Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ivonne Loeffler and Version 2 by Camila Xu.
Diabetic kidney disease (DKD) is a secondary disease of type 1 and type 2 diabetes mellitus (T1DM and T2DM). This microvascular complication develops in approximately 30% of patients with T1DM and 40% of patients with T2DM and is characterized by the presence of albuminuria and the progressive loss of renal function.
- diabetic kidney disease
- DKD
- sex differences
- gender
1. Introduction
The influence of biological sex differences on human disease has long been underestimated and underresearched. Until recent decades, the vast majority of clinical research was conducted with predominantly male participants. In addition, preclinical research using animal models has almost exclusively examined male animals. Despite this limited approach, it was often (and sometimes still is) assumed that research findings and medical treatments developed from those findings apply to the entire population [1]. However, the resulting lack of understanding limits the ability to treat with targeted and patient-centered therapies. This can have life-threatening consequences for many serious conditions, such as cancer or cardiovascular disease.
DKD is a secondary disease of type 1 and type 2 diabetes mellitus (T1DM and T2DM). This microvascular complication develops in approximately 30% of patients with T1DM and 40% of patients with T2DM [2] and is characterized by the presence of albuminuria and the progressive loss of renal function [3]. Persistent high blood glucose levels in patients with DM lead to the disruption and damage of the microvascular architecture of the kidneys [4]. As a result, small ultrastructural changes occur in the nephron, mainly localized in the glomerulus and proximal tubule compartment [5]. In renal biopsies of clinical patients with DKD, glomerular changes are most frequently observed [6]. Initial changes include thickening of the glomerular basement membrane (stage I), mild mesangial expansion (>25%), glomerular hypertrophy, and mild microalbuminuria (<30–300 mg/d, stage IIa) [5][6][5,6]. Progression of DKD increases the risk of cardiovascular disease and is characterized by an increase in albuminuria (macroalbuminuria > 300 mg/d), severe diffuse mesangial expansion, nodular sclerotic changes (Kimmelstiel–Wilson lesion), decrease in glomerular filtration rate, hyalinosis of afferent and efferent arterioles, loss of podocytes, thickening of tubular basement membrane, tubulointerstitial fibrosis/inflammation, and tubular atrophy (stage IIb-IV) [5][6][7][5,6,7]. Signs of tubulointerstitial fibrosis (TIF) include myofibroblast accumulation, excessive extracellular matrix (ECM) deposition, and renal tubule destruction [8][9][8,9].
 First and foremost, there are already sex differences in the development of diabetes. T2DM is diagnosed more often at a younger age and lower BMI in men, but the predominant risk factor, obesity, is more common in women [38][50]. Consistent with the analyses of this work, many studies have shown that women have higher body weights than men at diagnosis of T2DM [39][40][51,52]. In addition, newly diagnosed T2DM (>40 years) shows a positive association between small body size and the development of DKD in women [33][41][32,53]. There is also evidence that androgen acts directly in peripheral adipose tissue to promote insulin resistance [42][54]. This is evidenced, for example, by reduced insulin receptor autophosphorylation, decreased expression, and translocalization of the insulin-sensitive glucose transporter, and disruptions in insulin signaling pathways [42][54]. In contrast, premenopausal women have higher insulin sensitivity compared to postmenopausal women and estradiol has been shown to be protective against insulin resistance [43][55]. These data indicate that sensitivity to insulin in DM is influenced by sex hormones. Furthermore, the distribution of sex hormone receptors (estrogen and androgen) in subcutaneous adipose tissue is also different in men and women [44][45][56,57]. Thus, sex and sex hormones influence adipocyte development, adipogenesis, gene expression profiles responsible for insulin resistance, and lipolysis [44][56].
The quality of glycemic control in patients with T1DM also interacts with sex to determine renal prognosis. Interestingly, in one study, researchers found that among study participants who showed “good” metabolic control, females were more likely to develop DKD, while among participants with “poor” metabolic control, this likelihood was higher in males [12][22][35][46][12,22,47,58].
Studies have been able to demonstrate that the manifestation of diabetes disease differs in the sexes and the age of onset of DM, especially T1DM, plays an important role in sex differences in DKD risk [29][37][47][42,49,59]. While females are at a higher risk of developing microalbuminuria and even have a higher mortality rate if T1DM occurred in childhood [29][42], males are at higher risk for it if T1DM occurred with or after puberty [37][49]. A clear association has been found between higher testosterone levels in younger men and the development of microalbuminuria [48][60]. This association cannot be shown in an older population of patients with T1DM, reinforcing the concept that what happens in the early phase of diabetes has implications for events many years later [37][49][49,61].
There also appears to be a relationship between the onset of menarche and the risk of T1DM-induced microvascular complications: Women with menarche delayed more than 2 years had a 2.3-fold higher risk of DKD (as well as retinopathy) than women with menarche at the average age [50][62].
The literature on whether and to what extent the sex hormones estradiol and testosterone play a role in DKD is similarly confusing as that on sex differences in the DKD phenotype and is still controversial. However, there is now relative agreement that diabetes leads to an imbalance of sex hormones in both sexes [32][33][36][37][38][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][32,45,48,49,50,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82]. The vast majority of studies document that in men with T1DM or T2DM, estradiol levels increase, while testosterone levels decrease, although there are also T1DM data showing increased or unchanged testosterone (reviewed in [36][48]). In women, DM results in reduced or unchanged estradiol levels and increased testosterone or similar testosterone levels to non-diabetic controls (reviewed in ([36][48]). However, in postmenopausal women with T2DM, estradiol levels are elevated [33][32], which, together with the accelerated progression of DKD, may suggest a potential adverse effect of estradiol in the presence of DM.
It is not yet conclusively understood how testosterone and estrogen levels and their respective receptors relate to the progression of DKD in both sexes [33][36][37][38][51][32,48,49,50,63].
It is conceivable that an estradiol-mediated mechanism exacerbates the reduction in circulating testosterone in T2DM. This assumption is based on the fact that T2DM in men is associated with increased estradiol levels and that independent studies have shown that activation of the G protein-coupled estrogen receptor in isolated Leydig cells as well as in human testes can downregulate testosterone production [70][82].
A human study from Finland underlines that not as expected high testosterone levels in diabetic men are the cause of DKD, but that T1DM just leads to reduced serum testosterone concentrations, and that even with the progression of renal damage from micro- to macroalbuminuria, the reduction in testosterone is enhanced [49][61]. On the other hand, increased testosterone levels are detectable in premenopausal women with T2DM and are associated with insulin resistance and microvascular sequelae [36][48]. High androgen levels in diabetic women lead to susceptibility to microvascular damage, as DKD can do [70][82].
There is also a large body of research looking at genetic factors that may influence sex differences in DKD. Epidemiological studies have revealed familial clustering of DKD in both types of diabetes as well as a relevant influence of ethnic background [71][28]. The effects of sex chromosomes as well as the influence of gene–sex interactions with multiple susceptibility genes for DKD have been investigated and recently analyzed by Giandalia et al., for a review [71][28]. Among others, sex–gene interactions were found for a variant in the angiotensin gene or in the angiotensin II type 1 receptor gene and were described for genes implicated in inflammation and oxidation [71][28]. Sex differences were also found for variants in the carnosinase gene, CNDP1, on chromosome 18q [71][28]. A CNDP1 polymorphism associated with low CN1 activity correlates with a significantly reduced risk for DKD, especially in women with T2DM [72][83].
First and foremost, there are already sex differences in the development of diabetes. T2DM is diagnosed more often at a younger age and lower BMI in men, but the predominant risk factor, obesity, is more common in women [38][50]. Consistent with the analyses of this work, many studies have shown that women have higher body weights than men at diagnosis of T2DM [39][40][51,52]. In addition, newly diagnosed T2DM (>40 years) shows a positive association between small body size and the development of DKD in women [33][41][32,53]. There is also evidence that androgen acts directly in peripheral adipose tissue to promote insulin resistance [42][54]. This is evidenced, for example, by reduced insulin receptor autophosphorylation, decreased expression, and translocalization of the insulin-sensitive glucose transporter, and disruptions in insulin signaling pathways [42][54]. In contrast, premenopausal women have higher insulin sensitivity compared to postmenopausal women and estradiol has been shown to be protective against insulin resistance [43][55]. These data indicate that sensitivity to insulin in DM is influenced by sex hormones. Furthermore, the distribution of sex hormone receptors (estrogen and androgen) in subcutaneous adipose tissue is also different in men and women [44][45][56,57]. Thus, sex and sex hormones influence adipocyte development, adipogenesis, gene expression profiles responsible for insulin resistance, and lipolysis [44][56].
The quality of glycemic control in patients with T1DM also interacts with sex to determine renal prognosis. Interestingly, in one study, researchers found that among study participants who showed “good” metabolic control, females were more likely to develop DKD, while among participants with “poor” metabolic control, this likelihood was higher in males [12][22][35][46][12,22,47,58].
Studies have been able to demonstrate that the manifestation of diabetes disease differs in the sexes and the age of onset of DM, especially T1DM, plays an important role in sex differences in DKD risk [29][37][47][42,49,59]. While females are at a higher risk of developing microalbuminuria and even have a higher mortality rate if T1DM occurred in childhood [29][42], males are at higher risk for it if T1DM occurred with or after puberty [37][49]. A clear association has been found between higher testosterone levels in younger men and the development of microalbuminuria [48][60]. This association cannot be shown in an older population of patients with T1DM, reinforcing the concept that what happens in the early phase of diabetes has implications for events many years later [37][49][49,61].
There also appears to be a relationship between the onset of menarche and the risk of T1DM-induced microvascular complications: Women with menarche delayed more than 2 years had a 2.3-fold higher risk of DKD (as well as retinopathy) than women with menarche at the average age [50][62].
The literature on whether and to what extent the sex hormones estradiol and testosterone play a role in DKD is similarly confusing as that on sex differences in the DKD phenotype and is still controversial. However, there is now relative agreement that diabetes leads to an imbalance of sex hormones in both sexes [32][33][36][37][38][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][32,45,48,49,50,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82]. The vast majority of studies document that in men with T1DM or T2DM, estradiol levels increase, while testosterone levels decrease, although there are also T1DM data showing increased or unchanged testosterone (reviewed in [36][48]). In women, DM results in reduced or unchanged estradiol levels and increased testosterone or similar testosterone levels to non-diabetic controls (reviewed in ([36][48]). However, in postmenopausal women with T2DM, estradiol levels are elevated [33][32], which, together with the accelerated progression of DKD, may suggest a potential adverse effect of estradiol in the presence of DM.
It is not yet conclusively understood how testosterone and estrogen levels and their respective receptors relate to the progression of DKD in both sexes [33][36][37][38][51][32,48,49,50,63].
It is conceivable that an estradiol-mediated mechanism exacerbates the reduction in circulating testosterone in T2DM. This assumption is based on the fact that T2DM in men is associated with increased estradiol levels and that independent studies have shown that activation of the G protein-coupled estrogen receptor in isolated Leydig cells as well as in human testes can downregulate testosterone production [70][82].
A human study from Finland underlines that not as expected high testosterone levels in diabetic men are the cause of DKD, but that T1DM just leads to reduced serum testosterone concentrations, and that even with the progression of renal damage from micro- to macroalbuminuria, the reduction in testosterone is enhanced [49][61]. On the other hand, increased testosterone levels are detectable in premenopausal women with T2DM and are associated with insulin resistance and microvascular sequelae [36][48]. High androgen levels in diabetic women lead to susceptibility to microvascular damage, as DKD can do [70][82].
There is also a large body of research looking at genetic factors that may influence sex differences in DKD. Epidemiological studies have revealed familial clustering of DKD in both types of diabetes as well as a relevant influence of ethnic background [71][28]. The effects of sex chromosomes as well as the influence of gene–sex interactions with multiple susceptibility genes for DKD have been investigated and recently analyzed by Giandalia et al., for a review [71][28]. Among others, sex–gene interactions were found for a variant in the angiotensin gene or in the angiotensin II type 1 receptor gene and were described for genes implicated in inflammation and oxidation [71][28]. Sex differences were also found for variants in the carnosinase gene, CNDP1, on chromosome 18q [71][28]. A CNDP1 polymorphism associated with low CN1 activity correlates with a significantly reduced risk for DKD, especially in women with T2DM [72][83].
 Potential therapeutic strategies applicable to the different mechanisms of sexual dimorphism target, for example, sex hormone imbalance, hemodynamic alterations, oxidative stress, or disturbances in water–electrolyte homeostasis and channels [26].
Fortunately, there are more and more editorial provisions from science journals for sex analyses. Since 2016, the SAGER (Sex and Gender Equity in Research) guidelines have been developed by the Gender Policy Committee of EASE (European Association of Science Editors). The SAGER guidelines emphasize strictly separating research subjects and data analysis by biological sex and gender, revealing significant differences even when there were not expected to be any [79][90]. The relatively recent examples provided below illustrate that these guidelines have not yet been implemented extensively and that a rethinking of study design is urgently needed.
Potential therapeutic strategies applicable to the different mechanisms of sexual dimorphism target, for example, sex hormone imbalance, hemodynamic alterations, oxidative stress, or disturbances in water–electrolyte homeostasis and channels [26].
Fortunately, there are more and more editorial provisions from science journals for sex analyses. Since 2016, the SAGER (Sex and Gender Equity in Research) guidelines have been developed by the Gender Policy Committee of EASE (European Association of Science Editors). The SAGER guidelines emphasize strictly separating research subjects and data analysis by biological sex and gender, revealing significant differences even when there were not expected to be any [79][90]. The relatively recent examples provided below illustrate that these guidelines have not yet been implemented extensively and that a rethinking of study design is urgently needed.
2. Sex Differences in Human DKD
In IgA nephropathy and membranous nephropathy, as well as in nondiabetic chronic kidney disease of unknown etiology, a strong significant association between male sex and adverse renal outcome was observed in a meta-analysis [10]. Other multicenter and population-based studies confirmed that the loss of renal function occurs more slowly in women than in men and the female sex is associated with better survival [11][12][13][11,12,13]. Another meta-analysis indicated the opposite, that progression is faster rather than slower in women. However, the authors acknowledged that most of the women in their analysis were of postmenopausal age and their results may not be generalizable to younger women. Thus, the presumed estrogen-mediated protective effect against nondiabetic chronic kidney disease in younger women compared with men of the same age appears to be lost with the menopause [14][15][14,15]. Epidemiological studies show that worldwide, 80% of cases of end-stage kidney failure (ESKF) are due to diabetes, hypertension, or a combination of both. The incidence of ESKF in patients with diabetes is up to ten times higher compared to adults without diabetes [16][17][16,17]. Although it seems clear that diabetes-induced macrovascular complications, such as coronary heart disease or stroke, are more common in women [18], data on sex and DKD risk are inconsistent. Studies report either a higher risk in men, a higher risk in women, or no significant sex dimorphism [19][20][21][22][23][24][25][19,20,21,22,23,24,25].2.1. Sex Differences in Development, Progression, and ESKF in DKD
There are several reasons for the inconsistency of data on sex and DKD risk. First, there are different equations for calculating eGFR and gold standards, such as lohexol clearance, are often not used when measuring GFR. In addition, criteria for classifying CKD may need to take into account the distribution of GFR by age and sex [26]. Other, and perhaps the most important, reasons for inconsistency include the types of diabetes (T1DM, T2DM, or both) considered in the studies and the endpoints of interest considered (e.g., micro/macroalbuminuria, eGFR, ESKF, mortality). In addition, studies performing separate-sex analysis vary in sample size and length of follow-up and ethnic cohorts. Although many recent papers and guidelines on DKD generally mention male sex as a more invariant risk factor [16][27][16,27], the number of review articles analyzing individual studies on sex differences in DKD in more detail and helping to shed light on the literature jungle is increasing. The lower survival rate of individuals with diabetes-related CKD, compared with individuals without CKD, is primarily due to the increased risk of concomitant morbidity associated with CKD, particularly cardiovascular disease. The lack of high-quality population-based studies with validated measures of CKD is the main reason why large differences in the epidemiology of CKD have been observed in populations with diabetes worldwide [16]. For example, women in Israel and Sweden have higher mortality rates if their T1DM developed in childhood, before puberty [28][29][41,42]. For men with T2DM and low testosterone levels, testosterone replacement therapy showed reduced mortality [30][31][32][43,44,45].2.2. Factors That May Influence Sex Differences in DKD
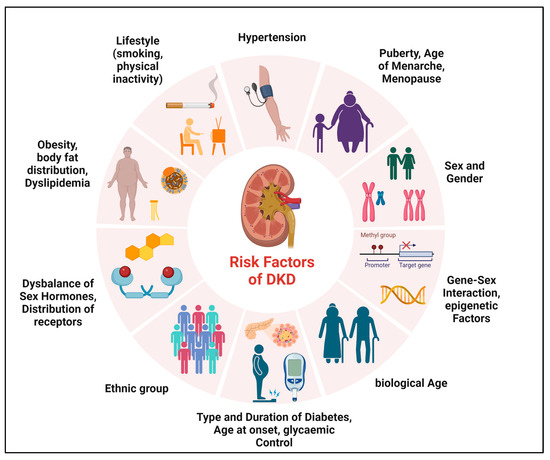
Diverse factors may be determinants of sex differences in microvascular complications of T1DM and T2DM, which may be unchangeable (such as sex, biological age, and genetic predisposition) or influenceable (such as smoking, physical activity, or glycemic control) (Figure 1). The onset and duration of DM, puberty, or menopause also appear to play a major role in sex differences [12][22][33][34][35][36][37][38][12,22,32,46,47,48,49,50].
Figure 1. Determinants of sex differences in DKD. Various factors may be determinants of sex differences in the development and progression of DKD.
2.3. Data on Possible Underlying Mechanisms in Human DKD
There are a number of mechanisms that are causative for sexual dimorphism. These include mechanisms of hemodynamics (hyperfiltration and the renin–angiotensin–aldosterone system (RAAS)), or in oxidative and substrate stress metabolism, and also the interaction of sex hormones with the signal transduction of TGF-β1 (transforming growth factor beta 1), the main mediator of DKD development and progression, is already known. As previously described, estradiol can downregulate testosterone production via activation of its receptor [70][82]. Sex-hormone-related mechanisms are also causal for the gender-specific differentiation of the RAAS, namely that men have a higher RAAS activity than women. While androgens can cause renal vasoconstriction through increased RAAS activity, estradiol on the one hand promotes higher angiotensinogen levels and the ACE2-angiotensin-1–7 axis and on the other hand reduces angiotensin-converting enzyme activity, renin levels, angiotensin II receptor type 1 (AT1R) density, aldosterone secretion, and angiotensin II activity [26]. In the case of oxidative stress, which is an essential pathophysiological feature of DKD, there are indications of sex differences to the disadvantage of the male sex (higher level of oxidative stress in men). The sex hormones play a regulating role in this context: estradiol acts as an antioxidant and androgens increase oxidative stress. Specifically in the kidney, hyperglycemia induces intracellular reactive oxygen species (ROS) in the renal mesangium and tubule cells. Advanced glycation end products (AGEs) and the cytokines TGF-β1 and ANGII are involved in this process. The ROS, in turn, are able to subsequently upregulate extracellular matrix expression via the transcription factors Nuclear Factor Kappa B (NF-κB) and Activator protein-1 (AP-1), which can lead to tubulointerstitial fibrosis (reviewed in [26]). TGF-β1 is a key factor for pathophysiological processes in DKD. In both type 1 and type 2 diabetes, increased tubular and glomerular TGF-β expression is found in the early as well as late phase of the disease [73][84]. TGF-β-mediated effects influence the pathology of mesangial cells, podocytes, and endothelial and tubular cells. This leads to cell proliferation, hypertrophy, and apoptosis, and further to inflammation, glomerulosclerosis, and tubulointerstitial fibrosis [73][84]. By binding and activating its receptor, TGF-β induces a variety of signaling pathways, including both the classical SMAD pathway, which results in the transcription of target genes, and SMAD-independent pathways, such as Ras, JNK, p38, and PI3K [73][84]. Through TGF-ß1 signaling pathways, the cell has versatile capabilities to control developmental programs autocrine and paracrine, but on the other hand, dysfunctions in this fine-tuned signaling can lead to severe diseases such as the development of DKD [74][85]. Studies indicate that an important underlying mechanism by which sex hormones mediate their effects in DKD is through the regulation of TGF-β1 [75][86]. Estrogen can bind SMAD2/3 proteins and inhibit the TGF-β1-induced accumulation of extracellular matrix through activation of the estrogen receptor [75][76][86,87]. Another work demonstrated that estradiol can influence TGF-β1-mediated CTGF expression [77][88]. Regarding the influence of testosterone on TGF-β1, one work was able to show that before puberty there are almost no differences between the two sexes, but after puberty, a threefold higher TGF-β1 production prevails in females than in males, with the activation of latent TGF-β1 dominating in the male sex [78][89]. Accordingly, it is possible that after puberty, there is much more efficient TGF-β1 activation in males than in females, and in the female sex, the lower activation rates are compensated for by higher basal TGF-β1 levels [78][89].2.4. Sex Aspects in Pharmacological Studies for Prevention and Treatment of DKD
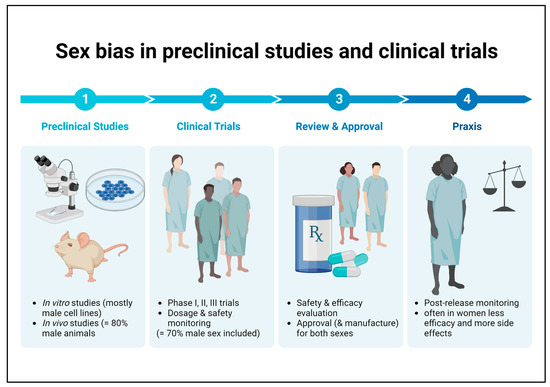
It is well known that there is an unequal sex distribution in preclinical research and clinical trials in favor of the male sex. Conducting studies on only one sex and extrapolating the results to the opposite sex can result in reduced efficacy to harmful side effects that may go undetected in the disregarded sex until market launch (Figure 2) [79][90]. Overall, this is a problem that also applies to medications recommended for patients with DKD, which is why there are no sex-specific guidelines on therapeutic aspects to date.
Figure 2. Sex bias in basic preclinical research and clinical trials. Male bias in animal studies as well as in human clinical trials may lead to reduced efficacy or harmful side effects in female patients that remain undetected until the post-marketing observation phase.
