
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hernán Cortés | -- | 2944 | 2023-04-20 21:04:09 | | | |
| 2 | Lindsay Dong | Meta information modification | 2944 | 2023-04-23 09:40:24 | | |
Video Upload Options
Lithium is a therapeutic cation used to treat bipolar disorders but also has some important features as an anti-cancer agent. Lithium formulations such as lithium acetoacetate (LiAcAc), lithium chloride (LiCl), lithium citrate (Li3C6H5O7), and lithium carbonate (Li2CO3) induce apoptosis, autophagy, and inhibition of tumor growth and also participate in the regulation of tumor proliferation, tumor invasion, and metastasis and cell cycle arrest. Moreover, lithium is synergistic with standard cancer therapies, enhancing their anti-tumor effects. In addition, lithium has a neuroprotective role in cancer patients, by improving their quality of life. Interestingly, nano-sized lithium enhances its anti-tumor activities and protects vital organs from the damage caused by lipid peroxidation during tumor development.
1. Introduction
2. The Effect of Lithium on Cancer Progression: Preclinical Approach
3. Lithium Transport
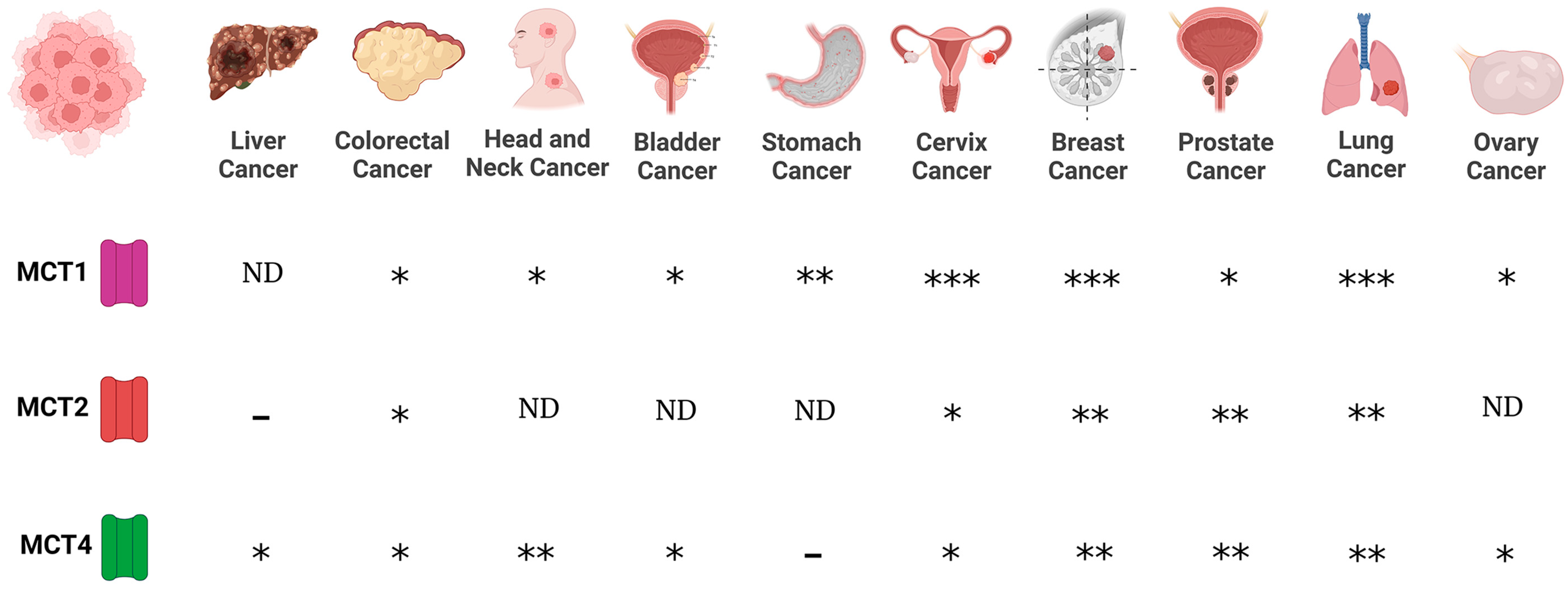
Lithium Transporters in Cancer
4. Lithium as a Specific Inhibitor of GSK3β
5. Effect of Lithium on Certain Cancer Hallmarks
5.1. Effect of Lithium on Apoptosis
5.2. Effect of Lithium on Autophagy
5.3. Effect of Lithium on Tumor Growth, Tumor Proliferation, Tumor Invasion and Metastasis, and Cell Cycle Arrest
6. Anti-Inflammatory Activity of Lithium
7. Synergism of Lithium with Standard Cancer Therapies
8. Contradictory Effects of Lithium
References
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46.
- Warburg, O. The Metabolism of Tumors; Richard R. Smith. Inc.: New York, NY, USA, 1931.
- Poff, A.; Koutnik, A.P.; Egan, K.M.; Sahebjam, S.; D’Agostino, D.; Kumar, N.B. Targeting the Warburg effect for cancer treatment: Ketogenic diets for management of glioma. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2019.
- Won, E.; Kim, Y.-K. An oldie but goodie: Lithium in the treatment of bipolar disorder through neuroprotective and neurotrophic mechanisms. Int. J. Mol. Sci. 2017, 18, 2679.
- Elbe, D. Stahl’s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. J. Can. Acad. Child Adolesc. Psychiatry 2010, 19, 230.
- de Freitas, D.M.; Leverson, B.D.; Goossens, J.L. Lithium in medicine: Mechanisms of action. In The Alkali Metal Ions: Their Role for Life; Springer: Berlin/Heidelberg, Germany, 2016; pp. 557–584.
- Edition, F. Diagnostic and statistical manual of mental disorders. Am. Psychiatr. Assoc. 2013, 21, 591–643.
- Walker, E.R.; McGee, R.E.; Druss, B.G. Mortality in mental disorders and global disease burden implications: A systematic review and meta-analysis. JAMA Psychiatry 2015, 72, 334–341.
- Wong, M.M. Management of bipolar II disorder. Indian J. Psychol. Med. 2011, 33, 18–28.
- Kessing, L.V.; Søndergård, L.; Forman, J.L.; Andersen, P.K. Lithium treatment and risk of dementia. Arch. Gen. Psychiatry 2008, 65, 1331–1335.
- Nitrini, R.; Caramelli, P.; Herrera, E.; Bahia, V.S.; Caixeta, L.F.; Radanovic, M.; Anghinah, R.; Charchat-Fichman, H.; Porto, C.S.; Carthery, M.T.; et al. Incidence of dementia in a community-dwelling Brazilian population. Alzheimer Dis. Assoc. Disord. 2004, 18, 241–246.
- Köhrle, J. Local activation and inactivation of thyroid hormones: The deiodinase family. Mol. Cell. Endocrinol. 1999, 151, 103–119.
- Berens, S.C.; Bernstein, R.S.; Robbins, J.; Wolff, J. Antithyroid effects of lithium. J. Clin. Investig. 1970, 49, 1357–1367.
- Kurita, M.; Mashiko, H.; Rai, M.; Kumasaka, T.; Kouno, S.-I.; Niwa, S.-I.; Nakahata, N. Lithium chloride at a therapeutic concentration reduces Ca2+ response in protein kinase C down-regulated human astrocytoma cells. Eur. J. Pharmacol. 2002, 442, 17–22.
- Briggs, K.T.; Giulian, G.G.; Li, G.; Kao, J.P.; Marino, J.P. A A Molecular Model for Lithium’s Bioactive Form. Biophys. J. 2016, 111, 294–300.
- Volkmann, C.; Bschor, T.; Köhler, S. Lithium treatment over the lifespan in bipolar disorders. Front. Psychiatry 2020, 11, 377.
- Ochoa, E.L.M. Lithium as a neuroprotective agent for bipolar disorder: An overview. Cell. Mol. Neurobiol. 2022, 42, 85–97.
- Vidali, S.; Aminzadeh-Gohari, S.; Vatrinet, R.; Iommarini, L.; Porcelli, A.M.; Kofler, B.; Feichtinger, R.G. Lithium and not acetoacetate influences the growth of cells treated with lithium acetoacetate. Int. J. Mol. Sci. 2019, 20, 3104.
- Jakobsson, E.; Argüello-Miranda, O.; Chiu, S.-W.; Fazal, Z.; Kruczek, J.; Nunez-Corrales, S.; Pandit, S.; Pritchet, L. Towards a unified understanding of lithium action in basic biology and its significance for applied biology. J. Membr. Biol. 2017, 250, 587–604.
- de Roos, N.M.; de Vries, J.H.; Katan, M.B. Serum lithium as a compliance marker for food and supplement intake. Am. J. Clin. Nutr. 2001, 73, 75–79.
- Pérez-Escuredo, J.; Van Hée, V.F.; Sboarina, M.; Falces, J.; Payen, V.L.; Pellerin, L.; Sonveaux, P. Monocarboxylate transporters in the brain and in cancer. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2481–2497.
- Bröer, S.; Bröer, A.; Schneider, H.-P.; Stegen, C.; Halestrap, A.P.; Deitmer, J.W. Characterization of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochem. J. 1999, 341, 529–535.
- Bröer, S.; Schneider, H.-P.; Bröer, A.; Rahman, B.; Hamprecht, B.; Deitmer, J.W. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem. J. 1998, 333, 167–174.
- Halestrap, A.P. The monocarboxylate transporter family—Structure and functional characterization. IUBMB Life 2012, 64, 1–9.
- Garcia, C.K.; Brown, M.S.; Pathak, R.K.; Goldstein, J.L. cDNA Cloning of MCT2, a Second Monocarboxylate Transporter Expressed in Different Cells than MCT1. J. Biol. Chem. 1995, 270, 1843–1849.
- Halestrap, A.P.; Meredith, D. The SLC16 gene family—From monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflügers Arch. 2004, 447, 619–628.
- Garcia, C.K.; Goldstein, J.L.; Pathak, R.K.; Anderson, R.G.; Brown, M.S. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: Implications for the Cori cycle. Cell 1994, 76, 865–873.
- Jóhannsson, E.; Nagelhus, E.A.; Mccullagh, K.J.; Sejersted, O.M.; Blackstad, T.W.; Bonen, A.; Ottersen, O.P. Cellular and subcellular expression of the monocarboxylate transporter MCT1 in rat heart: A high-resolution immunogold analysis. Circ. Res. 1997, 80, 400–407.
- Kirat, D.; Inoue, H.; Iwano, H.; Yokota, H.; Taniyama, H.; Kato, S. Monocarboxylate transporter 1 (MCT1) in the liver of pre-ruminant and adult bovines. Vet. J. 2007, 173, 124–130.
- Tamai, I.; Takanaga, H.; Ogihara, T.; Higashida, H.; Maeda, H.; Sai, Y.; Tsuji, A. Participation of a proton-cotransporter, MCT1, in the intestinal transport of monocarboxylic acids. Biochem. Biophys. Res. Commun. 1995, 214, 482–489.
- Ritzhaupt, A.; Wood, I.S.; Ellis, A.; Hosie, K.B.; Shirazi-Beechey, S.P. Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: Its potential to transport l-lactate as well as butyrate. J. Physiol. 1998, 513, 719–732.
- Orsenigo, M.N.; Tosco, M.; Bazzini, C.; Laforenza, U.; Faelli, A. A monocarboxylate transporter MCT1 is located at the basolateral pole of rat jejunum. Exp. Physiol. 1999, 84, 1033–1042.
- Kirat, D.; Inoue, H.; Iwano, H.; Hirayama, K.; Yokota, H.; Taniyama, H.; Kato, S. Monocarboxylate transporter 1 gene expression in the ovine gastrointestinal tract. Vet. J. 2006, 171, 462–467.
- Iwanaga, T.; Takebe, K.; Kato, I.; Karaki, S.-I.; Kuwahara, A. Cellular expression of monocarboxylate transporters (MCT) in the digestive tract of the mouse, rat, and humans, with special reference to slc5a8. Biomed. Res. 2006, 27, 243–254.
- Shimoyama, Y.; Kirat, D.; Akihara, Y.; Kawasako, K.; Komine, M.; Hirayama, K.; Matsuda, K.; Okamoto, M.; Iwano, H.; Kato, S.; et al. Expression of monocarboxylate transporter 1 (MCT1) in the dog intestine. J. Vet. Med. Sci. 2007, 69, 599–604.
- Welter, H.; Claus, R. Expression of the monocarboxylate transporter 1 (MCT1) in cells of the porcine intestine. Cell Biol. Int. 2008, 32, 638–645.
- Poole, R.C.; Halestrap, A.P. N-terminal protein sequence analysis of the rabbit erythrocyte lactate transporter suggests identity with the cloned monocarboxylate transport protein MCT1. Biochem. J. 1994, 303, 755–759.
- de Heredia, F.P.; Wood, I.S.; Trayhurn, P. Hypoxia stimulates lactate release and modulates monocarboxylate transporter (MCT1, MCT2, and MCT4) expression in human adipocytes. Pflügers Arch.-Eur. J. Physiol. 2010, 459, 509–518.
- Hajduch, E.; Heyes, R.R.; Watt, P.W.; Hundal, H.S. Lactate transport in rat adipocytes: Identification of monocarboxylate transporter 1 (MCT1) and its modulation during streptozotocin-induced diabetes. FEBS Lett. 2000, 479, 89–92.
- Takebe, K.; Nio-Kobayashi, J.; Takahashi-Iwanaga, H.; Yajima, T.; Iwanaga, T. Cellular expression of a monocarboxylate transporter (MCT1) in the mammary gland and sebaceous gland of mice. Histochem. Cell Biol. 2009, 131, 401–409.
- Mac, M.; Nałęcz, K.A. Expression of monocarboxylic acid transporters (MCT) in brain cells: Implication for branched chain α-ketoacids transport in neurons. Neurochem. Int. 2003, 43, 305–309.
- Hanu, R.; McKenna, M.; O’Neill, A.; Resneck, W.G.; Bloch, R.J. Monocarboxylic acid transporters, MCT1 and MCT2, in cortical astrocytes in vitro and in vivo. Am. J. Physiol. Physiol. 2000, 278, C921–C930.
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.-W.; et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 2012, 487, 443–448.
- Moreira, T.J.; Pierre, K.; Maekawa, F.; Repond, C.; Cebere, A.; Liljequist, S.; Pellerin, L. Enhanced cerebral expression of MCT1 and MCT2 in a rat ischemia model occurs in activated microglial cells. J. Cereb. Blood Flow Metab. 2009, 29, 1273–1283.
- Ainscow, E.K.; Mirshamsi, S.; Tang, T.; Ashford, M.L.; Rutter, G.A. Dynamic imaging of free cytosolic ATP concentration during fuel sensing by rat hypothalamic neurones: Evidence for ATP-independent control of ATP-sensitive K+ channels. J. Physiol. 2002, 544, 429–445.
- Domènech-Estévez, E.; Baloui, H.; Repond, C.; Rosafio, K.; Médard, J.-J.; Tricaud, N.; Pellerin, L.; Chrast, R. Distribution of monocarboxylate transporters in the peripheral nervous system suggests putative roles in lactate shuttling and myelination. J. Neurosci. 2015, 35, 4151–4156.
- Morrison, B.M.; Tsingalia, A.; Vidensky, S.; Lee, Y.; Jin, L.; Farah, M.H.; Lengacher, S.; Magistretti, P.J.; Pellerin, L.; Rothstein, J.D. Deficiency in monocarboxylate transporter 1 (MCT1) in mice delays regeneration of peripheral nerves following sciatic nerve crush. Exp. Neurol. 2015, 263, 325–338.
- Boussouar, F.; Mauduit, C.; Tabone, E.; Pellerin, L.; Magistretti, P.J.; Benahmed, M. Developmental and hormonal regulation of the monocarboxylate transporter 2 (MCT2) expression in the mouse germ cells. Biol. Reprod. 2003, 69, 1069–1078.
- Pierre, K.; Pellerin, L.; Debernardi, R.; Riederer, B.; Magistretti, P.J. Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience 2000, 100, 617–627.
- Pellerin, L.; Halestrap, A.P.; Pierre, K. Cellular and subcellular distribution of monocarboxylate transporters in cultured brain cells and in the adult brain. J. Neurosci. Res. 2005, 79, 55–64.
- Pierre, K.; Magistretti, P.J.; Pellerin, L. MCT2 is a major neuronal monocarboxylate transporter in the adult mouse brain. J. Cereb. Blood Flow Metab. 2002, 22, 586–595.
- Reiser, G.; Duhm, J. Transport pathways for lithium ions in neuroblastoma× glioma hybrid cells at ‘therapeutic’concentrations of Li+. Brain Res. 1982, 252, 247–258.
- Sonveaux, P.; Vegran, F.; Schroeder, T.; Wergin, M.C.; Verrax, J.; Rabbani, Z.N.; De Saedeleer, C.J.; Kennedy, K.M.; Diepart, C.; Jordan, B.F.; et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Investig. 2008, 118, 3930–3942.
- Baltazar, F.; Pinheiro, C.; Morais-Santos, F.; Azevedo-Silva, J.; Queiros, O.; Preto, A.; Casal, M. Monocarboxylate transporters as targets and mediators in cancer therapy response. Histol. Histopathol. 2014, 29, 1511–1524.
- Pinheiro, C.; Reis, R.M.; Ricardo, S.; Longatto-Filho, A.; Schmitt, F.; Baltazar, F. Expression of monocarboxylate transporters 1, 2, and 4 in human tumours and their association with CD147 and CD44. J. Biomed. Biotechnol. 2010, 2010, 427694.
- Kennedy, K.M.; Dewhirst, M.W. Tumor metabolism of lactate: The influence and therapeutic potential for MCT and CD147 regulation. Future Oncol. 2010, 6, 127–148.
- Pinheiro, C.; Longatto-Filho, A.; Azevedo-Silva, J.; Casal, M.; Schmitt, F.C.; Baltazar, F. Role of monocarboxylate transporters in human cancers: State of the art. J. Bioenerg. Biomembr. 2012, 44, 127–139.
- Miranda-Gonçalves, V.; Honavar, M.; Pinheiro, C.; Martinho, O.; Pires, M.M.; Pinheiro, C.; Cordeiro, M.; Bebiano, G.; Costa, P.; Palmeirim, I.; et al. Monocarboxylate transporters (MCTs) in gliomas: Expression and exploitation as therapeutic targets. Neuro-Oncol. 2013, 15, 172–188.
- Afonso, J.; Santos, L.L.; Miranda-Gonçalves, V.; Morais, A.; Amaro, T.; Longatto-Filho, A.; Baltazar, F. CD147 and MCT1-potential partners in bladder cancer aggressiveness and cisplatin resistance. Mol. Carcinog. 2015, 54, 1451–1466.
- Wang, Z.; Smith, K.S.; Murphy, M.; Piloto, O.; Somervaille, T.C.P.; Cleary, M.L. Glycogen synthase kinase 3 in MLL leukaemia maintenance and targeted therapy. Nature 2008, 455, 1205–1209.
- Woodgett, J.R. Regulation and functions of the glycogen synthase kinase-3 subfamily. Semin. Cancer Biol. 1994, 5, 269–275.
- Ougolkov, A.V.; Fernandez-Zapico, M.E.; Savoy, D.N.; Urrutia, R.A.; Billadeau, D.D. Glycogen synthase kinase-3β participates in nuclear factor κB–mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res. 2005, 65, 2076–2081.
- Kotliarova, S.; Pastorino, S.; Kovell, L.C.; Kotliarov, Y.; Song, H.; Zhang, W.; Bailey, R.; Maric, D.; Zenklusen, J.C.; Lee, J.; et al. Glycogen synthase kinase-3 inhibition induces glioma cell death through c-MYC, nuclear factor-κB, and glucose regulation. Cancer Res. 2008, 68, 6643–6651.
- Marchand, B.; Tremblay, I.; Cagnol, S.; Boucher, M.-J. Inhibition of glycogen synthase kinase-3 activity triggers an apoptotic response in pancreatic cancer cells through JNK-dependent mechanisms. Carcinogenesis 2012, 33, 529–537.
- Shakoori, A.; Ougolkov, A.; Yu, Z.W.; Zhang, B.; Modarressi, M.H.; Billadeau, D.D.; Mai, M.; Takahashi, Y.; Minamoto, T. Deregulated GSK3β activity in colorectal cancer: Its association with tumor cell survival and proliferation. Biochem. Biophys. Res. Commun. 2005, 334, 1365–1373.
- Mazor, M.; Kawano, Y.; Zhu, H.; Waxman, J.; Kypta, R.M. Inhibition of glycogen synthase kinase-3 represses androgen receptor activity and prostate cancer cell growth. Oncogene 2004, 23, 7882–7892.
- Ryves, W.; Harwood, A.J. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochem. Biophys. Res. Commun. 2001, 280, 720–725.
- Klein, P.S.; Melton, D.A. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA 1996, 93, 8455–8459.
- Li, X.; Friedman, A.B.; Zhu, W.; Wang, L.; Boswell, S.; May, R.S.; Davis, L.L.; Jope, R.S. Lithium regulates glycogen synthase kinase-3β in human peripheral blood mononuclear cells: Implication in the treatment of bipolar disorder. Biol. Psychiatry 2007, 61, 216–222.
- Qu, L.; Huang, S.; Baltzis, D.; Rivas-Estilla, A.-M.; Pluquet, O.; Hatzoglou, M.; Koumenis, C.; Taya, Y.; Yoshimura, A.; Koromilas, A.E. Endoplasmic reticulum stress induces p53 cytoplasmic localization and prevents p53-dependent apoptosis by a pathway involving glycogen synthase kinase-3β. Genes Dev. 2004, 18, 261–277.
- Watcharasit, P.; Bijur, G.N.; Song, L.; Zhu, J.; Chen, X.; Jope, R.S. Glycogen synthase kinase-3β (GSK3β) binds to and promotes the actions of p53. J. Biol. Chem. 2003, 278, 48872–48879.
- Watcharasit, P.; Bijur, G.N.; Zmijewski, J.W.; Song, L.; Zmijewska, A.; Chen, X.; Johnson, G.V.W.; Jope, R.S. Direct, activating interaction between glycogen synthase kinase-3β and p53 after DNA damage. Proc. Natl. Acad. Sci. USA 2002, 99, 7951–7955.
- Cao, Q.; Lu, X.; Feng, Y.-J. Glycogen synthase kinase-3β positively regulates the proliferation of human ovarian cancer cells. Cell Res. 2006, 16, 671–677.
- Kunnimalaiyaan, M.; Vaccaro, A.M.; Ndiaye, M.A.; Chen, H. Inactivation of glycogen synthase kinase-3β, a downstream target of the raf-1 pathway, is associated with growth suppression in medullary thyroid cancer cells. Mol. Cancer Ther. 2007, 6, 1151–1158.
- Beurel, E.; Kornprobst, M.; Eggelpoël, M.-J.B.-V.; Ruiz-Ruiz, C.; Cadoret, A.; Capeau, J.; Desbois-Mouthon, C. GSK-3β inhibition by lithium confers resistance to chemotherapy-induced apoptosis through the repression of CD95 (Fas/APO-1) expression. Exp. Cell Res. 2004, 300, 354–364.
- Bijur, G.N.; De Sarno, P.; Jope, R.S. Glycogen synthase kinase-3β facilitates staurosporine-and heat shock-induced apoptosis: Protection by lithium. J. Biol. Chem. 2000, 275, 7583–7590.
- Chen, R.-W.; Chuang, D.-M. Long term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expression: A prominent role in neuroprotection against excitotoxicity. J. Biol. Chem. 1999, 274, 6039–6042.
- Cross, D.A.; Alessi, D.R.; Cohen, P.; Andjelkovich, M.; Hemmings, B.A. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 1995, 378, 785–789.
- King, T.D.; Bijur, G.N.; Jope, R.S. Caspase-3 activation induced by inhibition of mitochondrial complex I is facilitated by glycogen synthase kinase-3β and attenuated by lithium. Brain Res. 2001, 919, 106–114.
- Karlovic, D.; Jakopec, S.; Dubravcic, K.; Batinic, D.; Buljan, D.; Osmak, M. Lithium increases expression of p21WAF/Cip1 and survivin in human glioblastoma cells. Cell Biol. Toxicol. 2007, 23, 83–90.
- Mora, A.; Sabio, G.; Alonso, J.C.; Soler, G.; Centeno, F. Different dependence of lithium and valproate on PI3K/PKB pathway. Bipolar Disord. 2002, 4, 195–200.
- Adler, J.T.; Hottinger, D.G.; Kunnimalaiyaan, M.; Chen, H. Inhibition of growth in medullary thyroid cancer cells with histone deacetylase inhibitors and lithium chloride. J. Surg. Res. 2010, 159, 640–644.
- Liao, X.; Zhang, L.; Thrasher, J.B.; Du, J.; Li, B. Glycogen synthase kinase-3β suppression eliminates tumor necrosis factor-related apoptosis-inducing ligand resistance in prostate cancer. Mol. Cancer Ther. 2003, 2, 1215–1222.
- Sarkar, S.; Rubinsztein, D.C. Inositol and IP3 levels regulate autophagy—Biology and therapeutic speculations. Autophagy 2006, 2, 132–134.
- Sarkar, S.; Floto, R.A.; Berger, Z.; Imarisio, S.; Cordenier, A.; Pasco, M.; Cook, L.J.; Rubinsztein, D.C. Lithium induces autophagy by inhibiting inositol monophosphatase. J. Cell Biol. 2005, 170, 1101–1111.
- Taskaeva, I.; Bgatova, N. Ultrastructural and immunofluorescent analysis of lithium effects on autophagy in hepatocellular carcinoma cells. Asian Pac. J. Cancer Biol. 2018, 3, 83–87.
- Taskaeva, I.S.; Bgatova, N.P.; Solovieva, A.O. Autophagy in Hepatocellular Carcinoma-29 after Single or Combined Administration of Lithium Carbonate and Rapamycin. Cell Tissue Biol. 2019, 13, 353–359.
- Taskaeva, Y.S.; Bgatova, N.P.; Dossymbekova, R.S.; Solovieva, A.O.; Miroshnichenko, S.M.; Sharipov, K.O.; Tungushbaeva, Z.B. In vitro effects of lithium carbonate on cell cycle, apoptosis, and autophagy in hepatocellular carcinoma-29 cells. Bull. Exp. Biol. Med. 2020, 170, 246–250.
- Kim, E.C.; Meng, H.; Jun, A.S. Lithium treatment increases endothelial cell survival and autophagy in a mouse model of Fuchs endothelial corneal dystrophy. Br. J. Ophthalmol. 2013, 97, 1068–1073.
- Liu, J.; Ju, P.; Zhou, Y.; Zhao, Y.; Xie, Y.; Long, Y.; Gu, Y.; Ni, D.; Lyv, Z.; Mao, Z.; et al. Six2 is a coordinator of LiCl-induced cell proliferation and apoptosis. Int. J. Mol. Sci. 2016, 17, 1504.
- Grimes, C.A.; Jope, R.S. The multifaceted roles of glycogen synthase kinase 3β in cellular signaling. Prog. Neurobiol. 2001, 65, 391–426.
- Ding, Q.; Xia, W.; Liu, J.-C.; Yang, J.-Y.; Lee, D.-F.; Xia, J.; Bartholomeusz, G.; Li, Y.; Pan, Y.; Li, Z.; et al. associates with and primes GSK-3β for its inactivation resulting in upregulation of β-catenin. Mol. Cell 2005, 19, 159–170.
- Díaz, M.; Vidal, F.; Cruz, A.L.; De Araujo, W.M.; Tanaka, M.N.; Viola, J.P.; Morgado-Diaz, J.A. Lithium reduces tumorigenic potential in response to EGF signaling in human colorectal cancer cells. Int. J. Oncol. 2011, 38, 1365–1373.
- Yao, R.; Sun, X.; Xie, Y.; Liu, L.; Han, D.; Yao, Y.; Li, H.; Li, Z.; Xu, K. Lithium chloride inhibits cell survival, overcomes drug resistance, and triggers apoptosis in multiple myeloma via activation of the Wnt/β-catenin pathway. Am. J. Transl. Res. 2018, 10, 2610.
- Farina, A.K.; Bong, Y.-S.; Feltes, C.M.; Byers, S.W. Post-transcriptional regulation of cadherin-11 expression by GSK-3 and β-catenin in prostate and breast cancer cells. PLoS ONE 2009, 4, e4797.
- Maeng, Y.-S.; Lee, R.; Lee, B.; Choi, S.-I.; Kim, E.K. Lithium inhibits tumor lymphangiogenesis and metastasis through the inhibition of TGFBIp expression in cancer cells. Sci. Rep. 2016, 6, 20739.
- Nowicki, M.O.; Dmitrieva, N.; Stein, A.M.; Cutter, J.L.; Godlewski, J.; Saeki, Y.; Nita, M.; Berens, M.E.; Sander, L.M.; Newton, H.B.; et al. Lithium inhibits invasion of glioma cells; possible involvement of glycogen synthase kinase-3. Neuro-Oncology 2008, 10, 690–699.
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444.
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121.
- Lee, J.-H.; Kim, S.-W.; Kim, J.-H.; Kim, H.-J.; Um, J.; Jung, D.-W.; Williams, D. Lithium chloride protects against sepsis-induced skeletal muscle atrophy and cancer cachexia. Cells 2021, 10, 1017.
- Wang, M.J.; Huang, H.Y.; Chen, W.F.; Chang, H.F.; Kuo, J.S. Glycogen synthase kinase-3β inactivation inhibits tumor necrosis factor-α production in microglia by modulating nuclear factor κB and MLK3/JNK signaling cascades. J. Neuroinflamm. 2010, 7, 99.
- Huang, W.-C.; Lin, Y.-S.; Wang, C.-Y.; Tsai, C.-C.; Tseng, H.-C.; Chen, C.-L.; Lu, P.-J.; Chen, P.-S.; Qian, L.; Hong, J.-S.; et al. Glycogen synthase kinase-3 negatively regulates anti-inflammatory interleukin-10 for lipopolysaccharide-induced iNOS/NO biosynthesis and RANTES production in microglial cells. Immunology 2009, 128, e275–e286.
- Nahman, S.; Belmaker, R.; Azab, A.N. Effects of lithium on lipopolysaccharide-induced inflammation in rat primary glia cells. Endotoxin Res. 2012, 18, 447–458.
- Nassar, A.; Azab, A.N. Effects of lithium on inflammation. ACS Chem. Neurosci. 2014, 5, 451–458.
- Novetsky, A.P.; Thompson, D.M.; Zighelboim, I.; Thaker, P.H.; Powell, M.A.; Mutch, D.G.; Goodfellow, P.J. Lithium chloride and inhibition of glycogen synthase kinase 3β as a potential therapy for serous ovarian cancer. Int. J. Gynecol. Cancer 2013, 23, 361–366.
- Khasraw, M.; Ashley, D.; Wheeler, G.; Berk, M. Using lithium as a neuroprotective agent in patients with cancer. BMC Med. 2012, 10, 131.
- Hossein, G.; Janzamin, E.; Azimian-Zavareh, V. Effect of lithium chloride and antineoplastic drugs on survival and cell cycle of androgen-dependent prostate cancer LNCap cells. Indian J. Pharmacol. 2012, 44, 714.
- Peng, Z.; Ji, Z.; Mei, F.; Lu, M.; Ou, Y.; Cheng, X. Lithium inhibits tumorigenic potential of PDA cells through targeting hedgehog-GLI signaling pathway. PLoS ONE 2013, 8, e61457.
- Boehmerle, W.; Zhang, K.; Sivula, M.; Heidrich, F.M.; Lee, Y.; Jordt, S.-E.; Ehrlich, B.E. Chronic exposure to paclitaxel diminishes phosphoinositide signaling by calpain-mediated neuronal calcium sensor-1 degradation. Proc. Natl. Acad. Sci. USA 2007, 104, 11103–11108.
- Ibrahim, E.Y.; Ehrlich, B.E. Prevention of chemotherapy-induced peripheral neuropathy: A review of recent findings. Crit. Rev. Oncol./Hematol. 2020, 145, 102831.
- Walker, R.J.; Weggery, S.; Bedford, J.J.; Mcdonald, F.J.; Ellis, G.; Leader, J.P. Lithium-induced reduction in urinary concentrating ability and urinary aquaporin 2 (AQP2) excretion in healthy volunteers. Kidney Int. 2005, 67, 291–294.
- Pinna, M.; Manchia, M.; Puddu, S.; Minnai, G.; Tondo, L.; Salis, P. Cutaneous adverse reaction during lithium treatment: A case report and updated systematic review with meta-analysis. Int. J. Bipolar Disord. 2017, 5, 20.
- Nair, C.G.; Menon, R.; Jacob, P.; Babu, M. Lithium-induced parathyroid dysfunction: A new case. Indian J. Endocrinol. Metab. 2013, 17, 930–932.
- Meehan, A.D.; Udumyan, R.; Kardell, M.; Landén, M.; Järhult, J.; Wallin, G. Lithium-associated hypercalcemia: Pathophysiology, prevalence, management. World J. Surg. 2018, 42, 415–424.
- Poels, E.M.P.; Bijma, H.H.; Galbally, M.; Bergink, V. Lithium during pregnancy and after delivery: A review. Int. J. Bipolar Disord. 2018, 6, 26.
- Matsebatlela, T.M.; Mogodiri, R.K.; Hart, D.A.; Gallicchio, V.S.; Becker, R.W. Resistance to lithium-induced apoptosis in a lithium tolerant clone of HL-60 promyelocytes. J. Trace Microprobe Tech. 2000, 18, 163–170.
- Lucas, K.C.; Hart, D.A.; Becker, R.W. Porcine proximal tubular cells (LLC-PK1) are able to tolerate high levels of lithium chloride in vitro: Assessment of the influence of 1–20 mM LiCl on cell death and alterations in cell biology and biochemistry. Cell Biol. Int. 2010, 34, 225–233.
- Matsebatlela, T.; Gallicchio, V.; Becker, R. Lithium modulates cancer cell growth, apoptosis, gene expression and cytokine production in HL-60 promyelocytic leukaemia cells and their drug-resistant sub-clones. Biol. Trace Element Res. 2012, 149, 323–330.




