
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jessica Ribeiro | -- | 7770 | 2023-04-19 22:42:00 | | | |
| 2 | Lindsay Dong | -4 word(s) | 7766 | 2023-04-21 03:15:52 | | | | |
| 3 | Lindsay Dong | -3 word(s) | 7763 | 2023-04-23 10:06:12 | | |
Video Upload Options
Chickens can acquire bacteria at different stages, and bacterial diversity can occur due to production practices, diet, and environment. The changes in consumer trends have led to increased animal production, and chicken meat is one of the most consumed meats. To ensure high levels of production, antimicrobials have been used in livestock for therapeutic purposes, disease prevention, and growth promotion, contributing to the development of antimicrobial resistance across the resident microbiota. Enterococcus spp. and Escherichia coli are normal inhabitants of the gastrointestinal microbiota of chickens that can develop strains capable of causing a wide range of diseases, i.e., opportunistic pathogens. Enterococcus spp. isolated from broilers have shown resistance to at least seven classes of antibiotics, while E. coli have shown resistance to at least four.
1. Gastrointestinal Bacteria in Chickens
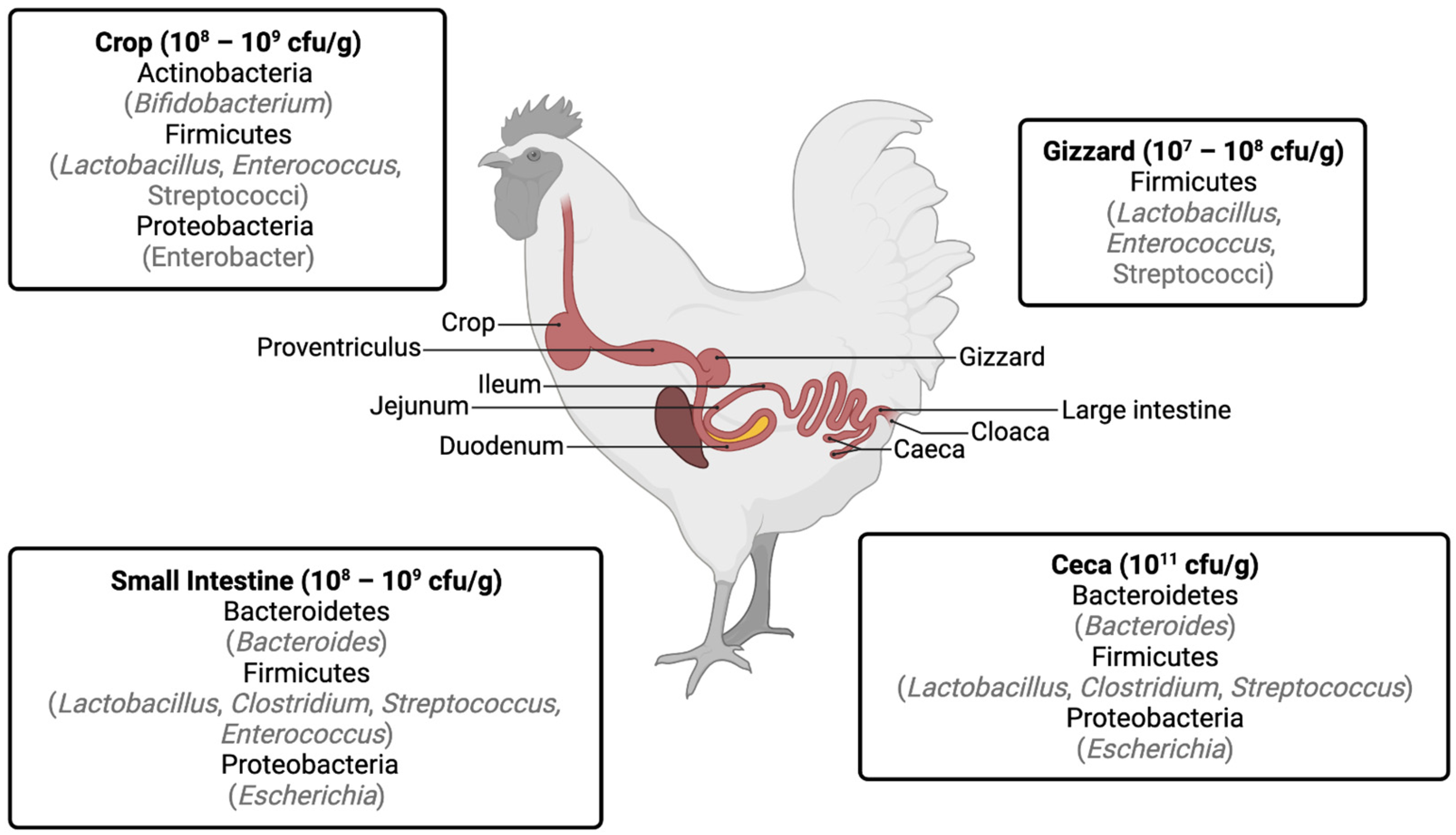
2. Development of Antibiotic Resistance by Gastrointestinal Bacteria in Broilers
3. Enterococcus spp.
3.1. Enterococci Species Diversity
3.2. Antimicrobial Resistance in Enterococci
3.3. Emergence and Dissemination of Vancomycin-Resistant Enterococci (VRE)
3.4. Molecular Characteristics of Enterococcus Clones
Stępień-Pyśniak et al. (2021) carried out a study that included 35 Polish E. faecalis and 41 Danish E. faecalis strains collected during post-mortem examination from broiler chicks showing lesions characteristic of yolk sac infection. The most prevalent clonal lineage among the Polish isolates was ST59, followed by ST282 and ST16. Regarding the Danish isolates, the most prevalent clonal lineages were ST116 and ST16. Only two Danish isolates were identified as VRE, and one belonged to the ST387 clonal lineage, while the other belonged to ST838 [127]. A Brazilian study that analyzed 12 E. faecalis strains isolated from natural cases of vertebral osteomyelitis in broilers revealed that almost half of these belonged to ST49. In addition, ST202 was represented by one strain that was vancomycin-resistant [93]. A study performed with 45 E. faecalis strains isolated from the cloaca of healthy broilers in Saudi Arabia reported that most of those strains belonged to ST16, ST302, and ST179, respectively. Two isolates were VRE, and these also belonged to ST16 [128]. In China, 61 strains of E. faecalis isolated from the cecal tissue of broiler chickens with swollen cecal lesions belonged to 34 sequence types, and the most prevalent was ST631 [129]. Kim et al. (2018) studied the molecular characteristics of 85 E. faecalis strains isolated from chicken meat samples, and ST256 was observed in over 50% of the isolates [130]. E. faecalis strains isolated from retail chicken carcasses in the Emirate of Abu Dhabi were assigned to five different sequence types, and half of them belonged to the clonal lineage ST476 [101]. ST314, followed by ST16, were the most prevalent clonal lineages reported among broilers across Australia [60]. Overall, the most frequent and wide-ranging clonal lineage that has been identified among E. faecalis isolated from broilers or broiler meat since 2018 is ST16. This sequence type has already been identified in Poland, the Netherlands, Saudi Arabia, China, and Australia, and in both vancomycin-resistant and vancomycin-susceptible E. faecalis.
Leinweber et al. (2018) isolated three vancomycin-resistant E. faecium (VREfm) strains from Danish chicken meat, and all the strains belonged to ST32 [120]. VREfm strains were also isolated from cecal samples from healthy broilers in Sweden, but all of these belonged to ST310 [111]. In Turkey, a study that included vancomycin-susceptible E. faecium and VREfm isolated from broiler cloaca reported that ST1346 was the most prevalent clonal lineage among vancomycin-susceptible E. faecium, while all VREfm presented different and novel STs (ST1341, ST1342, ST1343, ST1244, and ST1345) [131]. A study that included 30 E. faecium strains isolated from the cloaca of healthy broilers in Saudi Arabia reported that most of those strains belonged to ST194, ST82, and ST157, respectively [128]. Kim et al. (2018) isolated one E. faecium strain from chicken meat samples that was revealed to belong to ST451 [130]. E. faecium isolated from retail chicken carcasses in Abu Dhabi Emirate has been assigned to four different sequence types: one known ST (ST195) and three novel STs (ST2236, ST2238, and ST2239) [101]. ST492, followed by ST195 and ST241, were the most prevalent clonal lineages reported among broilers across Australia [60]. Overall, E. faecium isolates from broilers or broiler meat since 2018 do not share many clonal lineages. However, ST194 and ST195 were already identified in two different sources (broilers and broiler meat) on at least two different continents.
4. Escherichia coli
4.1. Antimicrobial Resistance in E. coli
4.2. ESBL-Producing E. coli
4.3. Molecular Characteristics of E. coli Clones
Päivärinta et al. (2020) collected broiler cecal samples from a high-capacity slaughterhouse and from vacuum-packed raw broiler meat without marinade intended for consumer use, all from the same high-capacity slaughterhouse. In total, three ESBL-producing E. coli strains were isolated: two from the ceca that belonged to ST1594, and one from the meat that belonged to ST351 [175]. Retail chicken meat was also studied in Egypt, and ST1196 was the most prevalent sequence type among ESBL-producing E. coli, while ST156 and ST189 were identified among non-ESBL-producing E.coli [198]. Broilers infected with colibacillosis were studied in Norway, Croatia, Tunisia, and Pakistan [144][147][199][200]. In the Norwegian study, ST429 accounted for over 60% of the clonal lineages identified in E. coli isolates [144]. However, in Croatia, ST429 was reported at a much lower rate (0.65%). The most prevalent sequence types in Croatia were ST95 and ST117 [199]. ST117 was also predominant among the Pakistani E. coli isolates from broilers with colibacillosis [201]. The Tunisian study reported four different sequence types in ESBL-producing E. coli strains, with the majority belonging to ST4187 [147]. Two different Pakistani studies that included cecal and fecal samples from broilers reported ST131 between the most prevalent sequence types in ESBL-producing E. coli strains [202][203]. A study carried out by Aslantaş (2020) in Turkey detected 19 sequence types in 28 ESBL-producing E. coli isolates, and the most prevalent were ST114 and ST354 [200]. In Australia, ESBL-producing E. coli isolated from healthy broilers belonged to different clonal lineages, while E. coli from chickens with colibacillosis belonged mainly to ST354 [143]. Overall, according to the studies, the most frequent and wide-ranging clonal lineage that was identified in both ESBL-producing and non-ESBL-producing E. coli isolated from broilers or broiler meat since 2020 was ST117.
5. Impact of Antibiotic Usage and Antibiotic-Resistant Bacteria in the Gastrointestinal Tract of Broilers: A One Health Approach
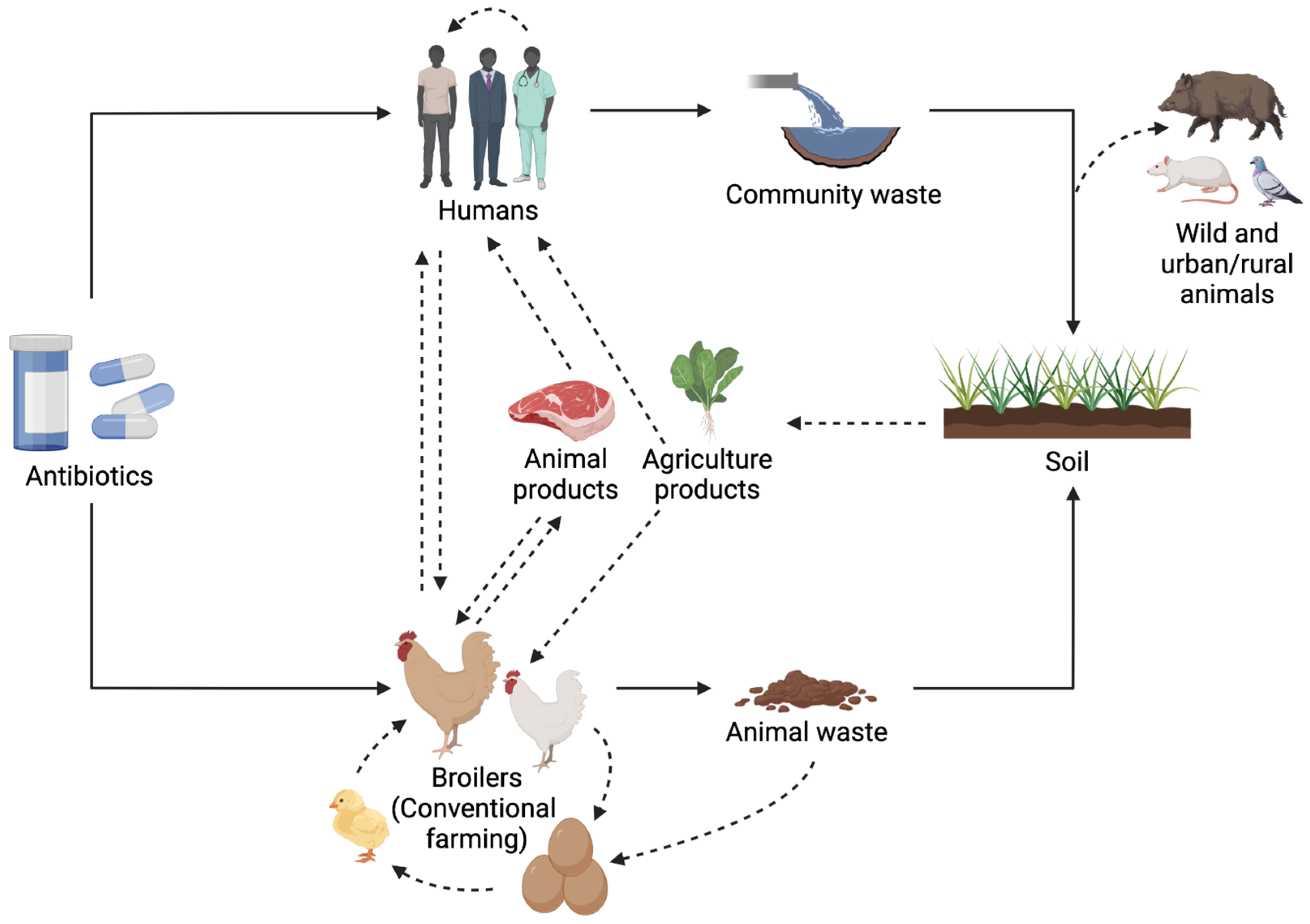
Recently, broilers have increased significantly as a meat source, and the largest broiler meat producers worldwide include the United States, China, and Brazil, respectively. Within the European Union, Poland, Spain, Germany, France, and Italy present the higher gross domestic production of broilers [217]. Broiler meat produced by some of these countries is exported globally [23]. For example, Brazil is the world’s largest poultry exporter; about a third of Brazil’s chicken production is exported—4.6 million out of 14.3 million metric tons in 2020—to over 150 countries worldwide [218]. Therefore, ongoing surveillance systems for antimicrobial resistance in broiler production are mandatory to avoid the spread of antimicrobial resistance among broiler meat or other foods derived from these animals.
5.1. Transmission of Enterococcus spp. and E. coli
5.2. Clonal Relationship from a One Health Perspective
6. Conclusions
References
- Stanley, D.; Hughes, R.J.; Moore, R.J. Microbiota of the chicken gastrointestinal tract: Influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014, 98, 4301–4310.
- Chen, C.Y.; Chen, C.K.; Chen, Y.Y.; Fang, A.; Shaw, G.T.W.; Hung, C.M.; Wang, D. Maternal gut microbes shape the early-life assembly of gut microbiota in passerine chicks via nests. Microbiome 2020, 8, 129.
- Olsen, R.; Kudirkiene, E.; Thøfner, I.; Pors, S.; Karlskov-Mortensen, P.; Li, L.; Papasolomontos, S.; Angastiniotou, C.; Christensen, J. Impact of egg disinfection of hatching eggs on the eggshell microbiome and bacterial load. Poult. Sci. 2017, 96, 3901–3911.
- Fathima, S.; Shanmugasundaram, R.; Adams, D.; Selvaraj, R.K. Gastrointestinal Microbiota and Their Manipulation for Improved Growth and Performance in Chickens. Foods 2022, 11, 1401.
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019, 10, 2.
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W.K. Chicken gut microbiota: Importance and detection technology. Front. Vet. Sci. 2018, 5.
- Hinton, A.; Buhr, R.J.; Ingram, K.D. Physical, Chemical, and Microbiological Changes in the Crop of Broiler Chickens Subjected to Incremental Feed Withdrawal. Poult. Sci. 2000, 79, 212–218.
- Gong, J.; Si, W.; Forster, R.J.; Huang, R.; Yu, H.; Yin, Y.; Yang, C.; Han, Y. 16S rRNA gene-based analysis of mucosa-associated bacterial community and phylogeny in the chicken gastrointestinal tracts: From crops to ceca. FEMS Microbiol. Ecol. 2006, 59, 147–157.
- Videnska, P.; Faldynova, M.; Juricova, H.; Babak, V.; Sisak, F.; Havlickova, H.; Rychlik, I. Chicken faecal microbiota and disturbances induced by single or repeated therapy with tetracycline and streptomycin. BMC Vet. Res. 2013, 9, 6–8.
- Takahashi, M.; Kametaka, M.; Mitsuoka, T. Influence of Diets Low in Protein or Lysine on the Intestinal Flora of Chicks with Reference to Cecal Contents. J. Nutr. Sci. Vitaminol. 1982, 28, 501–510.
- Lu, J.; Idris, U.; Harmon, B.; Hofacre, C.; Maurer, J.J.; Lee, M.D. Diversity and Succession of the Intestinal Bacterial Community of the Maturing Broiler Chicken. Appl. Environ. Microbiol. 2003, 69, 6816–6824.
- Gong, J.; Forster, R.J.; Yu, H.; Chambers, J.R.; Sabour, P.M.; Wheatcroft, R.; Chen, S. Diversity and phylogenetic analysis of bacteria in the mucosa of chicken ceca and comparison with bacteria in the cecal lumen. FEMS Microbiol. Lett. 2002, 208, 1–7.
- Subirats, J.; Murray, R.; Scott, A.; Lau, C.H.F.; Topp, E. Composting of chicken litter from commercial broiler farms reduces the abundance of viable enteric bacteria, Firmicutes, and selected antibiotic resistance genes. Sci. Total Environ. 2020, 746, 141113.
- Rychlik, I. Composition and function of chicken gut microbiota. Animals 2020, 10, 103.
- Dhama, K.; Rajagunalan, S.; Chakraborty, S.; Verma, A.K.; Kumar, A.; Tiwari, R.; Kapoor, S. Food-borne Pathogens of Animal Origin-Diagnosis, Prevention, Control and Their Zoonotic Significance: A Review. Pakistan J. Biol. Sci. 2013, 16, 1076–1085.
- Edwards, P.; Zhang, W.; Belton, B.; Little, D.C. Misunderstandings, myths and mantras in aquaculture: Its contribution to world food supplies has been systematically over reported. Mar. Policy 2019, 106, 103547.
- FAO. World Agriculture: Towards 2030/2050 Prospects for Food, Nutrition, Agriculture and Major Commodity Groups; FAO: Yokohama, Japan, 2006.
- GPP/GOV Análise Setorial Carne de Aves; Gabinete de Planeamento, Políticas e Administração Geral: Lisboa, Portugal, 2020.
- Pereira, J.L.S.; Ferreira, S.; Pinheiro, V.; Trindade, H. Ammonia, Nitrous Oxide, Carbon Dioxide and Methane Emissions from Commercial Broiler Houses in Mediterranean Portugal. Water. Air. Soil Pollut. 2018, 229, 377.
- INE Estatísticas Agrícolas 2021; Instituto Nacional De Estatística: Lisboa, Portugal, 2022.
- Patel, S.J.; Wellington, M.; Shah, R.M.; Ferreira, M.J. Antibiotic Stewardship in Food-producing Animals: Challenges, Progress, and Opportunities. Clin. Ther. 2020, 42, 1649–1658.
- ESVAC. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2019 and 2020. Trends from 2010 to 2020 (EMA/294674/2019); European Medicines Agency: Zuidas, Amsterdam, The Netherlands, 2019; ISBN 9789291550685.
- Roth, N.; Käsbohrer, A.; Mayrhofer, S.; Zitz, U.; Hofacre, C.; Domig, K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poult. Sci. 2019, 98, 1791–1804.
- Poole, T.; Sheffield, C. Use and Misuse of Antimicrobial Drugs in Poultry and Livestock: Mechanisms of Antimicrobial Resistance. Pak. Vet. J. 2013, 33, 85–92.
- Diarra, M.S.; Malouin, F. Antibiotics in Canadian poultry productions and anticipated alternatives. Front. Microbiol. 2014, 5, 282.
- European Commission. Ban on Antibiotics as Growth Promoters in Animal Feed Enters Into Effect; European Commission: Brussels, Belgium, 2005.
- Lee, J.H.; Cho, S.; Paik, H.D.; Choi, C.W.; Nam, K.T.; Hwang, S.G.; Kim, S.K. Investigation on antibacterial and antioxidant activities, phenolic and flavonoid contents of some thai edible plants as an alternative for antibiotics. Asian-Australas. J. Anim. Sci. 2014, 27, 1461–1468.
- Bager, F. DANMAP: Monitoring antimicrobial resistance in Denmark. Int. J. Antimicrob. Agents 2000, 14, 271–274.
- PHAC. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2017: Design and Methods; PHAC: Ottawa, ON, Canada, 2017.
- Shimazaki, Y.; Ozawa, M.; Matsuda, M.; Akama, R.; Shirakawa, T.; Furuya, Y.; Harada, S. Contributors: Report on the Japanese Veterinary Antimicrobial Resistance Monitoring System; National Veterinary Assay Laboratory Ministry of Agriculture, Forestry and Fisheries: Tokyo, Japan, 2020.
- Wallinga, D.; Smit, L.A.M.; Davis, M.F.; Casey, J.A.; Nachman, K.E. A Review of the Effectiveness of Current US Policies on Antimicrobial Use in Meat and Poultry Production. Curr. Environ. Health Rep. 2022, 9, 339–354.
- ESVAC. Sales of Veterinary Antimicrobial Agents in 30 European Countries in 2015. Trends from 2010 to 2015 (EMA/184855/2017); European Medicines Agency: Zuidas, Amsterdam, The Netherlands, 2017.
- Singer, R.S.; Porter, L.J.; Thomson, D.U.; Gage, M.; Beaudoin, A.; Wishnie, J.K. Raising Animals Without Antibiotics: U.S. Producer and Veterinarian Experiences and Opinions. Front. Vet. Sci. 2019, 6, 452.
- McKeith, A.; Loper, M.; Tarrant, K.J. Research Note: Stocking density effects on production qualities of broilers raised without the use of antibiotics. Poult. Sci. 2019, 99, 698–701.
- Seidavi, A.; Tavakoli, M.; Slozhenkina, M.; Gorlov, I.; Hashem, N.M.; Asroosh, F.; Taha, A.E.; Abd El-Hack, M.E.; Swelum, A.A. The use of some plant-derived products as effective alternatives to antibiotic growth promoters in organic poultry production: A review. Environ. Sci. Pollut. Res. 2021, 28, 47856–47868.
- Magnusson, U.; Lewerin, S.S.; Eklund, G.; Rozstalnyy, A. Prudent and Efficient Use of Antimicrobials in Pigs and Poultry; FAO: Rome, Italy, 2019.
- Rajput, D.S.; Zeng, D.; Khalique, A.; Rajput, S.S.; Wang, H.; Zhao, Y.; Sun, N.; Ni, X. Pretreatment with probiotics ameliorate gut health and necrotic enteritis in broiler chickens, a substitute to antibiotics. AMB Express 2020, 10, 220.
- Wijesekara, P.N.K.; Kumbukgolla, W.W.; Jayaweera, J.A.A.S.; Rawat, D. Review on usage of vancomycin in livestock and humans: Maintaining its efficacy, prevention of resistance and alternative therapy. Vet. Sci. 2017, 4, 6.
- Simm, R.; Slettemeås, J.S.; Norström, M.; Dean, K.R.; Kaldhusdal, M.; Urdahl, A.M. Significant reduction of vancomycin resistant E. faecium in the Norwegian broiler population coincided with measures taken by the broiler industry to reduce antimicrobial resistant bacteria. PLoS ONE 2019, 14, e0226101.
- Sabença, C.; de Sousa, T.; Oliveira, S.; Viala, D.; Théron, L.; Chambon, C.; Hébraud, M.; Beyrouthy, R.; Bonnet, R.; Caniça, M.; et al. Next-Generation Sequencing and MALDI Mass Spectrometry in the Study of Multiresistant Processed Meat Vancomycin-Resistant Enterococci (VRE). Biology 2020, 9, 89.
- Bacanlı, M.; Başaran, N. Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019, 125, 462–466.
- Rousham, E.K.; Asaduzzaman, M.; Amin Uddin Mozmader, T.I.M.; Amin, M.B.; Rahman, M.; Hossain, M.I.; Islam, M.R.; Mahmud, Z.H.; Unicomb, L.; Islam, M.A. Human colonization with extended-spectrum beta-lactamase-producing E. coli in relation to animal and environmental exposures in bangladesh: An observational one health study. Environ. Health Perspect. 2021, 129, 037001.
- Ferreira, M.; Leão, C.; Clemente, L.; Albuquerque, T.; Amaro, A. Antibiotic Susceptibility Profiles and Resistance Mechanisms to β-Lactams and Polymyxins of Escherichia coli from Broilers Raised under Intensive and Extensive Production Systems. Microorganisms 2022, 10, 2044.
- Van Hoek, A.H.A.M.; Veenman, C.; Florijn, A.; Huijbers, P.M.C.; Graat, E.A.M.; De Greeff, S.; Dierikx, C.M.; Van Duijkeren, E. Longitudinal study of ESBL Escherichia coli carriage on an organic broiler farm. J. Antimicrob. Chemother. 2018, 73, 3298–3304.
- Subramanya, S.H.; Bairy, I.; Metok, Y.; Baral, B.P.; Gautam, D.; Nayak, N. Detection and characterization of ESBL-producing Enterobacteriaceae from the gut of subsistence farmers, their livestock, and the surrounding environment in rural Nepal. Sci. Rep. 2021, 11, 2091.
- Dierikx, C.M.; van der Goot, J.; van Essen-Zandbergen, A.; Mevius, D.J. Dynamics of cefotaxime resistant Escherichia coli in broilers in the first week of life. Vet. Microbiol. 2018, 222, 64–68.
- Zhang, L.; Kinkelaar, D.; Huang, Y.; Li, Y.; Li, X.; Wang, H.H. Acquired antibiotic resistance: Are we born with it? Appl. Environ. Microbiol. 2011, 77, 7134–7141.
- Silva, V.; Igrejas, G.; Carvalho, I.; Peixoto, F.; Cardoso, L.; Pereira, J.E.; Del Campo, R.; Poeta, P. Genetic Characterization of vanA-Enterococcus faecium Isolates from Wild Red-Legged Partridges in Portugal. Microb. Drug Resist. 2017, 24, 89–94.
- Borgen, K.; Sorum, M.; Wasteson, Y.; Kruse, H. VanA-type vancomycin-resistant enterococci (VRE) remain prevalent in poultry carcasses 3 years after avoparcin was banned. Int. J. Food Microbiol. 2001, 64, 89–94.
- Haley, B.J.; Van Kessel, J.A.S. The resistome of the bovine gastrointestinal tract. Curr. Opin. Biotechnol. 2022, 73, 213–219.
- Silva, V.; Caniça, M.; Capelo, J.L.; Igrejas, G.; Poeta, P. Diversity and genetic lineages of environmental staphylococci: A surface water overview. FEMS Microbiol. Ecol. 2020, 96, fiaa191.
- European Commission. Commission Implementing Decision (EU) 2020/1729; European Commission: Brussels, Belgium, 2020.
- Comerlato, C.B.; Ritter, A.C.; Miyamoto, K.N.; Brandelli, A. Proteomic study of Enterococcus durans LAB18S growing on prebiotic oligosaccharides. Food Microbiol. 2020, 89, 103430.
- Torres, C.; Alonso, C.A.; Ruiz-Ripa, L.; León-Sampedro, R.; Del Campo, R.; Coque, T.M. Antimicrobial Resistance in Enterococcus spp. of animal origin. Microbiol. Spectr. 2018, 6, 185–227.
- Ramos, S.; Silva, V.; Dapkevicius, M.d.L.E.; Igrejas, G.; Poeta, P. Enterococci, from harmless bacteria to a pathogen. Microorganisms 2020, 8, 1118.
- Roy, K.; Islam, M.S.; Paul, A.; Ievy, S.; Talukder, M.; Sobur, M.A.; Ballah, F.M.; Khan, M.S.R.; Rahman, M.T. Molecular detection and antibiotyping of multi-drug resistant Enterococcus faecium from healthy broiler chickens in Bangladesh. Vet. Med. Sci. 2022, 8, 200–210.
- Tyson, G.H.; Nyirabahizi, E.; Crarey, E.; Kabera, C.; Lam, C.; Rice-Trujillo, C.; McDermott, P.F.; Tate, H. Prevalence and antimicrobial resistance of enterococci isolated from retail meats in the United States, 2002 to 2014. Appl. Environ. Microbiol. 2017, 84, e01902-17.
- Lengfelder, I.; Sava, I.G.; Hansen, J.J.; Kleigrewe, K.; Herzog, J.; Neuhaus, K.; Hofmann, T.; Sartor, R.B.; Haller, D. Complex bacterial consortia reprogram the colitogenic activity of Enterococcus faecalis in a gnotobiotic mouse model of chronic, immune-mediated colitis. Front. Immunol. 2019, 10, 1420.
- Hasan, K.A.; Ali, S.A.; Rehman, M.; Bin-Asif, H.; Zahid, S. The unravelled Enterococcus faecalis zoonotic superbugs: Emerging multiple resistant and virulent lineages isolated from poultry environment. Zoonoses Public Health 2018, 65, 921–935.
- O’Dea, M.; Sahibzada, S.; Jordan, D.; Laird, T.; Lee, T.; Hewson, K.; Pang, S.; Abraham, R.; Coombs, G.W.; Harris, T.; et al. Genomic, Antimicrobial Resistance, and Public Health Insights into Enterococcus spp. from Australian Chickens. J. Clin. Microbiol. 2019, 57, e00319-19.
- Daniel, D.S.; Lee, S.M.; Gan, H.M.; Dykes, G.A.; Rahman, S. Genetic diversity of Enterococcus faecalis isolated from environmental, animal and clinical sources in Malaysia. J. Infect. Public Health 2017, 10, 617–623.
- Robbins, K.M.; Suyemoto, M.M.; Lyman, R.L.; Martin, M.P.; Barnes, H.J.; Borst, L.B. An outbreak and source investigation of enterococcal spondylitis in broilers caused by Enterococcus cecorum. Avian Dis. 2012, 56, 768–773.
- Miranda, J.M.; Guarddon, M.; Vázquez, B.I.; Fente, C.A.; Barros-Velázquez, J.; Cepeda, A.; Franco, C.M. Antimicrobial resistance in Enterobacteriaceae strains isolated from organic chicken, conventional chicken and conventional turkey meat: A comparative survey. J. Food Prot. 2007, 70, 412–416.
- Diarra, M.S.; Rempel, H.; Champagne, J.; Masson, L.; Pritchard, J.; Topp, E. Distribution of antimicrobial resistance and virulence genes in Enterococcus spp. and characterization of isolates from broiler chickens. Appl. Environ. Microbiol. 2010, 76, 8033–8043.
- Michaux, C.; Hansen, E.E.; Jenniches, L.; Gerovac, M.; Barquist, L.; Vogel, J. Single-Nucleotide RNA Maps for the Two Major Nosocomial Pathogens Enterococcus faecalis and Enterococcus faecium. Front. Cell Infect. Microbiol. 2020, 10, 600325.
- Gregersen, R.H.; Petersen, A.; Christensen, H.; Bisgaard, M. Multilocus sequence typing of Enterococcus faecalis isolates demonstrating different lesion types in broiler breeders. Avian Pathol. 2010, 39, 435–440.
- Sandhu, T.S. Fecal streptococcal infection of commercial white Pekin ducklings. Avian Dis. 1988, 32, 570–573.
- Devriese, L.A.; Hommez, J.; Wijfels, R.; Haesebrouck, F. Composition of the enterococcal and streptococcal intestinal flora of poultry. J. Appl. Bacteriol. 1991, 71, 46–50.
- De Jong, A.; Simjee, S.; Rose, M.; Moyaert, H.; El Garch, F.; Youala, M.; Butty, P.; Haag-Diergarten, S.; Klein, U.; Pellet, T.; et al. Antimicrobial resistance monitoring in commensal enterococci from healthy cattle, pigs and chickens across Europe during 2004-14 (EASSA Study). J. Antimicrob. Chemother. 2019, 74, 921–930.
- Dolka, B.; Gołȩbiewska-Kosakowska, M.; Krajewski, K.; Kwieciński, P.; Nowak, T.; Szubstarski, J.; Wilczyński, J.; Szeleszczuk, P. Occurrence of Enterococcus spp. in poultry in Poland based on 2014-2015 data. Med. Weter. 2017, 73, 220–224.
- Dolka, B.; Cisek, A.A.; Szeleszczuk, P. The application of the loop-mediated isothermal amplification (LAMP) method for diagnosing Enterococcus hirae-associated endocarditis outbreaks in chickens. BMC Microbiol. 2019, 19, 48.
- Stȩpień-Pyśniak, D.; Marek, A.; Banach, T.; Adaszek, Ł.; Pyzik, E.; Wilczyński, J.; Winiarczyk, S. Prevalence and antibiotic resistance of Enterococcus strains isolated from poultry. Acta Vet. Hung. 2016, 64, 148–163.
- Talarmin, J.P.; Pineau, S.; Guillouzouic, A.; Boutoille, D.; Giraudeau, C.; Reynaud, A.; Lepelletier, D.; Corvec, S. Relapse of Enterococcus hirae prosthetic valve endocarditis. J. Clin. Microbiol. 2011, 49, 1182–1184.
- Kim, M.H.; Moon, D.C.; Kim, S.-J.; Mechesso, A.F.; Song, H.-J.; Kang, H.Y.; Choi, J.-H.; Yoon, S.-S.; Lim, S.-K. Nationwide Surveillance on Antimicrobial Resistance Profiles of Enterococcus faecium and Enterococcus faecalis Isolated from Healthy Food Animals in South Korea, 2010–2019. Microorganisms 2021, 9, 925.
- Dolka, B.; Chrobak-Chmiel, D.; Makrai, L.; Szeleszczuk, P. Phenotypic and genotypic characterization of Enterococcus cecorum strains associated with infections in poultry. BMC Vet. Res. 2016, 12, 129.
- Greub, G.; Devriese, L.A.; Pot, B.; Dominguez, J.; Bille, J. Enterococcus cecorum Septicemia in a Malnourished Adult Patient. Eur. J. Clin. Microbiol. Infect Dis. 1997, 16, 594–598.
- Warnke, P.; Köller, T.; Stoll, P.; Podbielski, A. Nosocomial infection due to Enterococcus cecorum identified by MALDI-TOF MS and Vitek 2 from a blood culture of a septic patient. Eur. J. Microbiol. Immunol. 2015, 5, 177–179.
- Jung, A.; Teske, L.; Rautenschlein, S. Enterococcus cecorum infection in a racing pigeon. Avian Dis. 2014, 58, 654–658.
- Jung, A.; Rautenschlein, S. Comprehensive report of an Enterococcus cecorum infection in a broiler flock in Northern Germany. BMC Vet. Res. 2014, 10, 311.
- Aitchison, H.; Poolman, P.; Coetzer, M.; Griffiths, C.; Jacobs, J.; Meyer, M.; Bisschop, S. Enterococcal-related vertebral osteoarthritis in South African broiler breeders: A case report. J. S. Afr. Vet. Assoc. 2014, 85, 5–9.
- Schreier, J.; Rautenschlein, S.; Jung, A. Different virulence levels of Enterococcus cecorum strains in experimentally infected meat-type chickens. PLoS ONE 2021, 16, e0259904.
- Schreier, J.; Karasova, D.; Crhanova, M.; Rychlik, I.; Rautenschlein, S.; Jung, A. Influence of lincomycin-spectinomycin treatment on the outcome of Enterococcus cecorum infection and on the cecal microbiota in broilers. Gut Pathog. 2022, 14, 3.
- Borst, L.B.; Suyemoto, M.M.; Sarsour, A.H.; Harris, M.C.; Martin, M.P.; Strickland, J.D.; Oviedo, E.O.; Barnes, H.J. Pathogenesis of Enterococcal Spondylitis Caused by Enterococcus cecorum in Broiler Chickens. Vet. Pathol. 2017, 54, 61–73.
- Kense, M.J.; Landman, W.J.M. Enterococcus cecorum infections in broiler breeders and their offspring: Molecular epidemiology. Avian Pathol. 2011, 40, 603–612.
- Stalker, M.J.; Brash, M.L.; Weisz, A.; Ouckama, R.M.; Slavic, D. Arthritis and osteomyelitis associated with Enterococcus cecorum infection in broiler and broiler breeder chickens in Ontario, Canada. J. Vet. Diagnostic Investig. 2010, 22, 643–645.
- Sanlibaba, P.; Senturk, E. Prevalence, characterization, and antibiotic resistance of enterococci from traditional cheeses in turkey. Int. J. Food Prop. 2018, 21, 1955–1963.
- Arias, C.A.; Murray, B.E. The rise of the Enterococcus: Beyond vancomycin resistance. Nat. Rev. Microbiol. 2012, 10, 266–278.
- Shang, Y.; Li, D.; Shan, X.; Schwarz, S.; Zhang, S.M.; Chen, Y.X.; Ouyang, W.; Du, X.D. Analysis of two pheromone-responsive conjugative multiresistance plasmids carrying the novel mobile optra locus from Enterococcus faecalis. Infect. Drug Resist. 2019, 12, 2355–2362.
- Poeta, P.; Costa, D.; Rodrigues, J.; Torres, C. Antimicrobial resistance and the mechanisms implicated in faecal enterococci from healthy humans, poultry and pets in Portugal. Int. J. Antimicrob. Agents 2006, 27, 131–137.
- Miller, W.R.; Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance in enterococci. Expert Rev. Anti. Infect. Ther. 2014, 12, 1221–1236.
- Semedo-Lemsaddek, T.; Bettencourt Cota, J.; Ribeiro, T.; Pimentel, A.; Tavares, L.; Bernando, F.; Oliveira, M. Resistance and virulence distribution in enterococci isolated from broilers reared in two farming systems. Ir. Vet. J. 2021, 74, 22.
- Ono, S.; Muratani, T.; Matsumoto, T. Mechanisms of resistance to imipenem and ampicillin in Enterococcus faecalis. Antimicrob. Agents Chemother. 2005, 49, 2954–2958.
- Braga, J.F.V.; Leal, C.A.G.; Silva, C.C.; Fernandes, A.A.; Martins, N.R.d.S.; Ecco, R. Genetic diversity and antimicrobial resistance profile of Enterococcus faecalis isolated from broilers with vertebral osteomyelitis in Southeast Brazil. Avian Pathol. 2018, 47, 14–22.
- Harada, T.; Mito, Y.; Otsuki, K.; Murase, T. Resistance to gentamicin and vancomycin in enterococcal strains isolated from retail broiler chickens in Japan. J. Food Prot. 2004, 67, 2292–2295.
- Aarestrup, F.M.; Agerso, Y.; Gerner-Smidt, P.; Madsen, M.; Jensen, L.B. Comparison of antimicrobial resistance phenotypes and resistance genes in Enterococcus faecalis and Enterococcus faecium from humans in the community, broilers, and pigs in Denmark. Diagn. Microbiol. Infect. Dis. 2000, 37, 127–137.
- Weinbren, M.J.; Johnson, A.P.; Woodford, N. Defining high-level gentamicin resistance in enterococci. J. Antimicrob. Chemother. 2000, 45, 404–405.
- Donabedian, S.M.; Thal, L.A.; Hershberger, E.; Perri, M.B.; Chow, J.W.; Bartlett, P.; Jones, R.; Joyce, K.; Rossiter, S.; Gay, K.; et al. Molecular characterization of gentamicin-resistant Enterococci in the United States: Evidence of spread from animals to humans through food. J. Clin. Microbiol. 2003, 41, 1109–1113.
- Schwarz, S.; Kehrenberg, C.; Doublet, B.; Cloeckaert, A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol. Rev. 2004, 28, 519–542.
- Baynes, R.E.; Dedonder, K.; Kissell, L.; Mzyk, D.; Marmulak, T.; Smith, G.; Tell, L.; Gehring, R.; Davis, J.; Riviere, J.E. Health concerns and management of select veterinary drug residues. Food Chem. Toxicol. 2016, 88, 112–122.
- Ünal, N.; Aşkar, Ş.; Yildirim, M. Antibiotic resistance profile of Enterococcus faecium and Enterococcus faecalis isolated from broiler cloacal samples. Turkish J. Vet. Anim. Sci. 2017, 41, 199–203.
- Habib, I.; Ghazawi, A.; Lakshmi, G.B.; Mohamed, M.I.; Li, D.; Khan, M.; Sahibzada, S. Emergence and Genomic Characterization of the First Reported optrA-Carrying Linezolid-Resistant Enterococci Isolated from Retail Broiler Meat in the United Arab Emirates. Foods 2022, 11, 3190.
- Hui, L.A.; Bodolea, C.; Vlase, L.; Hiriscau, E.I.; Popa, A. Linezolid Administration to Critically Ill Patients: Intermittent or Continuous Infusion? A Systematic Literature Search and Review. Antibiotics 2022, 11, 436.
- Yoon, S.; Kim, Y.B.; Seo, K.W.; Ha, J.S.; Noh, E.B.; Lee, Y.J. Characteristics of linezolid-resistant Enterococcus faecalis isolates from broiler breeder farms. Poult. Sci. 2020, 99, 6055–6061.
- Tyson, G.H.; Sabo, J.L.; Hoffmann, M.; Hsu, C.H.; Mukherjee, S.; Hernandez, J.; Tillman, G.; Wasilenko, J.L.; Haro, J.; Simmons, M.; et al. Novel linezolid resistance plasmids in Enterococcus from food animals in the USA. J. Antimicrob. Chemother. 2018, 73, 3254–3258.
- Gião, J.; Leão, C.; Albuquerque, T.; Clemente, L.; Amaro, A. Antimicrobial Susceptibility of Enterococcus Isolates from Cattle and Pigs in Portugal: Linezolid Resistance Genes optrA and poxtA. Antibiotics 2022, 11, 615.
- Wang, Y.; Lv, Y.; Cai, J.; Schwarz, S.; Cui, L.; Hu, Z.; Zhang, R.; Li, J.; Zhao, Q.; He, T.; et al. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J. Antimicrob. Chemother. 2015, 70, 2182–2190.
- Long, K.S.; Poehlsgaard, J.; Kehrenberg, C.; Schwarz, S.; Vester, B. The cfr rRNA methyltransferase confers resistance to phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin A antibiotics. Antimicrob. Agents Chemother. 2006, 50, 2500–2505.
- Kim, Y.B.; Seo, K.W.; Son, S.H.; Noh, E.B.; Lee, Y.J. Genetic characterization of high-level aminoglycoside-resistant Enterococcus faecalis and Enterococcus faecium isolated from retail chicken meat. Poult. Sci. 2019, 98, 5981–5988.
- Tamang, M.D.; Moon, D.C.; Kim, S.R.; Kang, H.Y.; Lee, K.; Nam, H.M.; Jang, G.C.; Lee, H.S.; Jung, S.C.; Lim, S.K. Detection of novel oxazolidinone and phenicol resistance gene optrA in enterococcal isolates from food animals and animal carcasses. Vet. Microbiol. 2017, 201, 252–256.
- Cauwerts, K.; Decostere, A.; De Graef, E.M.; Haesebrouck, F.; Pasmans, F. High prevalence of tetracycline resistance in Enterococcus isolates from broilers carrying the erm(B) gene. Avian Pathol. 2007, 36, 395–399.
- Nilsson, O.; Alm, E.; Greko, C.; Bengtsson, B. The rise and fall of a vancomycin-resistant clone of Enterococcus faecium among broilers in Sweden. J. Glob. Antimicrob. Resist. 2019, 17, 233–235.
- Skarzynska, M.; Leekitcharoenphon, P.; Hendriksen, R.S.; Aarestrup, F.M.; Wasyl, D. A metagenomic glimpse into the gut of wild and domestic animals: Quantification of antimicrobial resistance and more. PLoS ONE 2020, 15, e0242987.
- Nilsson, O.; Greko, C.; Top, J.; Franklin, A.; Bengtsson, B. Spread without known selective pressure of a vancomycin-resistant clone of Enterococcus faecium among broilers. J. Antimicrob. Chemother. 2009, 63, 868–872.
- Robredo, B.; Singh, K.V.; Baquero, F.; Murray, B.E.; Torres, C. From vanA Enterococcus hirae to vanA Enterococcus faecium: A study of feed supplementation with avoparcin and tylosin in young chickens. Antimicrob. Agents Chemother. 1999, 43, 1137–1143.
- Zhu, Y.; Huang, W.E.; Yang, Q. Clinical Perspective of Antimicrobial Resistance in Bacteria. Infect. Drug Resist. 2022, 15, 735–746.
- Dubin, K.; Pamer, E.G. Enterococci and Their Interactions with the Intestinal Microbiome. Microbiol. Spectr. 2017, 5, 5–6.
- Manson, J.M.; Keis, S.; Smith, J.M.B.; Cook, G.M. A clonal lineage of VanA-type Enterococcus faecalis predominates in vancomycin-resistant enterococci isolated in New Zealand. Antimicrob. Agents Chemother. 2003, 47, 204–210.
- Heuer, O.E.; Pedersen, K.; Jensen, L.B.; Madsen, M.; Olsen, J.E. Persistence of vancomycin-resistant enterococci (VRE) in broiler houses after the avoparcin ban. Microb. Drug Resist. 2002, 8, 355–361.
- Klare, I.; Badstübner, D.; Konstabel, C.; Böhme, G.; Claus, H.; Witte, W. Decreased incidence of VanA-type vancomycin-resistant enterococci isolated from poultry meat and from fecal samples of humans in the community after discontinuation of avoparcin usage in animal husbandry. Microb. Drug Resist. 1999, 5, 45–52.
- Leinweber, H.; Alotaibi, S.M.I.; Overballe-Petersen, S.; Hansen, F.; Hasman, H.; Bortolaia, V.; Hammerum, A.M.; Ingmer, H. Vancomycin resistance in Enterococcus faecium isolated from Danish chicken meat is located on a pVEF4-like plasmid persisting in poultry for 18 years. Int. J. Antimicrob. Agents 2018, 52, 283–286.
- Aun, E.; Kisand, V.; Laht, M.; Telling, K.; Kalmus, P.; Väli, Ü.; Brauer, A.; Remm, M.; Tenson, T. Molecular Characterization of Enterococcus Isolates From Different Sources in Estonia Reveals Potential Transmission of Resistance Genes Among Different Reservoirs. Front. Microbiol. 2021, 12, 601490.
- Ahmed, M.O.; Baptiste, K.E. Vancomycin-Resistant Enterococci: A Review of Antimicrobial Resistance Mechanisms and Perspectives of Human and Animal Health. Microb. Drug Resist. 2018, 24, 590–606.
- Morris, D.; Galvin, S.; Boyle, F.; Hickey, P.; Mulligan, M.; Cormican, M. Enterococcus faecium of the vanA genotype in rural drinking water, effluent, and the aqueous environment. Appl. Environ. Microbiol. 2011, 78, 596–598.
- Poeta, P.; Costa, D.; Rodrigues, J.; Torres, C. Study of faecal colonization by vanA-containing Enterococcus strains in healthy humans, pets, poultry and wild animals in Portugal. J. Antimicrob. Chemother. 2005, 55, 278–280.
- Wilson, I.G.; McAfee, G.G. Vancomycin-resistant enterococci in shellfish, unchlorinated waters, and chicken. Int. J. Food Microbiol. 2002, 79, 143–151.
- Dutta, I.; Reynolds, P.E. Biochemical and genetic characterization of the vanC-2 vancomycin resistance gene cluster of Enterococcus casseliflavus ATCC 25788. Antimicrob. Agents Chemother. 2002, 46, 3125–3132.
- Stępień-Pyśniak, D.; Hauschild, T.; Dec, M.; Marek, A.; Brzeski, M.; Kosikowska, U. Antimicrobial resistance and genetic diversity of Enterococcus faecalis from yolk sac infections in broiler chicks. Poult. Sci. 2021, 100, 101491.
- Alzahrani, O.M.; Fayez, M.; Alswat, A.S.; Alkafafy, M.; Mahmoud, S.F.; Al-Marri, T.; Almuslem, A.; Ashfaq, H.; Yusuf, S. Antimicrobial Resistance, Biofilm Formation, and Virulence Genes in Enterococcus Species from Small Backyard Chicken Flocks. Antibiotics 2022, 11, 380.
- Yu, L.; Liu, Y.; Liu, M.; Li, Z.; Li, L.; Wang, F. Research Note: Molecular characterization of antimicrobial resistance and virulence gene analysis of Enterococcus faecalis in poultry in Tai’an, China. Poult. Sci. 2022, 101, 101763.
- Kim, Y.B.; Seo, H.J.; Seo, K.W.; Jeon, H.Y.; Kim, D.K.; Kim, S.W.; Lim, S.K.; Lee, Y.J. Characteristics of high-Level ciprofloxacin-Resistant Enterococcus faecalis and Enterococcus faecium from retail chicken meat in Korea. J. Food Prot. 2018, 81, 1357–1363.
- Aslanta, Ö. Molecular and phenotypic characterization of enterococci isolated from broiler flocks in Turkey. Trop. Anim. Health Prod. 2019, 51, 1073–1082.
- Day, M.J.; Rodríguez, I.; van Essen-Zandbergen, A.; Dierikx, C.; Kadlec, K.; Schink, A.K.; Wu, G.; Chattaway, M.A.; DoNascimento, V.; Wain, J.; et al. Diversity of STs, plasmids and ESBL genes among Escherichia coli from humans, animals and food in Germany, the Netherlands and the UK. J. Antimicrob. Chemother. 2016, 71, 1178–1182.
- Badr, H.; Reda, R.M.; Hagag, N.M.; Kamel, E.; Elnomrosy, S.M.; Mansour, A.I.; Shahein, M.A.; Ali, S.F.; Ali, H.R. Multidrug-Resistant and Genetic Characterization of Recovered from Chickens and Humans in Egypt. Animals 2022, 12, 346.
- Ramos, S.; Silva, V.; Dapkevicius, M.d.L.E.; Caniça, M.; Tejedor-Junco, M.T.; Igrejas, G.; Poeta, P. Escherichia coli as Commensal and Pathogenic Bacteria among Food-Producing Animals: Health Implications of Extended Spectrum β-Lactamase (ESBL) Production. Animals 2020, 10, 2239.
- Poirel, L.; Madec, J.-Y.; Lupo, A.; Schink, A.-K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 4–6.
- Jouini, A.; Klibi, A.; Elarbi, I.; Chaabene, M.B.; Hamrouni, S.; Souiai, O.; Hanachi, M.; Ghram, A.; Maaroufi, A. First detection of human ST131-CTX-M-15-o25-b2 clone and high-risk clonal lineages of ESBL/pAmpC-producing E. coli isolates from diarrheic poultry in Tunisia. Antibiotics 2021, 10, 70.
- Lindstedt, B.A.; Finton, M.D.; Porcellato, D.; Brandal, L.T. High frequency of hybrid Escherichia coli strains with combined Intestinal Pathogenic Escherichia coli (IPEC) and Extraintestinal Pathogenic Escherichia coli (ExPEC) virulence factors isolated from human faecal samples. BMC Infect. Dis. 2018, 18, 544.
- Burke, D.A.; Axon, A.T.R. Ulcerative colitis and Escherichia coli with adhesive Properties. J. Clin. Pathol. 1987, 40, 782–786.
- Akya, A.; Ahmadi, M.; Khodamoradi, S.; Rezaei, M.R.; Karani, N.; Elahi, A.; Lorestani, R.C.; Rezaei, M. Prevalence of blaCTX-M, blaCTX-M-2, blaCTX-M-8, blaCTX-M-25 and blaCTX-M-3 Genes in Escherichia coli Isolated from Urinary Tract Infection in Kermanshah City, Iran. J. Clin. Diagnostic Res. 2019, 13, 13–16.
- Leverstein-van Hall, M.A.; Dierikx, C.M.; Cohen Stuart, J.; Voets, G.M.; van den Munckhof, M.P.; van Essen-Zandbergen, A.; Platteel, T.; Fluit, A.C.; van de Sande-Bruinsma, N.; Scharinga, J.; et al. Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin. Microbiol. Infect. 2011, 17, 873–880.
- Stromberg, Z.R.; Johnson, J.R.; Fairbrother, J.M.; Kilbourne, J.; Van Goor, A.; Curtiss, R.; Mellata, M. Evaluation of Escherichia coli isolates from healthy chickens to determine their potential risk to poultry and human health. PLoS ONE 2017, 12, e0180599.
- Mohamed, M.A.; Shehata, M.A.; Rafeek, E. Virulence genes content and antimicrobial resistance in Escherichia coli from broiler chickens. Vet. Med. Int. 2014, 2014, 195189.
- Awawdeh, L.; Turni, C.; Mollinger, J.L.; Henning, J.; Cobbold, R.N.; Trott, D.J.; Wakeham, D.L.; Gibson, J.S. Antimicrobial susceptibility, plasmid replicon typing, phylogenetic grouping, and virulence potential of avian pathogenic and faecal Escherichia coli isolated from meat chickens in Australia. Avian Pathol. 2022, 51, 349–360.
- Kravik, I.H.; Kaspersen, H.; Sjurseth, S.K.; Jonsson, M.; David, B.; Aspholm, M.; Sekse, C. High sequence similarity between avian pathogenic E. coli isolates from individual birds and within broiler chicken flocks during colibacillosis outbreaks. Vet. Microbiol. 2022, 267, 109378.
- Ahmed, A.M.; Shimamoto, T.; Shimamoto, T. Molecular characterization of multidrug-resistant avian pathogenic Escherichia coli isolated from septicemic broilers. Int. J. Med. Microbiol. 2013, 303, 475–483.
- Solà-Ginés, M.; Cameron-Veas, K.; Badiola, I.; Dolz, R.; Majó, N.; Dahbi, G.; Viso, S.; Mora, A.; Blanco, J.; Piedra-Carrasco, N.; et al. Diversity of multi-drug resistant avian pathogenic Escherichia coli (APEC) Causing outbreaks of colibacillosis in broilers during 2012 in Spain. PLoS ONE 2015, 10, e0143191.
- Dhaouadi, S.; Soufi, L.; Hamza, A.; Fedida, D.; Zied, C.; Awadhi, E.; Mtibaa, M.; Hassen, B.; Cherif, A.; Torres, C.; et al. Co-occurrence of mcr-1 mediated colistin resistance and β-lactamase-encoding genes in multidrug-resistant Escherichia coli from broiler chickens with colibacillosis in Tunisia. J. Glob. Antimicrob. Resist. 2020, 22, 538–545.
- Kim, Y.B.; Yoon, M.Y.; Ha, J.S.; Seo, K.W.; Noh, E.B.; Son, S.H.; Lee, Y.J. Molecular characterization of avian pathogenic Escherichia coli from broiler chickens with colibacillosis. Poult. Sci. 2020, 99, 1088–1095.
- Saif, Y.M.; Fadly, A.M.; Glisson, J.R.; McDougald, L.R.; Nolan, L.K.; Swayne, D.E. Diseases of Poultry, 12th ed.; Blackwell Publishing: Hoboken, NJ, USA, 2008; ISBN 9781119350927.
- Śmiałek, M.; Kowalczyk, J.; Koncicki, A. Influence of vaccination of broiler chickens against Escherichia coli with live attenuated vaccine on general properties of E. coli population, IBV vaccination efficiency, and production parameters—A field experiment. Poult. Sci. 2020, 99, 5452–5460.
- Ebrahimi-Nik, H.; Bassami, M.R.; Mohri, M.; Rad, M.; Khan, M.I. Bacterial ghost of avian pathogenic E. coli (APEC) serotype O78:K80 as a homologous vaccine against avian colibacillosis. PLoS ONE 2018, 13, e0194888.
- Oliveira, J.M.; Cardoso, M.F.; Moreira, F.A.; Müller, A. Phenotypic antimicrobial resistance (AMR) of avian pathogenic Escherichia coli (APEC) from broiler breeder flocks between 2009 and 2018. Avian Pathol. 2022, 51, 388–394.
- Sarker, M.S.; Mannan, M.S.; Ali, M.Y.; Bayzid, M.; Ahad, A.; Bupasha, Z.B. Antibiotic resistance of Escherichia coli isolated from broilers sold at live bird markets in Chattogram, Bangladesh. J. Adv. Vet. Anim. Res. 2019, 6, 272–277.
- Borges, C.A.; Tarlton, N.J.; Riley, L.W. Escherichia coli from Commercial Broiler and Backyard Chickens Share Sequence Types, Antimicrobial Resistance Profiles, and Resistance Genes with Human Extraintestinal Pathogenic Escherichia coli. Foodborne Pathog. Dis. 2019, 16, 813–822.
- Apostolakos, I.; Mughini-Gras, L.; Fasolato, L.; Piccirillo, A. Assessing the occurrence and transfer dynamics of ESBL/pAmpC-producing Escherichia coli across the broiler production pyramid. PLoS ONE 2019, 14, e0217174.
- Al Azad, M.A.R.; Rahman, M.M.; Amin, R.; Begum, M.I.A.; Fries, R.; Husna, A.; Khairalla, A.S.; Badruzzaman, A.T.M.; El Zowalaty, M.E.; Lampang, K.N.; et al. Susceptibility and multidrug resistance patterns of Escherichia coli isolated from cloacal swabs of live broiler chickens in Bangladesh. Pathogens 2019, 8, 118.
- Hojabri, Z.; Darabi, N.; Arab, M.; Saffari, F.; Pajand, O. Clonal diversity, virulence genes content and subclone status of Escherichia coli sequence type 131: Comparative analysis of E. coli ST131 and non-ST131 isolates from Iran. BMC Microbiol. 2019, 19, 117.
- Das, A.; Dhar, P.K.; Dutta, A.; Jalal, M.S.; Ghosh, P.; Das, T.; Barua, H.; Biswas, P.K. Circulation of oxytetracycline- and ciprofloxacin-resistant commensal Escherichia coli strains in broiler chickens and farm environments, Bangladesh. Vet. World 2020, 13, 2395–2400.
- Thorsteinsdottir, T.R.; Haraldsson, G.; Fridriksdottir, V.; Kristinsson, K.G.; Gunnarsson, E. Prevalence and genetic relatedness of antimicrobial-resistant Escherichia coli isolated from animals, foods and humans in Iceland. Zoonoses Public Health 2010, 57, 189–196.
- Yoon, M.Y.; Kim, Y.B.; Ha, J.S.; Seo, K.W.; Noh, E.B.; Son, S.H.; Lee, Y.J. Molecular characteristics of fluoroquinolone-resistant avian pathogenic Escherichia coli isolated from broiler chickens. Poult. Sci. 2020, 99, 3628–3636.
- Mahmud, S.; Nazir, K.H.M.N.H.; Rahman, M.T. Prevalence and molecular detection of fluoroquinolone-resistant genes (qnrA and qnrS) in Escherichia coli isolated from healthy broiler chickens. Vet. World 2018, 11, 1720–1724.
- De Koster, S.; Ringenier, M.; Lammens, C.; Stegeman, A.; Tobias, T.; Velkers, F.; Vernooij, H.; Kluytmans-Van Den Bergh, M.; Kluytmans, J.; Dewulf, J.; et al. ESBL-producing, carbapenem-and ciprofloxacin-resistant Escherichia coli in Belgian and Dutch broiler and pig farms: A cross-sectional and cross-border study. Antibiotics 2021, 10, 945.
- Börjesson, S.; Guillard, T.; Landén, A.; Bengtsson, B.; Nilsson, O. Introduction of quinolone resistant Escherichia coli to Swedish broiler population by imported breeding animals. Vet. Microbiol. 2016, 194, 74–78.
- Mendonça, N.; Figueiredo, R.; Mendes, C.; Card, R.M.; Anjum, M.F.; da Silva, G.J. Microarray evaluation of antimicrobial resistance and virulence of Escherichia coli isolates from Portuguese poultry. Antibiotics 2016, 5, 4.
- Trobos, M.; Jakobsen, L.; Olsen, K.E.P.; Frimodt-Møller, N.; Hammerum, A.M. Prevalence of sulphonamide resistance and class 1 integron genes in Escherichia coli isolates obtained from broilers, broiler meat, healthy humans and urinary infections in Denmark. Int. J. Antimicrob. Agents 2008, 32, 366–367.
- Amador, P.; Fernandes, R.; Prudêncio, C.; Duarte, I. Prevalence of antibiotic resistance genes in multidrug-resistant Enterobacteriaceae on portuguese livestock manure. Antibiotics 2019, 8, 23.
- Messaili, C.; Messai, Y.; Bakour, R. Virulence gene profiles, antimicrobial resistance and phylogenetic groups of fecal Escherichia coli strains isolated from broiler chickens in Algeria. Vet. Ital. 2019, 55, 35–46.
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-spectrum β-lactamases: An update on their characteristics, epidemiology and detection. JAC-Antimicrobial Resist. 2021, 3, dlab092.
- De Champs, C.; Sirot, D.; Chanal, C.; Bonnet, R.; Sirot, J.; Avril, J.L.; Cattoen, C.; Chardon, H.; Croix, J.L.; Dabernat, H.; et al. A 1998 survey of extended-spectrum β-lactamases in Enterobacteriaceae in France. Antimicrob. Agents Chemother. 2000, 44, 3177–3179.
- Subramanya, S.H.; Bairy, I.; Nayak, N.; Padukone, S.; Sathian, B.; Gokhale, S. Low rate of gut colonization by extended-spectrum β-lactamase producing Enterobacteriaceae in HIV infected persons as compared to healthy individuals in Nepal. PLoS ONE 2019, 14, e0212042.
- Cantón, R.; Novais, A.; Valverde, A.; Machado, E.; Peixe, L.; Baquero, F.; Coque, T.M. Prevalence and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 2008, 14, 144–153.
- Knothe, H.; Shah, P.; Krcmery, V.; Antal, M.; Mitsuhashi, S. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection 1983, 11, 315–317.
- Carvalho, I.; Silva, N.; Carrola, J.; Silva, V.; Currie, C.; Igrejas, G.; Poeta, P. Antibiotic resistance: Immunity-acquired resistance: Evolution of antimicrobial resistance among extended-spectrum β-lactamases and carbapenemases in Klebsiella pneumoniae and Escherichia coli. In Antibiotic Drug Resistance; CDC: Atlanta, GA, USA, 2019.
- Wibisono, F.J.; Sumiarto, B.; Untari, T.; Effendi, M.H.; Permatasari, D.A.; Witaningrum, A.M. CTX Gene of Extended Spectrum Beta-Lactamase (ESBL) Producing Escherichia coli on Broilers in Blitar, Indonesia. Syst. Rev. Pharm. 2020, 11, 396–403.
- Päivärinta, M.; Latvio, S.; Fredriksson-Ahomaa, M.; Heikinheimo, A. Whole genome sequence analysis of antimicrobial resistance genes, multilocus sequence types and plasmid sequences in ESBL/AmpC Escherichia coli isolated from broiler caecum and meat. Int. J. Food Microbiol. 2020, 315, 108361.
- Smet, A.; Martel, A.; Persoons, D.; Dewulf, J.; Heyndrickx, M.; Herman, L.; Haesebrouck, F.; Butaye, P. Broad-spectrum β-lactamases among Enterobacteriaceae of animal origin: Molecular aspects, mobility and impact on public health. FEMS Microbiol. Rev. 2010, 34, 295–316.
- Rawat, D.; Nair, D. Extended-spectrum ß-lactamases in gram negative bacteria. J. Glob. Infect. Dis. 2010, 2, 263.
- Sader, H.S.; Pfaller, M.A.; Jones, R.N. Prevalence of important pathogens and the antimicrobial activity of parenteral drugs at numerous medical centers in the United States. Diagn. Microbiol. Infect. Dis. 1994, 20, 203–208.
- Cantón, R.; González-Alba, J.M.; Galán, J.C. CTX-M enzymes: Origin and diffusion. Front. Microbiol. 2012, 3, 110.
- Seyedjavadi, S.S.; Goudarzi, M.; Sabzehali, F. Relation between blaTEM, blaSHV and blaCTX-M genes and acute urinary tract infections. J. Acute Dis. 2016, 5, 71–76.
- Nandagopal, B.; Sankar, S.; Sagadevan, K.; Arumugam, H.; Jesudason, M.V.; Aswathaman, K.; Nair, A. Frequency of extended spectrum β-lactamase producing urinary isolates of Gram-negative bacilli among patients seen in a multispecialty hospital in Vellore district, India. Indian J. Med. Microbiol. 2015, 33, 282–285.
- El Bouamri, M.C.; Arsalane, L.; Zerouali, K.; Katfy, K.; El Kamouni, Y.; Zouhair, S. Molecular characterization of extended spectrum β-lactamase-producing Escherichia coli in a university hospital in Morocco, North Africa. African J. Urol. 2015, 21, 161–166.
- Carvalho, I.; Tejedor-Junco, M.T.; González-Martín, M.; Corbera, J.A.; Silva, V.; Igrejas, G.; Torres, C.; Poeta, P. Escherichia coli producing extended-spectrum β-lactamases (ESBL) from domestic camels in the Canary Islands: A one health approach. Animals 2020, 10, 1295.
- O’Keefe, A.; Hutton, T.A.; Schifferli, D.M.; Rankin, S.C. First detection of CTX-M and SHV extended-spectrum β-lactamases in Escherichia coli urinary tract isolates from dogs and cats in the United States. Antimicrob. Agents Chemother. 2010, 54, 3489–3492.
- Seo, K.W.; Lee, Y.J. The occurrence of CTX-M–producing E. coli in the broiler parent stock in Korea. Poult. Sci. 2021, 100, 1008–1015.
- Cormier, A.; Zhang, P.L.C.; Chalmers, G.; Weese, J.S.; Deckert, A.; Mulvey, M.; McAllister, T.; Boerlin, P. Diversity of CTX-M-positive Escherichia coli recovered from animals in Canada. Vet. Microbiol. 2019, 231, 71–75.
- Maciuca, I.E.; Williams, N.J.; Tuchilus, C.; Dorneanu, O.; Guguianu, E.; Carp-Carare, C.; Rimbu, C.; Timofte, D. High prevalence of Escherichia coli-producing CTX-M-15 extended-spectrum beta-lactamases in poultry and human clinical isolates in Romania. Microb. Drug Resist. 2015, 21, 651–662.
- Girlich, D.; Poirel, L.; Carattoli, A.; Kempf, I.; Lartigue, M.F.; Bertini, A.; Nordmann, P. Extended-spectrum β-lactamase CTX-M-1 in Escherichia coli isolates from healthy poultry in France. Appl. Environ. Microbiol. 2007, 73, 4681–4685.
- Kola, A.; Kohler, C.; Pfeifer, Y.; Schwab, F.; Kühn, K.; Schulz, K.; Balau, V.; Breitbach, K.; Bast, A.; Witte, W.; et al. High prevalence of extended-spectrum-β-lactamase-producing Enterobacteriaceae in organic and conventional retail chicken meat, Germany. J. Antimicrob. Chemother. 2012, 67, 2631–2634.
- Liu, X.; Wei, X.; Liu, L.; Feng, X.; Shao, Z.; Han, Z.; Li, Y. Prevalence and characteristics of extended-spectrum β-lactamases-producing Escherichia coli from broiler chickens at different day-age. Poult. Sci. 2020, 99, 3688–3696.
- Clemente, L.; Leão, C.; Moura, L.; Albuquerque, T.; Amaro, A. Prevalence and characterization of ESBL/AmpC producing Escherichia coli from fresh meat in Portugal. Antibiotics 2021, 10, 1333.
- Subramanya, S.H.; Bairy, I.; Nayak, N.; Amberpet, R.; Padukone, S.; Metok, Y.; Bhatta, D.R.; Sathian, B. Detection and characterization of ESBLproducing Enterobacteriaceae from the gut of healthy chickens, Gallus gallus domesticus in rural Nepal: Dominance of CTX-M-15-non-ST131 Escherichia coli clones. PLoS ONE 2020, 15, e0227725.
- Hussain, A.; Shaik, S.; Ranjan, A.; Suresh, A.; Sarker, N.; Semmler, T.; Wieler, L.H.; Alam, M.; Watanabe, H.; Chakravortty, D.; et al. Genomic and Functional Characterization of Poultry Escherichia coli From India Revealed Diverse Extended-Spectrum β-Lactamase-Producing Lineages With Shared Virulence Profiles. Front. Microbiol. 2019, 10, 2766.
- ESVAC. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2021 (EMA/294674/2019); European Medicines Agency: Zuidas, Amsterdam, The Netherlands, 2021.
- FDA Withdrawal of Enrofloxacin for Poultry. Available online: https://www.fda.gov/animal-veterinary/recalls-withdrawals/withdrawal-enrofloxacin-poultry (accessed on 25 March 2023).
- Cheng, A.C.; Turnidge, J.; Collignon, P.; Looke, D.; Barton, M.; Gottlieb, T. Control of fluoroquinolone resistance through successful regulation, Australia. Emerg. Infect. Dis. 2012, 18, 1453–1460.
- Chauvin, C.; Le Devendec, L.; Jouy, E.; Le Cornec, M.; Francart, S.; Marois-Créhan, C.; Kempf, I. National prevalence of resistance to third-generation cephalosporins in Escherichia coli isolates from layer flocks in France. Antimicrob. Agents Chemother. 2013, 57, 6351–6353.
- Ramadan, H.; Jackson, C.R.; Frye, J.G.; Hiott, L.M.; Samir, M.; Awad, A.; Woodley, T.A. Antimicrobial resistance, genetic diversity and multilocus sequence typing of Escherichia coli from humans, retail chicken and ground beef in Egypt. Pathogens 2020, 9, 357.
- Lozica, L.; Repar, J.; Gottstein, Ž. Longitudinal study on the effect of autogenous vaccine application on the sequence type and virulence profiles of Escherichia coli in broiler breeder flocks. Vet. Microbiol. 2021, 259, 109159.
- Aslantaş, Ö. High occurrence of CMY-2-type beta-lactamase-producing Escherichia coli among broiler flocks in Turkey. Trop. Anim. Health Prod. 2020, 52, 1681–1689.
- Azam, M.; Mohsin, M.; Johnson, T.J.; Smith, E.A.; Johnson, A.; Umair, M.; Saleemi, M.K. Sajjad-ur-Rahman Genomic landscape of multi-drug resistant avian pathogenic Escherichia coli recovered from broilers. Vet. Microbiol. 2020, 247, 108766.
- Ilyas, S.; Rasool, M.H.; Arshed, M.J.; Qamar, M.U.; Aslam, B.; Almatroudi, A.; Khurshid, M. The Escherichia coli sequence type 131 harboring extended-spectrum beta-lactamases and carbapenemases genes from poultry birds. Infect. Drug Resist. 2021, 14, 805–813.
- Jamil, A.; Zahoor, M.A.; Nawaz, Z.; Siddique, A.B.; Khurshid, M. Genetic Diversity of Escherichia coli Coharboring mcr-1 and Extended Spectrum Beta-Lactamases from Poultry. Biomed Res. Int. 2022, 2022, 8224883.
- Bengtsson-Palme, J. Antibiotic resistance in the food supply chain: Where can sequencing and metagenomics aid risk assessment? Curr. Opin. Food Sci. 2017, 14, 66–71.
- Gantois, I.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F.; Gast, R.; Humphrey, T.J.; Van Immerseel, F. Mechanisms of egg contamination by Salmonella enteritidis. FEMS Microbiol. Rev. 2009, 33, 718–738.
- Bertelloni, F.; Salvadori, C.; Moni, A.; Cerri, D.; Mani, P.; Ebani, V.V. Antimicrobial resistance in Enterococcus spp. Isolated from laying hens of backyard poultry flocks. Ann. Agric. Environ. Med. 2015, 22, 665–669.
- Rouger, A.; Tresse, O.; Zagorec, M. Bacterial contaminants of poultry meat: Sources, species, and dynamics. Microorganisms 2017, 5, 50.
- Adeyanju, G.T.; Ishola, O. Salmonella and Escherichia coli contamination of poultry meat from a processing plant and retail markets in Ibadan, Oyo state, Nigeria. Springerplus 2014, 3, 139.
- Almeida, A.; Duarte, S.; Nunes, R.; Rocha, H.; Pena, A.; Meisel, L. Human and veterinary antibiotics used in Portugal-A ranking for ecosurveillance. Toxics 2014, 2, 188–225.
- Hu, Y.; Gao, G.F.; Zhu, B. The antibiotic resistome: Gene flow in environments, animals and human beings. Front. Med. 2017, 11, 161–168.
- Scott, A.; Tien, Y.C.; Drury, C.F.; Reynolds, W.D.; Topp, E. Enrichment of antibiotic resistance genes in soil receiving composts derived from swine manure, yard wastes, or food wastes, and evidence for multiyear persistence of swine Clostridium spp. Can. J. Microbiol. 2018, 64, 201–208.
- Silva, V.; Ribeiro, J.; Rocha, J.; Manaia, C.M.; Silva, A.; Pereira, J.E.; Maltez, L.; Capelo, J.L.; Igrejas, G.; Poeta, P. High Frequency of the EMRSA-15 Clone (ST22-MRSA-IV) in Hospital Wastewater. Microorganisms 2022, 10, 147.
- Stępień-Pyśniak, D.; Hauschild, T.; Kosikowska, U.; Dec, M.; Urban-Chmiel, R. Biofilm formation capacity and presence of virulence factors among commensal Enterococcus spp. from wild birds. Sci. Rep. 2019, 9, 11204.
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A.; et al. Antibiotic Resistance: One Health One World Outlook. Front. Cell Infect. Microbiol. 2021, 11, 1153.
- Park, S.Y.; Woodward, C.L.; Kubena, L.F.; Nisbet, D.J.; Birkhold, S.G.; Ricke, S.C. Environmental dissemination of foodborne Salmonella in preharvest poultry production: Reservoirs, critical factors, and research strategies. Crit. Rev. Environ. Sci. Technol. 2008, 38, 73–111.
- Trudeau, S.; Thibodeau, A.; Côté, J.C.; Gaucher, M.L.; Fravalo, P. Contribution of the Broiler Breeders’ Fecal Microbiota to the Establishment of the Eggshell Microbiota. Front. Microbiol. 2020, 11, 666.
- AVEC Annual Report 2022; AVEC: Brussels, Belgium, 2022.
- Poultry. Brazilian Farmers Poultry. 2022. Available online: brazilianfarmers.com/discover/poultry–2/ (accessed on 27 March 2023).
- Getachew, Y.; Hassan, L.; Zakaria, Z.; Abdul Aziz, S. Genetic variability of vancomycin-resistant Enterococcus faecium and Enterococcus faecalis isolates from humans, chickens, and pigs in Malaysia. Appl. Environ. Microbiol. 2013, 79, 4528–4533.
- Brinkwirth, S.; Ayobami, O.; Eckmanns, T.; Markwart, R. Hospital-acquired infections caused by enterococci: A systematic review and meta-analysis, WHO European region, 1 January 2010 to 4 February 2020. Eurosurveillance 2021, 26, 2001628.
- Hammerum, A.M. Enterococci of animal origin and their significance for public health. Clin. Microbiol. Infect. 2012, 18, 619–625.
- Anghinah, R.; Watanabe, R.G.S.; Simabukuro, M.M.; Guariglia, C.; Pinto, L.F.; de Menezes e Gonçalves, D.C. Native Valve Endocarditis due to Enterococcus hirae Presenting as a Neurological Deficit. Case Rep. Neurol. Med. 2013, 2013, 636070.
- Poyart, C.; Lambert, T.; Morand, P.; Abassade, P.; Quesne, G.; Baudouy, Y.; Trieu-Cuot, P. Native valve endocarditis due to Enterococcus hirae. J. Clin. Microbiol. 2002, 40, 2689–2690.
- Ahmed, F.Z.; Baig, M.W.; Gascoyne-Binzi, D.; Sandoe, J.A.T. Enterococcus cecorum aortic valve endocarditis. Diagn. Microbiol. Infect. Dis. 2011, 70, 525–527.
- Woo, P.C.Y.; Tam, D.M.W.; Lau, S.K.P.; Fung, A.M.Y.; Yuen, K.Y. Enterococcus cecorum Empyema Thoracis Successfully Treated with Cefotaxime. J. Clin. Microbiol. 2004, 42, 919–922.
- Hsueh, P.R.; Teng, L.J.; Chen, Y.C.; Yang, P.C.; Ho, S.W.; Luh, K.T. Recurrent bacteremic peritonitis caused by Enterococcus cecorum in a patient with liver cirrhosis. J. Clin. Microbiol. 2000, 38, 2450–2452.
- De Baere, T.; Claeys, G.; Verschraegen, G.; Devriese, L.A.; Baele, M.; Van Vlem, B.; Vanholder, R.; Dequidt, C.; Vaneechoutte, M. Continuous ambulatory peritoneal dialysis peritonitis due to Enterococcus cecorum. J. Clin. Microbiol. 2000, 38, 3511–3512.
- Sørensen, T.L.; Blom, M.; Monnet, D.L.; Frimodt-Møller, N.; Poulsen, R.L.; Espersen, F. Transient intestinal carriage after ingestion of Antibiotic-resistant Enterococcus faecium from chicken and pork. N. Engl. J. Med. 2001, 345, 1161–1166.
- Tzavaras, I.; Siarkou, V.I.; Zdragas, A.; Kotzamanidis, C.; Vafeas, G.; Bourtzi-Hatzopoulou, E.; Pournaras, S.; Sofianou, D. Diversity of vanA-type vancomycin-resistant Enterococcus faecium isolated from broilers, poultry slaughterers and hospitalized humans in Greece. J. Antimicrob. Chemother. 2012, 67, 1811–1818.
- Nowakiewicz, A.; Ziółkowska, G.; Trościańczyk, A.; Zięba, P.; Gnat, S. Determination of resistance and virulence genes in Enterococcus faecalis and E. faecium strains isolated from poultry and their genotypic characterization by ADSRRS-fingerprinting. Poult. Sci. 2017, 96, 986–996.
- Pormohammad, A.; Nasiri, M.J.; Azimi, T. Prevalence of antibiotic resistance in Escherichia coli strains simultaneously isolated from humans, animals, food, and the environment: A systematic review and meta-analysis. Infect. Drug Resist. 2019, 12, 1181–1197.
- Winokur, P.L.; Vonstein, D.L.; Hoffman, L.J.; Uhlenhopp, E.K.; Doern, G.V. Evidence for transfer of CMY-2 AmpC β-lactamase plasmids between Escherichia coli and Salmonella isolates from food animals and humans. Antimicrob. Agents Chemother. 2001, 45, 2716–2722.
- Fei, X.; Yin, K.; Yin, C.; Hu, Y.; Li, J.; Zhou, Z.; Tian, Y.; Geng, S.; Chen, X.; Pan, Z.; et al. Analyses of prevalence and molecular typing reveal the spread of antimicrobial-resistant Salmonella infection across two breeder chicken farms. Poult. Sci. 2018, 97, 4374–4383.
- Noh, E.B.; Kim, Y.B.; Seo, K.W.; Son, S.H.; Ha, J.S.; Lee, Y.J. Antimicrobial resistance monitoring of commensal Enterococcus faecalis in broiler breeders. Poult. Sci. 2020, 99, 2675–2683.
- Ferreira, J.C.; Penha Filho, R.A.C.; Kuaye, A.P.Y.; Andrade, L.N.; Chang, Y.F.; Darini, A.L.C. Virulence potential of commensal multidrug resistant Escherichia coli isolated from poultry in Brazil. Infect. Genet. Evol. 2018, 65, 251–256.
- Díaz-Jiménez, D.; García-Meniño, I.; Fernández, J.; García, V.; Mora, A. Chicken and turkey meat: Consumer exposure to multidrug-resistant Enterobacteriaceae including mcr-carriers, uropathogenic E. coli and high-risk lineages such as ST131. Int. J. Food Microbiol. 2020, 331, 108750.
- Johnson, J.R.; Porter, S.B.; Johnston, B.; Thuras, P.; Clock, S.; Crupain, M.; Ranganb, U. Extraintestinal Pathogenic and Antimicrobial-Resistant Escherichia coli, Including Sequence Type 131 (ST131), from Retail Chicken Breasts in the United States in 2013. Appl. Enviromental Microbiol. 2017, 83, e02956-16.
- Falgenhauer, L.; Imirzalioglu, C.; Oppong, K.; Akenten, C.W.; Hogan, B.; Krumkamp, R.; Poppert, S.; Levermann, V.; Schwengers, O.; Sarpong, N.; et al. Detection and characterization of ESBL-producing Escherichia coli from humans and poultry in Ghana. Front. Microbiol. 2019, 10, 3358.
- Mughini-Gras, L.; Dorado-García, A.; van Duijkeren, E.; van den Bunt, G.; Dierikx, C.M.; Bonten, M.J.M.; Bootsma, M.C.J.; Schmitt, H.; Hald, T.; Evers, E.G.; et al. Attributable sources of community-acquired carriage of Escherichia coli containing β-lactam antibiotic resistance genes: A population-based modelling study. Lancet Planet. Health 2019, 3, e357–e369.
- Wada, Y.; Harun, A.B.; Yean, C.Y.; Zaidah, A.R. Vancomycin-Resistant Enterococcus: Issues in Human Health, Animal Health, Resistant Mechanisms and the Malaysian Paradox. Adv. Anim. Vet. Sci. 2019, 7, 1021–1034.
- Mollenkopf, D.F.; Cenera, J.K.; Bryant, E.M.; King, C.A.; Kashoma, I.; Kumar, A.; Funk, J.A.; Rajashekara, G.; Wittum, T.E. Organic or antibiotic-free labeling does not impact the recovery of enteric pathogens and antimicrobial-resistant Escherichia coli from fresh retail chicken. Foodborne Pathog. Dis. 2014, 11, 920–929.
- Musa, L.; Proietti, P.C.; Marenzoni, M.L.; Stefanetti, V.; Kika, T.S.; Blasi, F.; Magistrali, C.F.; Toppi, V.; Ranucci, D.; Branciari, R.; et al. Susceptibility of commensal E. coli isolated from conventional, antibiotic-free, and organic meat chickens on farms and at slaughter toward antimicrobials with public health relevance. Antibiotics 2021, 10, 1321.
- Kawalec, M.; Pietras, Z.; Daniłowicz, E.; Jakubczak, A.; Gniadkowski, M.; Hryniewicz, W.; Willems, R.J.L. Clonal structure of Enterococcus faecalis isolated from Polish hospitals: Characterization of epidemic clones. J. Clin. Microbiol. 2007, 45, 147–153.
- Kuch, A.; Willems, R.J.L.; Werner, G.; Coque, T.M.; Hammerum, A.M.; Sundsfjord, A.; Klare, I.; Ruiz-Garbajosa, P.; Simonsen, G.S.; van Luit-Asbroek, M.; et al. Insight into antimicrobial susceptibility and population structure of contemporary human Enterococcus faecalis isolates from Europe. J. Antimicrob. Chemother. 2012, 67, 551–558.
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124.
- Tedim, A.P.; Ruiz-Garbajosa, P.; Corander, J.; Rodríguez, C.M.; Cantón, R.; Willems, R.J.; Baquero, F.; Coque, T.M. Population biology of intestinal Enterococcus Isolates from hospitalized and nonhospitalized individuals in different age groups. Appl. Environ. Microbiol. 2015, 81, 1820–1831.
- Farman, M.; Yasir, M.; Al-Hindi, R.R.; Farraj, S.A.; Jiman-Fatani, A.A.; Alawi, M.; Azhar, E.I. Genomic analysis of multidrug-resistant clinical Enterococcus faecalis isolates for antimicrobial resistance genes and virulence factors from the western region of Saudi Arabia. Antimicrob. Resist. Infect. Control 2019, 8, 55.
- He, T.; Shen, Y.; Schwarz, S.; Cai, J.; Lv, Y.; Li, J.; Feßler, A.T.; Zhang, R.; Wu, C.; Shen, J.; et al. Genetic environment of the transferable oxazolidinone/phenicol resistance gene optrA in Enterococcus faecalis isolates of human and animal origin. J. Antimicrob. Chemother. 2016, 71, 1466–1473.
- Lee, J.H.; Shin, D.; Lee, B.; Lee, H.; Lee, I.; Jeong, D.W. Genetic diversity and antibiotic resistance of Enterococcus faecalis isolates from traditional Korean fermented soybean foods. J. Microbiol. Biotechnol. 2017, 27, 916–924.
- Freitas, A.R.; Tedim, A.P.; Novais, C.; Lanza, V.F.; Peixe, L. Comparative genomics of global optrA-carrying Enterococcus faecalis uncovers a common chromosomal hotspot for optrA acquisition within a diversity of core and accessory genomes. Microb. Genomics 2020, 6, e000350.
- Sakai, Y.; Tsukahara, T.; Ushida, K. Possibility of vancomycin-resistant enterococci transmission from human to broilers, and possibility of using the vancomycin-resistant gram-positive cocci as a model in a screening study of vancomycin-resistant enterococci infection in the broiler chick. Anim. Sci. J. 2006, 77, 538–544.
- Yu, F.; Chen, X.; Zheng, S.; Han, D.; Wang, Y.; Wang, R.; Wang, B.; Chen, Y. Prevalence and genetic diversity of human diarrheagenic Escherichia coli isolates by multilocus sequence typing. Int. J. Infect. Dis. 2018, 67, 7–13.
- Papouskova, A.; Papouskova, A.; Masarikova, M.; Masarikova, M.; Valcek, A.; Valcek, A.; Senk, D.; Cejkova, D.; Jahodarova, E.; Cizek, A.; et al. Genomic analysis of Escherichia coli strains isolated from diseased chicken in the Czech Republic. BMC Vet. Res. 2020, 16, 189.
- Jørgensen, S.L.; Stegger, M.; Kudirkiene, E.; Lilje, B.; Poulsen, L.L.; Ronco, T.; Pires Dos Santos, T.; Kiil, K.; Bisgaard, M.; Pedersen, K.; et al. Diversity and Population Overlap between Avian and Human Escherichia coli Belonging to Sequence Type 95. mSphere 2019, 4, e00333-18.
- Danzeisen, J.L.; Wannemuehler, Y.; Nolan, L.K.; Johnson, T.J. Comparison of multilocus sequence analysis and virulence genotyping of Escherichia coli from live birds, retail poultry meat, and human extraintestinal infection. Avian Dis. 2013, 57, 104–108.
- Bert, F.; Johnson, J.R.; Ouattara, B.; Leflon-Guibout, V.; Johnston, B.; Marcon, E.; Valla, D.; Moreau, R.; Nicolas-Chanoine, M.H. Genetic diversity and virulence profiles of Escherichia coli isolates causing spontaneous bacterial peritonitis and bacteremia in patients with cirrhosis. J. Clin. Microbiol. 2010, 48, 2709–2714.
- Pires-dos-Santos, T.; Bisgaard, M.; Christensen, H. Genetic diversity and virulence profiles of Escherichia coli causing salpingitis and peritonitis in broiler breeders. Vet. Microbiol. 2013, 162, 873–880.
- Nicolas-Chanoine, M.H.; Bertrand, X.; Madec, J.Y. Escherichia coli ST131, an intriguing clonal group. Clin. Microbiol. Rev. 2014, 27, 543–574.
- Ronco, T.; Stegger, M.; Olsen, R.H.; Sekse, C.; Nordstoga, A.B.; Pohjanvirta, T.; Lilje, B.; Lyhs, U.; Andersen, P.S.; Pedersen, K. Spread of avian pathogenic Escherichia coli ST117 O78: H4 in Nordic broiler production. BMC Genom. 2017, 18, 13.
- Kieffer, N.; Nordmann, P.; Aires-De-Sousa, M.; Poirel, L. High prevalence of carbapenemase-producing Enterobacteriaceae among hospitalized children in Luanda, Angola. Antimicrob. Agents Chemother. 2016, 60, 6189–6192.
- Báez, J.; Hernández-García, M.; Guamparito, C.; Díaz, S.; Olave, A.; Guerrero, K.; Cantón, R.; Baquero, F.; Gahona, J.; Valenzuela, N.; et al. Molecular characterization and genetic diversity of ESBL-producing Escherichia coli colonizing the migratory Franklin’s Gulls (Leucophaeus pipixcan) in Antofagasta, North of Chile. Microb. Drug Resist. 2015, 21, 111–116.




