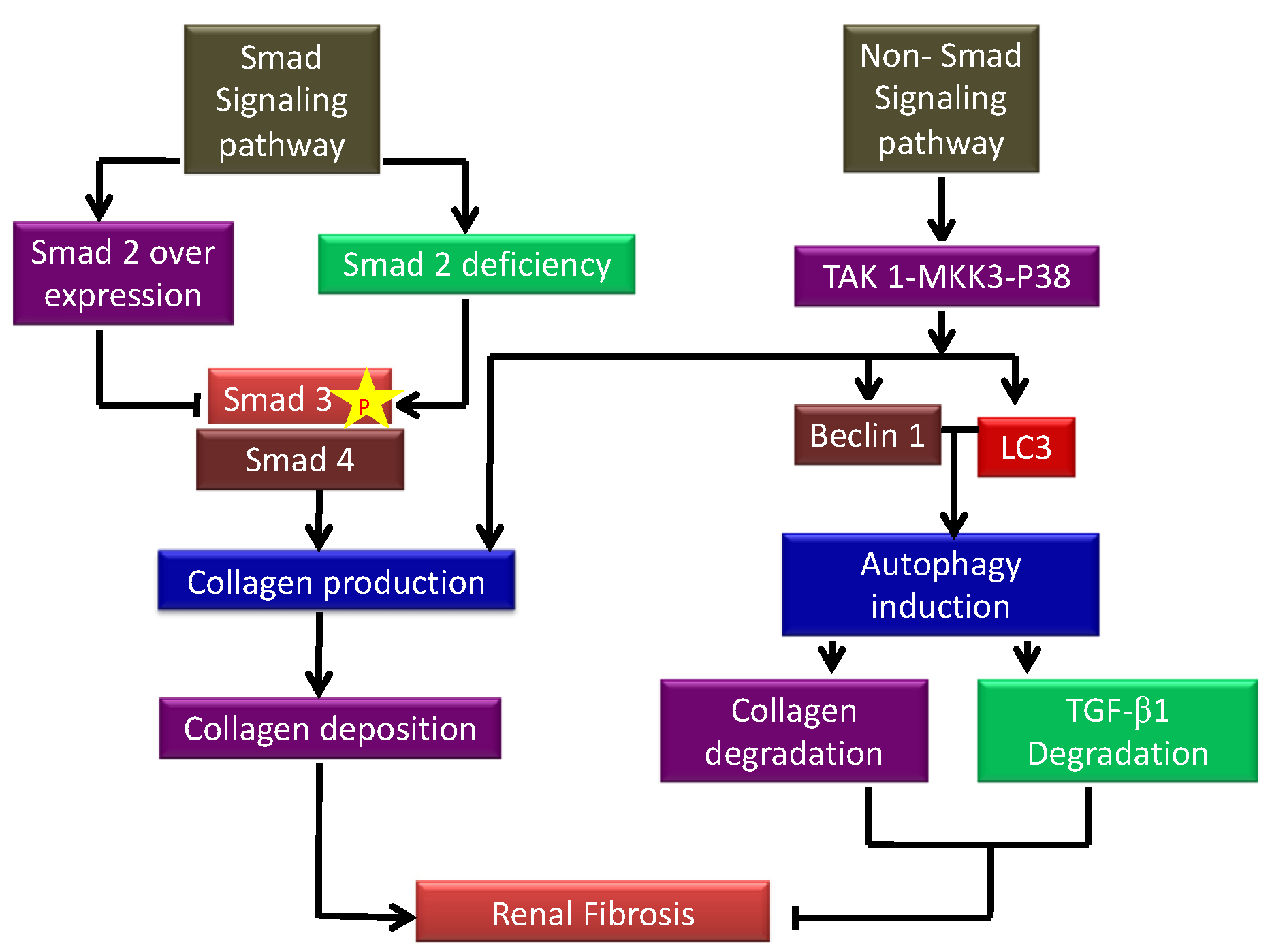
2.2. Autophagy in Chronic Kidney Disease
2.2.1. TGF-β1 as an Inducer of Autophagy
2.2.2. Autophagy: A Regulator of TGF-β1
2.2.3. Other Regulators of Autophagy
2.2.4. Autophagy Deficient Kidney
References
- Wang, C.-W.; Klionsky, D.J. The Molecular Mechanism of Autophagy. Mol. Med. 2003, 9, 65–76.
- Liang, S.; Wu, Y.S.; Li, D.Y.; Tang, J.X.; Liu, H.F. Autophagy and Renal Fibrosis. Aging Dis. 2022, 13, 712–731.
- Tang, C.; Livingston, M.J.; Liu, Z.; Dong, Z. Autophagy in kidney homeostasis and disease. Nat. Rev. Nephrol. 2020, 16, 489–508.
- Zhao, X.C.; Livingston, M.J.; Liang, X.L.; Dong, Z. Cell Apoptosis and Autophagy in Renal Fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 557–584.
- Lin, T.A.; Wu, V.C.; Wang, C.Y. Autophagy in Chronic Kidney Diseases. Cells 2019, 8, 61.
- Kaushal, G.P.; Shah, S.V. Autophagy in acute kidney injury. Kidney Int. 2016, 89, 779–791.
- Bao, J.; Shi, Y.; Tao, M.; Liu, N.; Zhuang, S.; Yuan, W. Pharmacological inhibition of autophagy by 3-MA attenuates hyperuricemic nephropathy. Clin. Sci. 2018, 132, 2299–2322.
- Ferenbach, D.A.; Bonventre, J.V. Acute kidney injury and chronic kidney disease: From the laboratory to the clinic. Nephrol. Ther. 2016, 12 (Suppl. 1), S41–S48.
- Humphreys, B.D. Mechanisms of Renal Fibrosis. Annu. Rev. Physiol. 2018, 80, 309–326.
- He, L.; Wei, Q.; Liu, J.; Yi, M.; Liu, Y.; Liu, H.; Sun, L.; Peng, Y.; Liu, F.; Venkatachalam, M.A.; et al. AKI on CKD: Heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 2017, 92, 1071–1083.
- Ding, Y.; Kim, S.L.; Lee, S.Y.; Koo, J.K.; Wang, Z.; Choi, M.E. Autophagy regulates TGF-β expression and suppresses kidney fibrosis induced by unilateral ureteral obstruction. J. Am. Soc. Nephrol. 2014, 25, 2835–2846.
- Yang, X.; Wang, H.; Tu, Y.; Li, Y.; Zou, Y.; Li, G.; Wang, L.; Zhong, X. WNT1-inducible signaling protein-1 mediates TGF-β1-induced renal fibrosis in tubular epithelial cells and unilateral ureteral obstruction mouse models via autophagy. J. Cell Physiol. 2020, 235, 2009–2022.
- Yang, C.; Wu, H.L.; Li, Z.H.; Chen, X.C.; Su, H.Y.; Guo, X.Y.; An, N.; Jing, K.P.; Pan, Q.J.; Liu, H.F. Autophagy Inhibition Sensitizes Renal Tubular Epithelial Cell to G1 Arrest Induced by Transforming Growth Factor beta (TGF-β). Med. Sci. Monit. 2020, 26, e922673.
- Nam, S.A.; Kim, W.Y.; Kim, J.W.; Kang, M.G.; Park, S.H.; Lee, M.S.; Kim, H.W.; Yang, C.W.; Kim, J.; Kim, Y.K. Autophagy in FOXD1 stroma-derived cells regulates renal fibrosis through TGF-β and NLRP3 inflammasome pathway. Biochem. Biophys. Res. Commun. 2019, 508, 965–972.
- Deng, X.; Xie, Y.; Zhang, A. Advance of autophagy in chronic kidney diseases. Ren. Fail. 2017, 39, 306–313.
- Liu, B.C.; Tang, T.T.; Lv, L.L.; Lan, H.Y. Renal tubule injury: A driving force toward chronic kidney disease. Kidney Int. 2018, 93, 568–579.
- Delles, C.; Vanholder, R. Chronic kidney disease. Clin. Sci. 2017, 131, 225–226.
- Wang, X.; Garrett, M.R. Nephron number, hypertension, and CKD: Physiological and genetic insight from humans and animal models. Physiol. Genom. 2017, 49, 180–192.
- Yang, L.; Besschetnova, T.Y.; Brooks, C.R.; Shah, J.V.; Bonventre, J.V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 2010, 16, 535–543.
- Zager, R.A.; Johnson, A.C. Renal ischemia-reperfusion injury upregulates histone-modifying enzyme systems and alters histone expression at proinflammatory/profibrotic genes. Am. J. Physiol. Renal Physiol. 2009, 296, F1032–F1041.
- van Timmeren, M.M.; van den Heuvel, M.C.; Bailly, V.; Bakker, S.J.; van Goor, H.; Stegeman, C.A. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J. Pathol. 2007, 212, 209–217.
- Humphreys, B.D.; Xu, F.; Sabbisetti, V.; Grgic, I.; Movahedi Naini, S.; Wang, N.; Chen, G.; Xiao, S.; Patel, D.; Henderson, J.M.; et al. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J. Clin. Investig. 2013, 123, 4023–4035.
- Sureshbabu, A.; Muhsin, S.A.; Choi, M.E. TGF-β signaling in the kidney: Profibrotic and protective effects. Am. J. Physiol. Renal Physiol. 2016, 310, F596–F606.
- Ma, T.T.; Meng, X.M. TGF-β/Smad and Renal Fibrosis. Adv. Exp. Med. Biol. 2019, 1165, 347–364.
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873.
- Sun, Y.B.; Qu, X.; Caruana, G.; Li, J. The origin of renal fibroblasts/myofibroblasts and the signals that trigger fibrosis. Differentiation 2016, 92, 102–107.
- Suárez-Álvarez, B.; Liapis, H.; Anders, H.J. Links between coagulation, inflammation, regeneration, and fibrosis in kidney pathology. Lab. Investig. 2016, 96, 378–390.
- Deelman, L.; Sharma, K. Mechanisms of kidney fibrosis and the role of antifibrotic therapies. Curr. Opin. Nephrol. Hypertens 2009, 18, 85–90.
- Gao, N.; Wang, H.; Yin, H.; Yang, Z. Angiotensin II induces calcium-mediated autophagy in podocytes through enhancing reactive oxygen species levels. Chem. Biol. Interact. 2017, 277, 110–118.
- Hung, T.W.; Chu, C.Y.; Yu, C.L.; Lee, C.C.; Hsu, L.S.; Chen, Y.S.; Hsieh, Y.H.; Tsai, J.P. Endothelial Cell-Specific Molecule 1 Promotes Endothelial to Mesenchymal Transition in Renal Fibrosis. Toxins 2020, 12, 506.
- Oliva Trejo, J.A.; Asanuma, K.; Kim, E.H.; Takagi-Akiba, M.; Nonaka, K.; Hidaka, T.; Komatsu, M.; Tada, N.; Ueno, T.; Tomino, Y. Transient increase in proteinuria poly-ubiquitylated proteins ER stress markers in podocyte-specific autophagy-deficient mice following unilateral nephrectomy. Biochem. Biophys. Res. Commun. 2014, 446, 1190–1196.
- Nolin, A.C.; Mulhern, R.M.; Panchenko, M.V.; Pisarek-Horowitz, A.; Wang, Z.; Shirihai, O.; Borkan, S.C.; Havasi, A. Proteinuria causes dysfunctional autophagy in the proximal tubule. Am. J. Physiol. Renal Physiol. 2016, 311, F1271–F1279.
- Yamamoto, T.; Takabatake, Y.; Minami, S.; Sakai, S.; Fujimura, R.; Takahashi, A.; Namba-Hamano, T.; Matsuda, J.; Kimura, T.; Matsui, I.; et al. Eicosapentaenoic acid attenuates renal lipotoxicity by restoring autophagic flux. Autophagy 2021, 17, 1700–1713.
- Satriano, J.; Sharma, K. Autophagy and metabolic changes in obesity-related chronic kidney disease. Nephrol. Dial. Transplant. 2013, 28 (Suppl. 4), iv29–iv36.
- Kim, W.Y.; Nam, S.A.; Song, H.C.; Ko, J.S.; Park, S.H.; Kim, H.L.; Choi, E.J.; Kim, Y.S.; Kim, J.; Kim, Y.K. The role of autophagy in unilateral ureteral obstruction rat model. Nephrology 2012, 17, 148–159.
- Kaushal, G.P.; Chandrashekar, K.; Juncos, L.A.; Shah, S.V. Autophagy Function and Regulation in Kidney Disease. Biomolecules 2020, 10, 100.
- Kim, S.I.; Na, H.J.; Ding, Y.; Wang, Z.; Lee, S.J.; Choi, M.E. Autophagy promotes intracellular degradation of type I collagen induced by transforming growth factor (TGF)-β1. J. Biol. Chem. 2012, 287, 11677–11688.
- Shi, M.; Maique, J.; Shepard, S.; Li, P.; Seli, O.; Moe, O.W.; Hu, M.C. In vivo evidence for therapeutic applications of beclin 1 to promote recovery and inhibit fibrosis after acute kidney injury. Kidney Int. 2022, 101, 63–78.
- Livingston, M.J.; Ding, H.F.; Huang, S.; Hill, J.A.; Yin, X.M.; Dong, Z. Persistent activation of autophagy in kidney tubular cells promotes renal interstitial fibrosis during unilateral ureteral obstruction. Autophagy 2016, 12, 976–998.
- Ding, Y.; Kim, J.K.; Kim, S.I.; Na, H.J.; Jun, S.Y.; Lee, S.J.; Choi, M.E. TGF-1 protects against mesangial cell apoptosis via induction of autophagy. J. Bio. Chem. 2010, 285, 37909–37919.
- Takaesu, G.; Kobayashi, T.; Yoshimura, A. TGFβ-activated kinase 1 (TAK1)-binding proteins (TAB) 2 and 3 negatively regulate autophagy. J. Biochem. 2012, 151, 157–166.
- Andrzejewska, Z.; Nevo, N.; Thomas, L.; Chhuon, C.; Bailleux, A.; Chauvet, V.; Courtoy, P.J.; Chol, M.; Guerrera, I.C.; Antignac, C. Cystinosin is a Component of the Vacuolar H+-ATPase-Ragulator-Rag Complex Controlling Mammalian Target of Rapamycin Complex 1 Signaling. J. Am. Soc. Nephrol. 2016, 27, 1678–1688.
- Lamark, T.; Svenning, S.; Johansen, T. Regulation of selective autophagy: The p62/SQSTM1 paradigm. Essays Biochem. 2017, 61, 609–624.
- Odle, R.I.; Florey, O.; Ktistakis, N.T.; Cook, S.J. CDK1, the Other ‘Master Regulator’ of Autophagy. Trends Cell Biol. 2021, 31, 95–107.
- Son, S.M.; Park, S.J.; Stamatakou, E.; Vicinanza, M.; Menzies, F.M.; Rubinsztein, D.C. Leucine regulates autophagy via acetylation of the mTORC1 component raptor. Nat. Commun. 2020, 11, 3148.
- Gros, F.; Muller, S. Pharmacological regulators of autophagy and their link with modulators of lupus disease. Br. J. Pharmacol. 2014, 171, 4337–4359.
- Bonventre, J.V. Maladaptive proximal tubule repair: Cell cycle arrest. Nephron. Clin. Pract. 2014, 127, 61–64.




