Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ivonne Loeffler | -- | 3137 | 2023-04-21 17:18:15 | | | |
| 2 | Camila Xu | Meta information modification | 3137 | 2023-04-23 09:48:54 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Loeffler, I.; Ziller, N. Sex Differences in Human Diabetic Kidney Disease. Encyclopedia. Available online: https://encyclopedia.pub/entry/43331 (accessed on 08 February 2026).
Loeffler I, Ziller N. Sex Differences in Human Diabetic Kidney Disease. Encyclopedia. Available at: https://encyclopedia.pub/entry/43331. Accessed February 08, 2026.
Loeffler, Ivonne, Nadja Ziller. "Sex Differences in Human Diabetic Kidney Disease" Encyclopedia, https://encyclopedia.pub/entry/43331 (accessed February 08, 2026).
Loeffler, I., & Ziller, N. (2023, April 21). Sex Differences in Human Diabetic Kidney Disease. In Encyclopedia. https://encyclopedia.pub/entry/43331
Loeffler, Ivonne and Nadja Ziller. "Sex Differences in Human Diabetic Kidney Disease." Encyclopedia. Web. 21 April, 2023.
Copy Citation
Diabetic kidney disease (DKD) is a secondary disease of type 1 and type 2 diabetes mellitus (T1DM and T2DM). This microvascular complication develops in approximately 30% of patients with T1DM and 40% of patients with T2DM and is characterized by the presence of albuminuria and the progressive loss of renal function.
diabetic kidney disease
DKD
sex differences
gender
1. Introduction
The influence of biological sex differences on human disease has long been underestimated and underresearched. Until recent decades, the vast majority of clinical research was conducted with predominantly male participants. In addition, preclinical research using animal models has almost exclusively examined male animals. Despite this limited approach, it was often (and sometimes still is) assumed that research findings and medical treatments developed from those findings apply to the entire population [1]. However, the resulting lack of understanding limits the ability to treat with targeted and patient-centered therapies. This can have life-threatening consequences for many serious conditions, such as cancer or cardiovascular disease.
DKD is a secondary disease of type 1 and type 2 diabetes mellitus (T1DM and T2DM). This microvascular complication develops in approximately 30% of patients with T1DM and 40% of patients with T2DM [2] and is characterized by the presence of albuminuria and the progressive loss of renal function [3]. Persistent high blood glucose levels in patients with DM lead to the disruption and damage of the microvascular architecture of the kidneys [4]. As a result, small ultrastructural changes occur in the nephron, mainly localized in the glomerulus and proximal tubule compartment [5]. In renal biopsies of clinical patients with DKD, glomerular changes are most frequently observed [6]. Initial changes include thickening of the glomerular basement membrane (stage I), mild mesangial expansion (>25%), glomerular hypertrophy, and mild microalbuminuria (<30–300 mg/d, stage IIa) [5][6]. Progression of DKD increases the risk of cardiovascular disease and is characterized by an increase in albuminuria (macroalbuminuria > 300 mg/d), severe diffuse mesangial expansion, nodular sclerotic changes (Kimmelstiel–Wilson lesion), decrease in glomerular filtration rate, hyalinosis of afferent and efferent arterioles, loss of podocytes, thickening of tubular basement membrane, tubulointerstitial fibrosis/inflammation, and tubular atrophy (stage IIb-IV) [5][6][7]. Signs of tubulointerstitial fibrosis (TIF) include myofibroblast accumulation, excessive extracellular matrix (ECM) deposition, and renal tubule destruction [8][9].
2. Sex Differences in Human DKD
In IgA nephropathy and membranous nephropathy, as well as in nondiabetic chronic kidney disease of unknown etiology, a strong significant association between male sex and adverse renal outcome was observed in a meta-analysis [10]. Other multicenter and population-based studies confirmed that the loss of renal function occurs more slowly in women than in men and the female sex is associated with better survival [11][12][13]. Another meta-analysis indicated the opposite, that progression is faster rather than slower in women. However, the authors acknowledged that most of the women in their analysis were of postmenopausal age and their results may not be generalizable to younger women. Thus, the presumed estrogen-mediated protective effect against nondiabetic chronic kidney disease in younger women compared with men of the same age appears to be lost with the menopause [14][15].
Epidemiological studies show that worldwide, 80% of cases of end-stage kidney failure (ESKF) are due to diabetes, hypertension, or a combination of both. The incidence of ESKF in patients with diabetes is up to ten times higher compared to adults without diabetes [16][17].
Although it seems clear that diabetes-induced macrovascular complications, such as coronary heart disease or stroke, are more common in women [18], data on sex and DKD risk are inconsistent. Studies report either a higher risk in men, a higher risk in women, or no significant sex dimorphism [19][20][21][22][23][24][25].
2.1. Sex Differences in Development, Progression, and ESKF in DKD
There are several reasons for the inconsistency of data on sex and DKD risk. First, there are different equations for calculating eGFR and gold standards, such as lohexol clearance, are often not used when measuring GFR. In addition, criteria for classifying CKD may need to take into account the distribution of GFR by age and sex [26]. Other, and perhaps the most important, reasons for inconsistency include the types of diabetes (T1DM, T2DM, or both) considered in the studies and the endpoints of interest considered (e.g., micro/macroalbuminuria, eGFR, ESKF, mortality). In addition, studies performing separate-sex analysis vary in sample size and length of follow-up and ethnic cohorts. Although many recent papers and guidelines on DKD generally mention male sex as a more invariant risk factor [16][27], the number of review articles analyzing individual studies on sex differences in DKD in more detail and helping to shed light on the literature jungle is increasing.
The lower survival rate of individuals with diabetes-related CKD, compared with individuals without CKD, is primarily due to the increased risk of concomitant morbidity associated with CKD, particularly cardiovascular disease. The lack of high-quality population-based studies with validated measures of CKD is the main reason why large differences in the epidemiology of CKD have been observed in populations with diabetes worldwide [16]. For example, women in Israel and Sweden have higher mortality rates if their T1DM developed in childhood, before puberty [28][29]. For men with T2DM and low testosterone levels, testosterone replacement therapy showed reduced mortality [30][31][32].
2.2. Factors That May Influence Sex Differences in DKD
Diverse factors may be determinants of sex differences in microvascular complications of T1DM and T2DM, which may be unchangeable (such as sex, biological age, and genetic predisposition) or influenceable (such as smoking, physical activity, or glycemic control) (Figure 1). The onset and duration of DM, puberty, or menopause also appear to play a major role in sex differences [12][22][33][34][35][36][37][38].
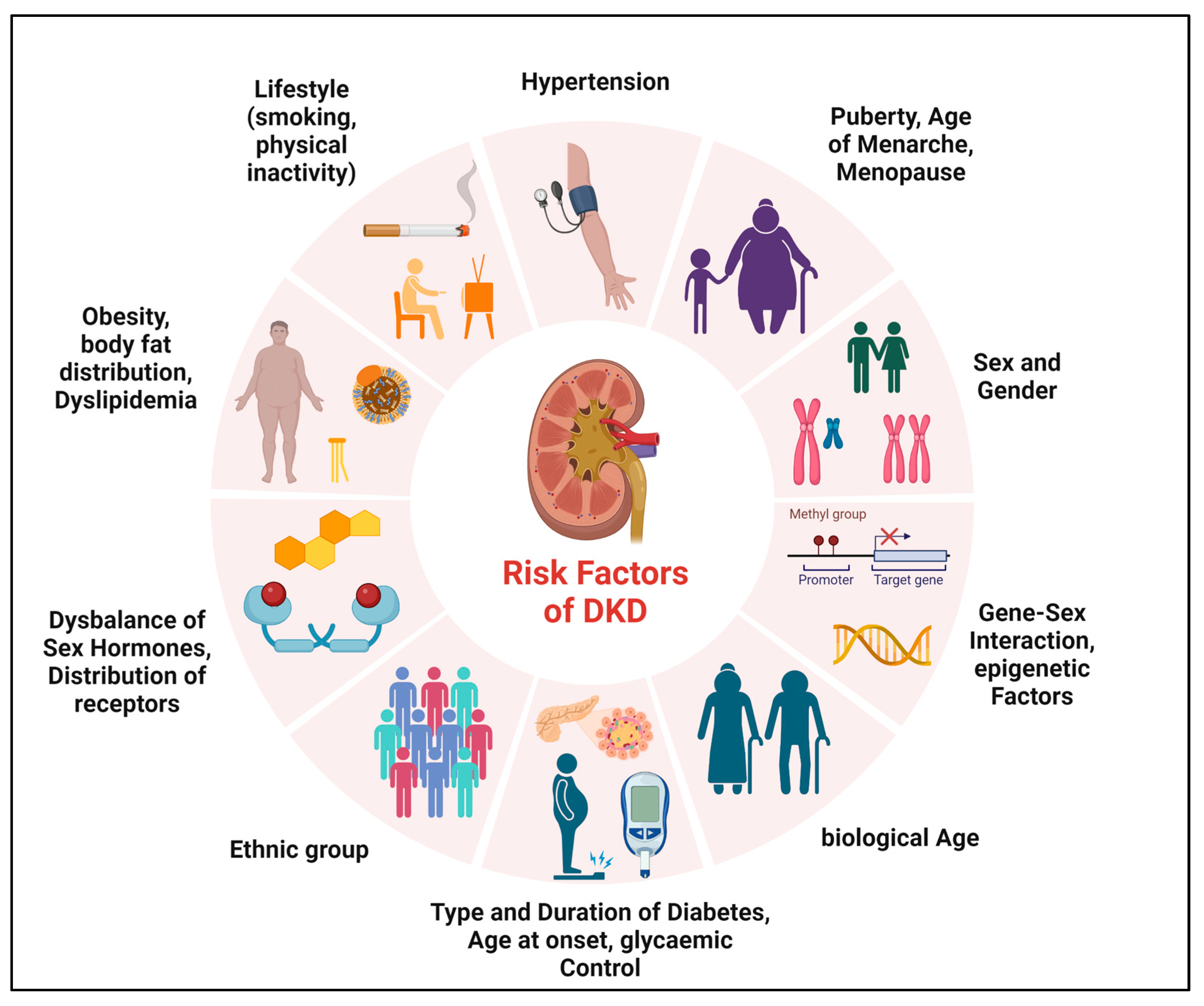
Figure 1. Determinants of sex differences in DKD. Various factors may be determinants of sex differences in the development and progression of DKD.
First and foremost, there are already sex differences in the development of diabetes. T2DM is diagnosed more often at a younger age and lower BMI in men, but the predominant risk factor, obesity, is more common in women [38]. Consistent with the analyses of this work, many studies have shown that women have higher body weights than men at diagnosis of T2DM [39][40]. In addition, newly diagnosed T2DM (>40 years) shows a positive association between small body size and the development of DKD in women [33][41]. There is also evidence that androgen acts directly in peripheral adipose tissue to promote insulin resistance [42]. This is evidenced, for example, by reduced insulin receptor autophosphorylation, decreased expression, and translocalization of the insulin-sensitive glucose transporter, and disruptions in insulin signaling pathways [42]. In contrast, premenopausal women have higher insulin sensitivity compared to postmenopausal women and estradiol has been shown to be protective against insulin resistance [43]. These data indicate that sensitivity to insulin in DM is influenced by sex hormones. Furthermore, the distribution of sex hormone receptors (estrogen and androgen) in subcutaneous adipose tissue is also different in men and women [44][45]. Thus, sex and sex hormones influence adipocyte development, adipogenesis, gene expression profiles responsible for insulin resistance, and lipolysis [44].
The quality of glycemic control in patients with T1DM also interacts with sex to determine renal prognosis. Interestingly, in one study, researchers found that among study participants who showed “good” metabolic control, females were more likely to develop DKD, while among participants with “poor” metabolic control, this likelihood was higher in males [12][22][35][46].
Studies have been able to demonstrate that the manifestation of diabetes disease differs in the sexes and the age of onset of DM, especially T1DM, plays an important role in sex differences in DKD risk [29][37][47]. While females are at a higher risk of developing microalbuminuria and even have a higher mortality rate if T1DM occurred in childhood [29], males are at higher risk for it if T1DM occurred with or after puberty [37]. A clear association has been found between higher testosterone levels in younger men and the development of microalbuminuria [48]. This association cannot be shown in an older population of patients with T1DM, reinforcing the concept that what happens in the early phase of diabetes has implications for events many years later [37][49].
There also appears to be a relationship between the onset of menarche and the risk of T1DM-induced microvascular complications: Women with menarche delayed more than 2 years had a 2.3-fold higher risk of DKD (as well as retinopathy) than women with menarche at the average age [50].
The literature on whether and to what extent the sex hormones estradiol and testosterone play a role in DKD is similarly confusing as that on sex differences in the DKD phenotype and is still controversial. However, there is now relative agreement that diabetes leads to an imbalance of sex hormones in both sexes [32][33][36][37][38][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70]. The vast majority of studies document that in men with T1DM or T2DM, estradiol levels increase, while testosterone levels decrease, although there are also T1DM data showing increased or unchanged testosterone (reviewed in [36]). In women, DM results in reduced or unchanged estradiol levels and increased testosterone or similar testosterone levels to non-diabetic controls (reviewed in ([36]). However, in postmenopausal women with T2DM, estradiol levels are elevated [33], which, together with the accelerated progression of DKD, may suggest a potential adverse effect of estradiol in the presence of DM.
It is not yet conclusively understood how testosterone and estrogen levels and their respective receptors relate to the progression of DKD in both sexes [33][36][37][38][51].
It is conceivable that an estradiol-mediated mechanism exacerbates the reduction in circulating testosterone in T2DM. This assumption is based on the fact that T2DM in men is associated with increased estradiol levels and that independent studies have shown that activation of the G protein-coupled estrogen receptor in isolated Leydig cells as well as in human testes can downregulate testosterone production [70].
A human study from Finland underlines that not as expected high testosterone levels in diabetic men are the cause of DKD, but that T1DM just leads to reduced serum testosterone concentrations, and that even with the progression of renal damage from micro- to macroalbuminuria, the reduction in testosterone is enhanced [49]. On the other hand, increased testosterone levels are detectable in premenopausal women with T2DM and are associated with insulin resistance and microvascular sequelae [36]. High androgen levels in diabetic women lead to susceptibility to microvascular damage, as DKD can do [70].
There is also a large body of research looking at genetic factors that may influence sex differences in DKD. Epidemiological studies have revealed familial clustering of DKD in both types of diabetes as well as a relevant influence of ethnic background [71]. The effects of sex chromosomes as well as the influence of gene–sex interactions with multiple susceptibility genes for DKD have been investigated and recently analyzed by Giandalia et al., for a review [71]. Among others, sex–gene interactions were found for a variant in the angiotensin gene or in the angiotensin II type 1 receptor gene and were described for genes implicated in inflammation and oxidation [71]. Sex differences were also found for variants in the carnosinase gene, CNDP1, on chromosome 18q [71]. A CNDP1 polymorphism associated with low CN1 activity correlates with a significantly reduced risk for DKD, especially in women with T2DM [72].
2.3. Data on Possible Underlying Mechanisms in Human DKD
There are a number of mechanisms that are causative for sexual dimorphism. These include mechanisms of hemodynamics (hyperfiltration and the renin–angiotensin–aldosterone system (RAAS)), or in oxidative and substrate stress metabolism, and also the interaction of sex hormones with the signal transduction of TGF-β1 (transforming growth factor beta 1), the main mediator of DKD development and progression, is already known.
As previously described, estradiol can downregulate testosterone production via activation of its receptor [70]. Sex-hormone-related mechanisms are also causal for the gender-specific differentiation of the RAAS, namely that men have a higher RAAS activity than women. While androgens can cause renal vasoconstriction through increased RAAS activity, estradiol on the one hand promotes higher angiotensinogen levels and the ACE2-angiotensin-1–7 axis and on the other hand reduces angiotensin-converting enzyme activity, renin levels, angiotensin II receptor type 1 (AT1R) density, aldosterone secretion, and angiotensin II activity [26].
In the case of oxidative stress, which is an essential pathophysiological feature of DKD, there are indications of sex differences to the disadvantage of the male sex (higher level of oxidative stress in men). The sex hormones play a regulating role in this context: estradiol acts as an antioxidant and androgens increase oxidative stress. Specifically in the kidney, hyperglycemia induces intracellular reactive oxygen species (ROS) in the renal mesangium and tubule cells. Advanced glycation end products (AGEs) and the cytokines TGF-β1 and ANGII are involved in this process. The ROS, in turn, are able to subsequently upregulate extracellular matrix expression via the transcription factors Nuclear Factor Kappa B (NF-κB) and Activator protein-1 (AP-1), which can lead to tubulointerstitial fibrosis (reviewed in [26]).
TGF-β1 is a key factor for pathophysiological processes in DKD. In both type 1 and type 2 diabetes, increased tubular and glomerular TGF-β expression is found in the early as well as late phase of the disease [73]. TGF-β-mediated effects influence the pathology of mesangial cells, podocytes, and endothelial and tubular cells. This leads to cell proliferation, hypertrophy, and apoptosis, and further to inflammation, glomerulosclerosis, and tubulointerstitial fibrosis [73]. By binding and activating its receptor, TGF-β induces a variety of signaling pathways, including both the classical SMAD pathway, which results in the transcription of target genes, and SMAD-independent pathways, such as Ras, JNK, p38, and PI3K [73]. Through TGF-ß1 signaling pathways, the cell has versatile capabilities to control developmental programs autocrine and paracrine, but on the other hand, dysfunctions in this fine-tuned signaling can lead to severe diseases such as the development of DKD [74]. Studies indicate that an important underlying mechanism by which sex hormones mediate their effects in DKD is through the regulation of TGF-β1 [75]. Estrogen can bind SMAD2/3 proteins and inhibit the TGF-β1-induced accumulation of extracellular matrix through activation of the estrogen receptor [75][76]. Another work demonstrated that estradiol can influence TGF-β1-mediated CTGF expression [77]. Regarding the influence of testosterone on TGF-β1, one work was able to show that before puberty there are almost no differences between the two sexes, but after puberty, a threefold higher TGF-β1 production prevails in females than in males, with the activation of latent TGF-β1 dominating in the male sex [78]. Accordingly, it is possible that after puberty, there is much more efficient TGF-β1 activation in males than in females, and in the female sex, the lower activation rates are compensated for by higher basal TGF-β1 levels [78].
2.4. Sex Aspects in Pharmacological Studies for Prevention and Treatment of DKD
It is well known that there is an unequal sex distribution in preclinical research and clinical trials in favor of the male sex. Conducting studies on only one sex and extrapolating the results to the opposite sex can result in reduced efficacy to harmful side effects that may go undetected in the disregarded sex until market launch (Figure 2) [79]. Overall, this is a problem that also applies to medications recommended for patients with DKD, which is why there are no sex-specific guidelines on therapeutic aspects to date.
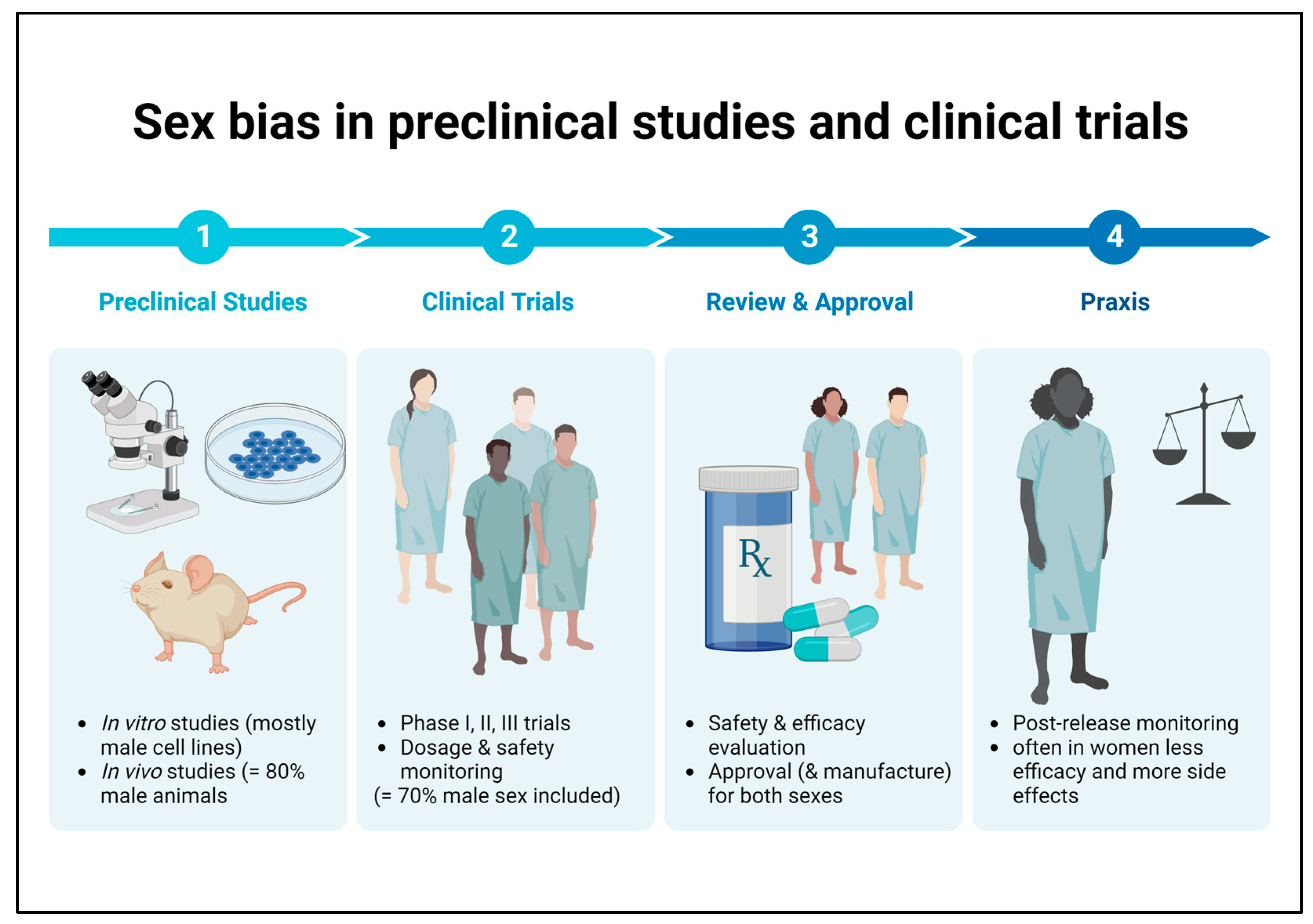
Figure 2. Sex bias in basic preclinical research and clinical trials. Male bias in animal studies as well as in human clinical trials may lead to reduced efficacy or harmful side effects in female patients that remain undetected until the post-marketing observation phase.
Potential therapeutic strategies applicable to the different mechanisms of sexual dimorphism target, for example, sex hormone imbalance, hemodynamic alterations, oxidative stress, or disturbances in water–electrolyte homeostasis and channels [26].
Fortunately, there are more and more editorial provisions from science journals for sex analyses. Since 2016, the SAGER (Sex and Gender Equity in Research) guidelines have been developed by the Gender Policy Committee of EASE (European Association of Science Editors). The SAGER guidelines emphasize strictly separating research subjects and data analysis by biological sex and gender, revealing significant differences even when there were not expected to be any [79]. The relatively recent examples provided below illustrate that these guidelines have not yet been implemented extensively and that a rethinking of study design is urgently needed.
2.4.1. Medications with Primary Reno-Protective Action
Most spironolactone, eplerenone, and finerenone trials of combined RAAS blockade with mineralocorticoid receptor antagonists included 65–98% men, and data were not analyzed separately by sex [80]. Moreover, in the two very important studies, FIDELIO and FIGARO, on the long-term effects of finerenone on kidney and cardiovascular outcomes, the overall population was predominately male (70%) [81][82].
In some important studies, although the sexes were equally distributed, the data were not analyzed separately by sex (e.g., the multicenter study of enalapril and losartan in T1DM; the BENEDICT study of the ACE inhibitor trandolapril and calcium channel blocker verapamil in T2DM; the olmesartan study; the captopril study) [83][84][85][86]. The importance of a separate-sex analysis is shown by a study of irbesartan in DKD (Irbesartan in DN Trial). This study, which actually analyzed data by sex, showed that the progression of DKD is more rapid in women than in men and that women benefit less from treatment than men [33][87].
Blockade of endothelin receptor-A has shown significant antiproteinuric effects, and while the trial of the first endothelin receptor-A antagonist tested in phase 3, avosentan, had to be stopped because of side effects, atrasentan seems more promising. Unfortunately, there is also a clear sex bias to the disadvantage of women in the available studies of endothelin receptor type A blockade, as highlighted in the 2019 multicenter study of the effect of atrasentan in DKD in T2DM published in The Lancet. Here, 971 men vs. 352 women were treated with a placebo and 994 men vs. 331 women were treated with atrasentan [88].
2.4.2. Antidiabetic Medications with Reno-Protective Effect
Studies of glucose-reducing agents with reno-protective effects, such as the GLP1 (glucagon-like peptide 1) receptor agonists and sodium–glucose transporter 2 (SGLT2) inhibitors, also show a clear sex bias. In a 2017 multicenter study of liraglutide, 64% of the approximately 4600 patients with T2DM were men, and in a study from Denmark, as many as 84% of the patients with T2DM treated were men (reviewed in [89]).
A 2019 Italian meta-analysis of seven trials involving 56,004 patients with T2DM treated with lixisenatide, liraglutide, exenatide, albiglutide, dulaglutide, and semaglutide did not report the proportion of men and women included or the effects of treatment on each sex [90].
In addition to their antihyperglycemic properties, SGLT2 inhibitors also show protective renal effects. They affect hemodynamics, oxidative stress, water–electrolyte homeostasis, and disruption in adiponectin [26]. Except for the CANTATA-SU study in which equal numbers of women and men with T2DM were treated with canagliflozin and glimepiride for nearly 1 year, the studies of empagliflozin (EMPA-KIDNEY, EMPA-REG OUTCOME), dapagliflozin (DAPA-CKD), and canagliflozin (CREDENCE) either included two-thirds men or did not report [89][91][92][93][94][95]. A recent meta-analysis showed that reductions in major adverse cardiac events with SGLT2 inhibitors were lower in women with diabetes than in men with diabetes [96]. Interestingly, in animal studies, higher expression of SGLT2 was found in female rats than in male rats [97]. To what extent the effects shown in the clinical studies can be explained by a possible sex-specific expression of SGLT2 also present in humans certainly requires more intensive research.
References
- ORWH. Sex & Gender. Available online: https://orwh.od.nih.gov/sex-gender (accessed on 12 February 2019).
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045.
- Ioannou, K. Diabetic nephropathy: Is it always there? Assumptions, weaknesses and pitfalls in the diagnosis. Hormones 2017, 16, 351–361.
- Sagoo, M.K.; Gnudi, L. Diabetic Nephropathy: An Overview. Methods Mol. Biol. 2020, 2067, 3–7.
- Qi, C.; Mao, X.; Zhang, Z.; Wu, H. Classification and Differential Diagnosis of Diabetic Nephropathy. J. Diabetes Res. 2017, 2017, 8637138.
- Najafian, B.; Alpers, C.E.; Fogo, A.B. Pathology of human diabetic nephropathy. Contrib. Nephrol. 2011, 170, 36–47.
- Wolf, G.; Ritz, E. Diabetic nephropathy in type 2 diabetes prevention and patient management. J. Am. Soc. Nephrol. 2003, 14, 1396–1405.
- Iwano, M.; Neilson, E.G. Mechanisms of tubulointerstitial fibrosis. Curr. Opin. Nephrol. Hypertens. 2004, 13, 279–284.
- Li, R.; Chung, A.C.; Dong, Y.; Yang, W.; Zhong, X.; Lan, H.Y. The microRNA miR-433 promotes renal fibrosis by amplifying the TGF-β/Smad3-Azin1 pathway. Kidney Int. 2013, 84, 1129–1144.
- Neugarten, J.; Acharya, A.; Silbiger, S.R. Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. J. Am. Soc. Nephrol. 2000, 11, 319–329.
- Coggins, C.H.; Breyer Lewis, J.; Caggiula, A.W.; Castaldo, L.S.; Klahr, S.; Wang, S.R. Differences between women and men with chronic renal disease. Nephrol. Dial. Transpl. 1998, 13, 1430–1437.
- Silbiger, S.; Neugarten, J. Gender and human chronic renal disease. Gend. Med. 2008, 5 (Suppl. S1), S3–S10.
- Eriksen, B.O.; Ingebretsen, O.C. The progression of chronic kidney disease: A 10-year population-based study of the effects of gender and age. Kidney Int. 2006, 69, 375–382.
- Jafar, T.H.; Schmid, C.H.; Stark, P.C.; Toto, R.; Remuzzi, G.; Ruggenenti, P.; Marcantoni, C.; Becker, G.; Shahinfar, S.; De Jong, P.E.; et al. The rate of progression of renal disease may not be slower in women compared with men: A patient-level meta-analysis. Nephrol. Dial. Transpl. 2003, 18, 2047–2053.
- Garovic, V.D.; August, P. Sex Differences and Renal Protection: Keeping in Touch with Your Feminine Side. J. Am. Soc. Nephrol. 2016, 27, 2921–2924.
- Koye, D.N.; Magliano, D.J.; Nelson, R.G.; Pavkov, M.E. The Global Epidemiology of Diabetes and Kidney Disease. Adv. Chronic Kidney Dis. 2018, 25, 121–132.
- Brück, K.; Stel, V.S.; Gambaro, G.; Hallan, S.; Völzke, H.; Ärnlöv, J.; Kastarinen, M.; Guessous, I.; Vinhas, J.; Stengel, B.; et al. CKD Prevalence Varies across the European General Population. J. Am. Soc. Nephrol. 2016, 27, 2135–2147.
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258.
- Retnakaran, R.; Cull, C.A.; Thorne, K.I.; Adler, A.I.; Holman, R.R. Risk factors for renal dysfunction in type 2 diabetes: U.K. Prospective Diabetes Study 74. Diabetes 2006, 55, 1832–1839.
- Raile, K.; Galler, A.; Hofer, S.; Herbst, A.; Dunstheimer, D.; Busch, P.; Holl, R.W. Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: Effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes Care 2007, 30, 2523–2528.
- Schultz, C.J.; Konopelska-Bahu, T.; Dalton, R.N.; Carroll, T.A.; Stratton, I.; Gale, E.A.; Neil, A.; Dunger, D.B. Microalbuminuria prevalence varies with age, sex, and puberty in children with type 1 diabetes followed from diagnosis in a longitudinal study. Oxford Regional Prospective Study Group. Diabetes Care 1999, 22, 495–502.
- Zhang, L.; Krzentowski, G.; Albert, A.; Lefèbvre, P.J. Factors predictive of nephropathy in DCCT Type 1 diabetic patients with good or poor metabolic control. Diabet. Med. 2003, 20, 580–585.
- Yu, M.K.; Katon, W.; Young, B.A. Associations between sex and incident chronic kidney disease in a prospective diabetic cohort. Nephrology 2015, 20, 451–458.
- Finne, P.; Reunanen, A.; Stenman, S.; Groop, P.H.; Gronhagen-Riska, C. Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA 2005, 294, 1782–1787.
- Okada, K.; Yanai, M.; Takeuchi, K.; Matsuyama, K.; Nitta, K.; Hayashi, K.; Takahashi, S. Sex differences in the prevalence, progression, and improvement of chronic kidney disease. Kidney Blood Press Res. 2014, 39, 279–288.
- Piani, F.; Melena, I.; Tommerdahl, K.L.; Nokoff, N.; Nelson, R.G.; Pavkov, M.E.; van Raalte, D.H.; Cherney, D.Z.; Johnson, R.J.; Nadeau, K.J.; et al. Sex-related differences in diabetic kidney disease: A review on the mechanisms and potential therapeutic implications. J. Diabetes Complicat. 2021, 35, 107841.
- von Bundesärztekammer, E.P.; Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF). Nationale VersorgungsLeitlinie Typ-2-Diabetes—Teilpublikation der Langfassung, 2. Auflage; Version 1. Available online: www.leitlinien.de/diabetes (accessed on 13 September 2021).
- Laron-Kenet, T.; Shamis, I.; Weitzman, S.; Rosen, S.; Laron, Z.V. Mortality of patients with childhood onset (0–17 years) Type I diabetes in Israel: A population-based study. Diabetologia 2001, 44 (Suppl. S3), B81–B86.
- Dahlquist, G.; Källén, B. Mortality in childhood-onset type 1 diabetes: A population-based study. Diabetes Care 2005, 28, 2384–2387.
- Hackett, G.; Cole, N.; Mulay, A.; Strange, R.C.; Ramachandran, S. Long-term testosterone therapy in type 2 diabetes is associated with reduced mortality without improvement in conventional cardiovascular risk factors. BJU Int. 2019, 123, 519–529.
- Alwani, M.; Al-Zoubi, R.M.; Al-Qudimat, A.; Yassin, A.; Aboumarzouk, O.; Al-Rumaihi, K.; Talib, R.; Al-Ansari, A. The impact of long-term Testosterone Therapy (TTh) in renal function (RF) among hypogonadal men: An observational cohort study. Ann. Med. Surg. 2021, 69, 102748.
- Maric-Bilkan, C. Sex differences in micro- and macro-vascular complications of diabetes mellitus. Clin. Sci. 2017, 131, 833–846.
- Maric, C.; Sullivan, S. Estrogens and the diabetic kidney. Gend. Med. 2008, 5 (Suppl. S1), S103–S113.
- Holl, R.W.; Grabert, M.; Thon, A.; Heinze, E. Urinary excretion of albumin in adolescents with type 1 diabetes: Persistent versus intermittent microalbuminuria and relationship to duration of diabetes, sex, and metabolic control. Diabetes Care 1999, 22, 1555–1560.
- Kautzky-Willer, A.; Handisurya, A. Metabolic diseases and associated complications: Sex and gender matter! Eur. J. Clin. Investig. 2009, 39, 631–648.
- Maric, C. Sex, diabetes and the kidney. Am. J. Physiol. Ren. Physiol. 2009, 296, F680–F688.
- Harvey, J.N. The influence of sex and puberty on the progression of diabetic nephropathy and retinopathy. Diabetologia 2011, 54, 1943–1945.
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316.
- Wannamethee, S.G.; Papacosta, O.; Lawlor, D.A.; Whincup, P.H.; Lowe, G.D.; Ebrahim, S.; Sattar, N. Do women exhibit greater differences in established and novel risk factors between diabetes and non-diabetes than men? The British Regional Heart Study and British Women’s Heart Health Study. Diabetologia 2012, 55, 80–87.
- Paul, S.K.; Owusu Adjah, E.S.; Samanta, M.; Patel, K.; Bellary, S.; Hanif, W.; Khunti, K. Comparison of body mass index at diagnosis of diabetes in a multi-ethnic population: A case-control study with matched non-diabetic controls. Diabetes Obes. Metab. 2017, 19, 1014–1023.
- Olivarius, N.d.F.; Vestbo, E.; Andreasen, A.H.; Mogensen, C.E. Renal involvement is related to body height in newly diagnosed diabetic women aged 40 years or over. Diabetes Metab. 2001, 27, 14–18.
- Livingstone, C.; Collison, M. Sex steroids and insulin resistance. Clin. Sci. 2002, 102, 151–166.
- Yan, H.; Yang, W.; Zhou, F.; Li, X.; Pan, Q.; Shen, Z.; Han, G.; Newell-Fugate, A.; Tian, Y.; Majeti, R.; et al. Estrogen Improves Insulin Sensitivity and Suppresses Gluconeogenesis via the Transcription Factor Foxo1. Diabetes 2019, 68, 291–304.
- Chang, E.; Varghese, M.; Singer, K. Gender and Sex Differences in Adipose Tissue. Curr. Diabetes Rep. 2018, 18, 69.
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206.
- Sibley, S.D.; Thomas, W.; de Boer, I.; Brunzell, J.D.; Steffes, M.W. Gender and elevated albumin excretion in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort: Role of central obesity. Am. J. Kidney Dis. 2006, 47, 223–232.
- Shepard, B.D. Sex differences in diabetes and kidney disease: Mechanisms and consequences. Am. J. Physiol. Ren. Physiol. 2019, 317, F456–F462.
- Amin, R.; Schultz, C.; Ong, K.; Frystyk, J.; Dalton, R.N.; Perry, L.; Ørskov, H.; Dunger, D.B. Low IGF-I and elevated testosterone during puberty in subjects with type 1 diabetes developing microalbuminuria in comparison to normoalbuminuric control subjects: The Oxford Regional Prospective Study. Diabetes Care 2003, 26, 1456–1461.
- Maric, C.; Forsblom, C.; Thorn, L.; Wadén, J.; Groop, P.H. Association between testosterone, estradiol and sex hormone binding globulin levels in men with type 1 diabetes with nephropathy. Steroids 2010, 75, 772–778.
- Harjutsalo, V.; Maric-Bilkan, C.; Forsblom, C.; Groop, P.H.; FinnDiane Study Group. Age at menarche and the risk of diabetic microvascular complications in patients with type 1 diabetes. Diabetologia 2016, 59, 472–480.
- Xu, Q.; Wells, C.C.; Garman, J.H.; Asico, L.; Escano, C.S.; Maric, C. Imbalance in sex hormone levels exacerbates diabetic renal disease. Hypertension 2008, 51, 1218–1224.
- Stamataki, K.E.; Spina, J.; Rangou, D.B.; Chlouverakis, C.S.; Piaditis, G.P. Ovarian function in women with non-insulin dependent diabetes mellitus. Clin. Endocrinol. 1996, 45, 615–621.
- Chin, M.; Isono, M.; Isshiki, K.; Araki, S.; Sugimoto, T.; Guo, B.; Sato, H.; Haneda, M.; Kashiwagi, A.; Koya, D. Estrogen and raloxifene, a selective estrogen receptor modulator, ameliorate renal damage in db/db mice. Am. J. Pathol. 2005, 166, 1629–1636.
- Lamon-Fava, S.; Barnett, J.B.; Woods, M.N.; McCormack, C.; McNamara, J.R.; Schaefer, E.J.; Longcope, C.; Rosner, B.; Gorbach, S.L. Differences in serum sex hormone and plasma lipid levels in Caucasian and African-American premenopausal women. J. Clin. Endocrinol. Metab. 2005, 90, 4516–4520.
- Tamura, K.; Osada, S.; Matsushita, M.; Abe, K.; Kogo, H. Changes in ovarian steroidogenesis in insulin-resistant, type 2 diabetic Goto-Kakizaki rats after thyroidectomy and gonadotropin treatment. Eur. J. Pharmacol. 2005, 513, 151–157.
- Wells, C.C.; Riazi, S.; Mankhey, R.W.; Bhatti, F.; Ecelbarger, C.; Maric, C. Diabetic nephropathy is associated with decreased circulating estradiol levels and imbalance in the expression of renal estrogen receptors. Gend. Med. 2005, 2, 227–237.
- Ding, E.L.; Song, Y.; Malik, V.S.; Liu, S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: A systematic review and meta-analysis. JAMA 2006, 295, 1288–1299.
- Kim, N.N.; Stankovic, M.; Cushman, T.T.; Goldstein, I.; Munarriz, R.; Traish, A.M. Streptozotocin-induced diabetes in the rat is associated with changes in vaginal hemodynamics, morphology and biochemical markers. BMC Physiol. 2006, 6, 4.
- Salonia, A.; Lanzi, R.; Scavini, M.; Pontillo, M.; Gatti, E.; Petrella, G.; Licata, G.; Nappi, R.E.; Bosi, E.; Briganti, A.; et al. Sexual function and endocrine profile in fertile women with type 1 diabetes. Diabetes Care 2006, 29, 312–316.
- Sun, J.; Devish, K.; Langer, W.J.; Carmines, P.K.; Lane, P.H. Testosterone treatment promotes tubular damage in experimental diabetes in prepubertal rats. Am. J. Physiol. Ren. Physiol. 2007, 292, F1681–F1690.
- Prabhu, A.; Xu, Q.; Manigrasso, M.B.; Biswas, M.; Flynn, E.; Iliescu, R.; Lephart, E.D.; Maric, C. Expression of aromatase, androgen and estrogen receptors in peripheral target tissues in diabetes. Steroids 2010, 75, 779–787.
- Grossmann, M.E.; Mizuno, N.K.; Bonorden, M.J.; Ray, A.; Sokolchik, I.; Narasimhan, M.L.; Cleary, M.P. Role of the adiponectin leptin ratio in prostate cancer. Oncol. Res. 2009, 18, 269–277.
- Grossmann, M.; Panagiotopolous, S.; Sharpe, K.; MacIsaac, R.J.; Clarke, S.; Zajac, J.D.; Jerums, G.; Thomas, M.C. Low testosterone and anaemia in men with type 2 diabetes. Clin. Endocrinol. 2009, 70, 547–553.
- Grossmann, M.; Thomas, M.C.; Panagiotopoulos, S.; Sharpe, K.; Macisaac, R.J.; Clarke, S.; Zajac, J.D.; Jerums, G. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1834–1840.
- Maric-Bilkan, C.; Galis, Z.S. Trends in NHLBI-Funded Research on Sex Differences in Hypertension. Circ. Res. 2016, 119, 591–595.
- Maric-Bilkan, C.; Arnold, A.P.; Taylor, D.A.; Dwinell, M.; Howlett, S.E.; Wenger, N.; Reckelhoff, J.F.; Sandberg, K.; Churchill, G.; Levin, E.; et al. Report of the National Heart, Lung, and Blood Institute Working Group on Sex Differences Research in Cardiovascular Disease: Scientific Questions and Challenges. Hypertension 2016, 67, 802–807.
- Andersson, B.; Mårin, P.; Lissner, L.; Vermeulen, A.; Björntorp, P. Testosterone concentrations in women and men with NIDDM. Diabetes Care 1994, 17, 405–411.
- Stellato, R.K.; Feldman, H.A.; Hamdy, O.; Horton, E.S.; McKinlay, J.B. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: Prospective results from the Massachusetts male aging study. Diabetes Care 2000, 23, 490–494.
- Tsai, E.C.; Matsumoto, A.M.; Fujimoto, W.Y.; Boyko, E.J. Association of bioavailable, free, and total testosterone with insulin resistance: Influence of sex hormone-binding globulin and body fat. Diabetes Care 2004, 27, 861–868.
- Clotet, S.; Riera, M.; Pascual, J.; Soler, M.J. RAS and sex differences in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2016, 310, F945–F957.
- Giandalia, A.; Giuffrida, A.E.; Gembillo, G.; Cucinotta, D.; Squadrito, G.; Santoro, D.; Russo, G.T. Gender Differences in Diabetic Kidney Disease: Focus on Hormonal, Genetic and Clinical Factors. Int. J. Mol. Sci. 2021, 22, 5808.
- Peters, V.; Zschocke, J.; Schmitt, C.P. Carnosinase, diabetes mellitus and the potential relevance of carnosinase deficiency. J. Inherit. Metab. Dis. 2018, 41, 39–47.
- Loeffler, I.; Wolf, G. Transforming growth factor-beta and the progression of renal disease. Nephrol. Dial. Transpl. 2014, 29 (Suppl. S1), i37–i45.
- Massagué, J. TGFβ signalling in context. Nat. Rev. Mol. Cell Biol. 2012, 13, 616–630.
- Diamond-Stanic, M.K.; You, Y.H.; Sharma, K. Sugar, sex, and TGF-β in diabetic nephropathy. Semin. Nephrol. 2012, 32, 261–268.
- Ito, I.; Hanyu, A.; Wayama, M.; Goto, N.; Katsuno, Y.; Kawasaki, S.; Nakajima, Y.; Kajiro, M.; Komatsu, Y.; Fujimura, A.; et al. Estrogen inhibits transforming growth factor beta signaling by promoting Smad2/3 degradation. J. Biol. Chem. 2010, 285, 14747–14755.
- Kumar, A.; Ruan, M.; Clifton, K.; Syed, F.; Khosla, S.; Oursler, M.J. TGF-β mediates suppression of adipogenesis by estradiol through connective tissue growth factor induction. Endocrinology 2012, 153, 254–263.
- Lane, P.H.; Snelling, D.M.; Babushkina-Patz, N.; Langer, W.J. Sex differences in the renal transforming growth factor-beta 1 system after puberty. Pediatr. Nephrol. 2001, 16, 61–68.
- Lee, S.K. Sex as an important biological variable in biomedical research. BMB Rep. 2018, 51, 167–173.
- Sarafidis, P.A.; Memmos, E.; Alexandrou, M.E.; Papagianni, A. Mineralocorticoid Receptor Antagonists for Nephroprotection: Current Evidence and Future Perspectives. Curr. Pharm. Des. 2018, 24, 5528–5536.
- Ruilope, L.M.; Agarwal, R.; Anker, S.D.; Bakris, G.L.; Filippatos, G.; Nowack, C.; Kolkhof, P.; Joseph, A.; Mentenich, N.; Pitt, B.; et al. Design and Baseline Characteristics of the Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease Trial. Am. J. Nephrol. 2019, 50, 345–356.
- Bakris, G.L.; Agarwal, R.; Anker, S.D.; Pitt, B.; Ruilope, L.M.; Rossing, P.; Kolkhof, P.; Nowack, C.; Schloemer, P.; Joseph, A.; et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N. Engl. J. Med. 2020, 383, 2219–2229.
- Lewis, E.J.; Hunsicker, L.G.; Bain, R.P.; Rohde, R.D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 1993, 329, 1456–1462.
- Ruggenenti, P.; Fassi, A.; Ilieva, A.P.; Bruno, S.; Iliev, I.P.; Brusegan, V.; Rubis, N.; Gherardi, G.; Arnoldi, F.; Ganeva, M.; et al. Preventing microalbuminuria in type 2 diabetes. N. Engl. J. Med. 2004, 351, 1941–1951.
- Mauer, M.; Zinman, B.; Gardiner, R.; Suissa, S.; Sinaiko, A.; Strand, T.; Drummond, K.; Donnelly, S.; Goodyer, P.; Gubler, M.C.; et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N. Engl. J. Med. 2009, 361, 40–51.
- Haller, H.; Ito, S.; Izzo, J.L., Jr.; Januszewicz, A.; Katayama, S.; Menne, J.; Mimran, A.; Rabelink, T.J.; Ritz, E.; Ruilope, L.M.; et al. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N. Engl. J. Med. 2011, 364, 907–917.
- Lewis, E.J.; Hunsicker, L.G.; Rodby, R.A.; The Collaborative Study Group. A clinical trial in type 2 diabetic nephropathy. Am. J. Kidney Dis. 2001, 38, S191–S194.
- Heerspink, H.J.L.; Parving, H.H.; Andress, D.L.; Bakris, G.; Correa-Rotter, R.; Hou, F.F.; Kitzman, D.W.; Kohan, D.; Makino, H.; McMurray, J.J.V.; et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): A double-blind, randomised, placebo-controlled trial. Lancet 2019, 393, 1937–1947.
- Rossing, P.; Persson, F.; Frimodt-Møller, M. Prognosis and treatment of diabetic nephropathy: Recent advances and perspectives. Nephrol. Ther. 2018, 14 (Suppl. S1), S31–S37.
- Giugliano, D.; De Nicola, L.; Maiorino, M.I.; Bellastella, G.; Esposito, K. Type 2 diabetes and the kidney: Insights from cardiovascular outcome trials. Diabetes Obes. Metab. 2019, 21, 1790–1800.
- Marso, S.P.; Poulter, N.R.; Nissen, S.E.; Nauck, M.A.; Zinman, B.; Daniels, G.H.; Pocock, S.; Steinberg, W.M.; Bergenstal, R.M.; Mann, J.F.; et al. Design of the liraglutide effect and action in diabetes: Evaluation of cardiovascular outcome results (LEADER) trial. Am. Heart J. 2013, 166, 823–830.e825.
- Heerspink, H.J.; Desai, M.; Jardine, M.; Balis, D.; Meininger, G.; Perkovic, V. Canagliflozin Slows Progression of Renal Function Decline Independently of Glycemic Effects. J. Am. Soc. Nephrol. 2017, 28, 368–375.
- Takashima, H.; Yoshida, Y.; Nagura, C.; Furukawa, T.; Tei, R.; Maruyama, T.; Maruyama, N.; Abe, M. Renoprotective effects of canagliflozin, a sodium glucose cotransporter 2 inhibitor, in type 2 diabetes patients with chronic kidney disease: A randomized open-label prospective trial. Diabetes Vasc. Dis. Res. 2018, 15, 469–472.
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306.
- Davidson, J.A. SGLT2 inhibitors in patients with type 2 diabetes and renal disease: Overview of current evidence. Postgrad. Med. 2019, 131, 251–260.
- Singh, A.K.; Singh, R. Gender difference in cardiovascular outcomes with SGLT-2 inhibitors and GLP-1 receptor agonist in type 2 diabetes: A systematic review and meta-analysis of cardio-vascular outcome trials. Diabetes Metab. Syndr. 2020, 14, 181–187.
- Sabolic, I.; Vrhovac, I.; Eror, D.B.; Gerasimova, M.; Rose, M.; Breljak, D.; Ljubojevic, M.; Brzica, H.; Sebastiani, A.; Thal, S.C.; et al. Expression of Na+-D-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am. J. Physiol. Cell Physiol. 2012, 302, C1174–C1188.
More
Information
Subjects:
Urology & Nephrology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
23 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




