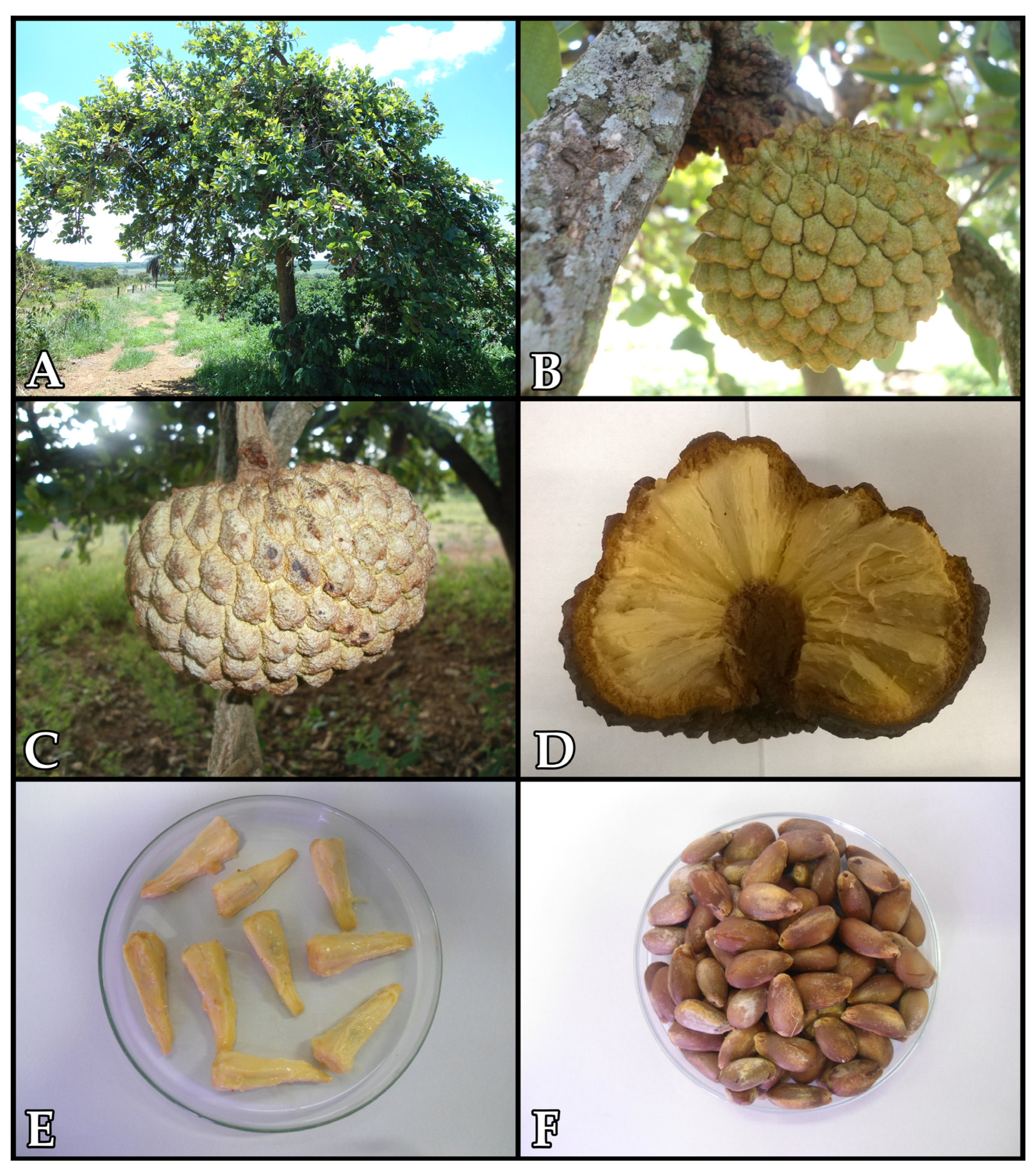
2. Bioactive Compounds Found in Araticum Fruit Parts
Bioactive compounds are naturally occurring chemical substances found in plants, animals, and microorganisms that have specific biological activities and can exert a wide spectrum of beneficial effects on human health, including antioxidant, anti-inflammatory, antimicrobial, anti-aging, and anticarcinogenic effects. They are classified into diverse classes according to chemical structure, namely phenolic compounds, alkaloids, carotenoids, tocols, phytosterols, organosulfur compounds, polysaccharides, amino acids, and peptides, among others
[10][11]. As shown in
Table 1, bioactive compounds such as phenolic compounds, alkaloids, annonaceous acetogenins, carotenoids, phytosterols, and tocols were observed in the pulp, peel, and seed of araticum fruit, and they will be discussed in more detail throughout this section.
Table 1. A summary of studies showing the bioactive compounds found in araticum fruit.
2.1. Phenolic Compounds
Phenolic compounds are an important class of phytochemicals formed from secondary plant metabolites containing hydroxyl (−OH) substituents on an aromatic hydrocarbon chain. This class of bioactive compounds shows a large diversity of structures, including rather simple molecules (phenolic acids) and polyphenols such as stilbenes, flavonoids, lignans, and tannins, that can be found free or associated with carbohydrates, lipids, cell wall components, amines, and organic acids
[34]. Phenolic compounds have multiple biochemical actions as antioxidant agents, and they are involved in the modulation of signaling pathways, gene expression, and the modification of epigenetic changes mainly related to chronic non-communicable diseases (e.g., metabolic syndromes, cancers, and neurodegenerative diseases)
[35]. Therefore, phenolic compounds can be alternative or complementary tools for the prevention or management of non-communicable chronic diseases.
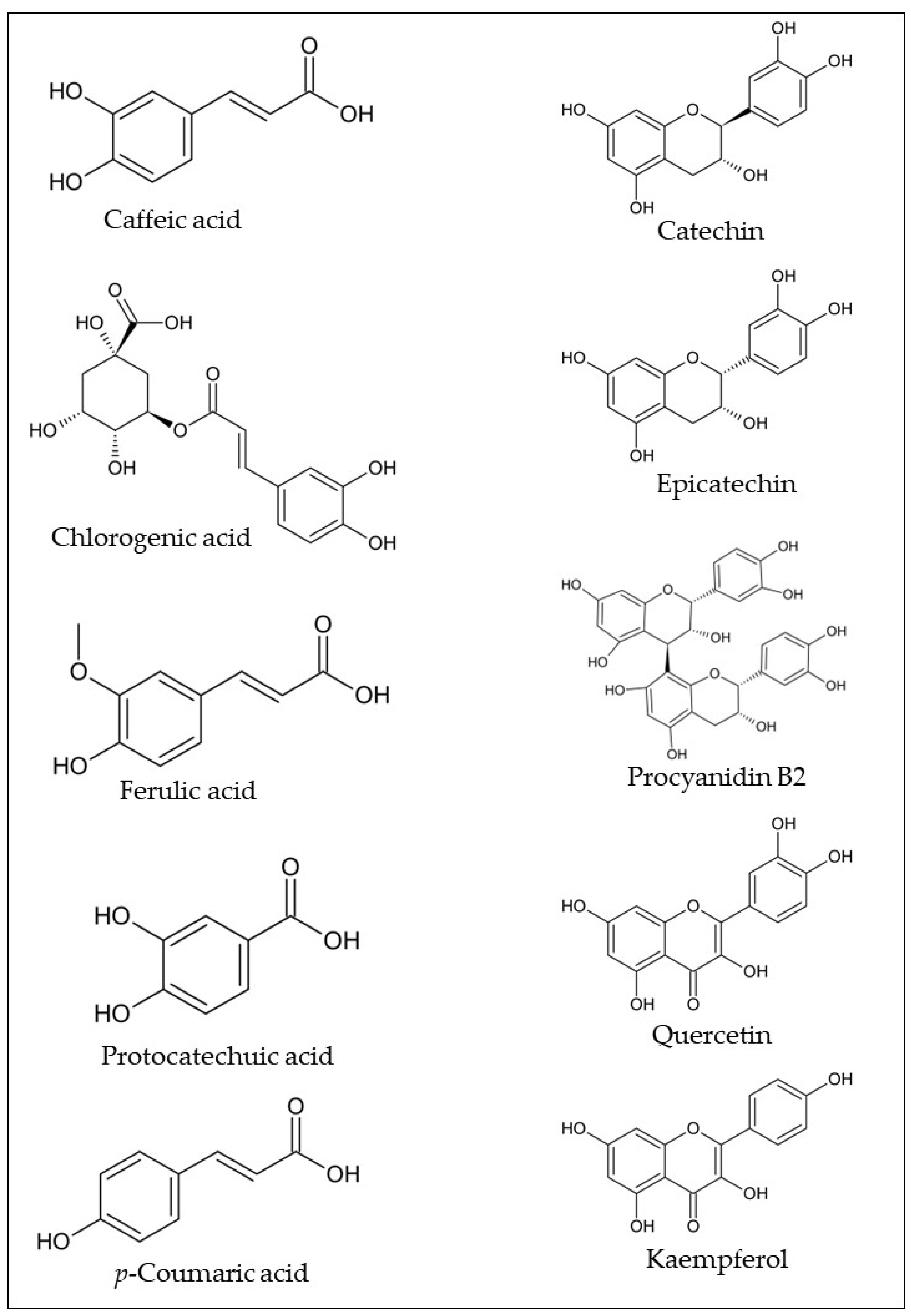
Phenolic compounds are the most studied bioactive compounds class in the araticum fruit parts. As can be seen in Table 1, several phenolic compounds have been identified in araticum fruit, with the most common being caffeic acid, chlorogenic acid, ferulic acid, protocatechuic acid, p-coumaric acid, catechin, epicatechin, procyanidin B2, quercetin, kaempferol, and their derivatives (Figure 2). Sophisticated analytical techniques, including high-performance liquid chromatography coupled with diode array detector (HPLC-DAD), high-performance liquid chromatography coupled with mass spectrometry (HPLC-MS), and paper spray ionization mass spectrometry (PS-MS), have been used for their identification and quantification.
Figure 2. Chemical structures of the most common phenolic compounds found in araticum fruit. Own authorship created by ChemSketch software.
Overall, the profile of phenolic compounds from the different parts of the araticum fruit (pulp, peel, and seeds) is diverse. As shown in
Table 1, several phenolic compounds have been identified in the pulp (derivatives of 4-hydroxybenzoic acid,
p-coumaric acid, ferulic acid, gallic acid, sinapic acid, caffeic acid, (epi)catechin, quercetin, kaempferol, rutin, tangeretin, syringic acid, apigenin, and naringenin), peel (derivatives of syringic acid, ferulic acid, protocatechuic acid, hydroxybenzoic acid, caffeic acid, vanillic acid, chlorogenic acid,
p-coumaric acid, (epi)catechin, kuwanon G, quercetin, kaempferol, apigenin, tangeretin, neocarthamin, isorharmnetin, luteolin, naringenin, vanillin, and lariciresinol), and seeds (derivatives of 4-hydroxybenzoic acid, gallic acid, chlorogenic acid, protocatechuic acid, caffeic acid,
p-coumaric acid, synaptic acid, ferulic acid, trans-cinnamic acid, catechin, epicatechin, kaempferol, vanillin, quercetin, rutin, naringenin, and tangeretin). Arruda et al.
[15] carried out an in-depth exploratory study that allowed for the identification of 112 phenolic compounds (73 flavonoids, 33 phenolic acids, and 6 other phenolics) in a hydroethanolic extract (50% ethanol) from araticum peel.
In general, the content of phenolic compounds can diverge according to fruit part (pulp, peel, and seeds), ripening stage, edaphoclimatic conditions, and storage conditions, as well as the extraction procedures and conditions (e.g., composition and polarity of the solvent, time and temperature of extraction, pH, number of re-extractions, particle size, etc.) and extraction methods (e.g., water bath shaker, maceration, ultrasound, etc.). For example, Ramos et al.
[23], evaluating pulp, peel, and seeds of araticum fruit obtained from different regions, found variations of 2-fold, 2.5-fold, and 3.5-fold in the total phenolic content, respectively. In the same study, the araticum peel showed the highest content of total phenolics (837.53–1926.56 mg GAE/100 g), followed by the seeds (358.28–1186.07 mg GAE/100 g) and pulp (480.81–1007.62 mg GAE/100 g). Similarly, Arruda et al.
[24] found the highest total phenolic contents in the araticum peel, followed by the pulp and the seeds. Furthermore, Arruda et al.
[15] observed a 2-fold increase in total phenolic content from araticum peel when optimizing the ultrasonic power and process time. Another study by Arruda et al.
[36] demonstrated that optimization extraction conditions (solvent composition, temperature, and extraction time) increase the total phenolic content extracted from araticum pulp 3-fold.
Regarding the quantification of phenolic compounds, Guimarães et al.
[14] observed that catechin (16.79 mg/100 g fw) and gallic acid (1.89 mg/100 g fw) were the major compounds in araticum pulp, followed by amounts lower than 0.55 mg/100 g fw for other phenolic acids (chlorogenic acid, caffeic acid,
p-coumaric acid, ferulic acid, trans-cinnamic acid,
m-coumaric acid, and
o-coumaric acid) and flavonoids (quercetin, vanillin, and rutin). Besides that, Arruda et al.
[24] quantified 10 phenolic compounds, namely catechin (768.42 µg/g dw), epicatechin (661.81 µg/g dw), caffeic acid (124.31 µg/g dw), protocatechuic acid (97.92 µg/g dw), ferulic acid (53.71 µg/g dw), chlorogenic acid (43.45 µg/g dw), gentisic acid (14.00 µg/g dw),
p-coumaric acid (11.86 µg/g dw), rutin (9.31 µg/g dw), and quercetin (7.80 µg/g dw), in araticum pulp.
There has been increasing interest in the utilization of araticum fruit byproducts (peel and seed). Arruda et al.
[15] found 14 phenolic compounds in the peel, namely epicatechin (136.47 µg/g dw), rutin (58.53 µg/g dw), chlorogenic acid (16.83 µg/g dw), catechin (15.23 µg/g dw), ferulic acid (10.96 µg/g dw), vicenin-2 (2.60 µg/g dw), vanillin (3.47 µg/g dw), naringenin (2.15 µg/g dw), protocatechuic acid (1.64 µg/g dw), caffeic acid (1.22 µg/g dw), luteolin (0.91 µg/g dw),
p-coumaric acid (0.49 µg/g dw), 4-hydroxybenzoic acid (0.49 µg/g dw), and vitexin (0.18 µg/g dw). Additionally, Prado et al.
[22] showed the presence of 12 phenolic compounds in the peel, such as epicatechin (6221.63 µg/g extract), catechin (579.40 µg/g extract), chlorogenic acid (305.15 µg/g extract), rutin (133.31 µg/g extract),
p-coumaric acid (14.97 µg/g extract), and traces of quercetin, naringenin, protocatechuic acid, 4-hydroxybenzoic acid, vanillic acid, caffeic acid, and ferulic acid. Moreover, Menezes et al.
[19] quantified 11 phenolic compounds in araticum seeds, namely
o-coumaric acid (3822.5 mg/kg dw), rutin (2209.4 mg/kg dw),
p-coumaric acid (188.5 mg/kg dw), gallic acid (135.6 mg/kg dw), trans-cinnamic acid (102.6 mg/kg dw), quercetin (83.5 mg/kg dw), ferulic acid (63.9 mg/kg dw), caffeic acid (40.5 mg/kg dw), catechin (35.1 mg/kg dw), chlorogenic acid (14.7 mg/kg dw), and vanillin (3.1 mg/kg dw). Furthermore, Formagio et al.
[20] observed the presence of rutin (493 mg/mL), caffeic acid (302 mg/mL), sinapic acid (248 mg/mL), ferulic acid (176 µg/mL), and
p-coumaric acid (106 µg/mL). Although there is a variation in the content of phenolic compounds due to different ways of obtaining the extract and different analytical methods, as well as other conditions described above, which may influence their content, catechin, epicatechin, rutin, chlorogenic acid, and caffeic acid appear to be the major phenolic compounds found in pulp and peel, whereas seed shows coumaric acid, caffeic acid, rutin, catechin, and epicatechin as its main phenolics. Recent studies have reported the ability of phenolic-rich extracts obtained from araticum fruit to exert beneficial effects on human health, including antitumor, antioxidant, anti-inflammatory, antimicrobial, antihypertensive, and hepatoprotective properties. Therefore, this fruit offers a wide spectrum of technological applications in the area of functional foods, pharmaceuticals, and cosmetics.
2.2. Alkaloids
Alkaloids are a huge class of natural compounds commonly found in the Annonaceae family
[37]. This class of secondary metabolites is grouped under heterocyclic and non-heterocyclic compounds based on the position of the nitrogen atom in their chemical structure
[38]. Alkaloids are considered bioactive compounds capable of exerting multiple biological activities, such as antioxidant, antidiabetic, anti-inflammatory, antimicrobial, antitumoral, anti-hypertensive, antidiarrheal, antimalarial, and antidiabetic activities
[6][38][39]. Additionally, there are already several drugs available on the market produced from natural plant alkaloids
[40].
As can be seen in
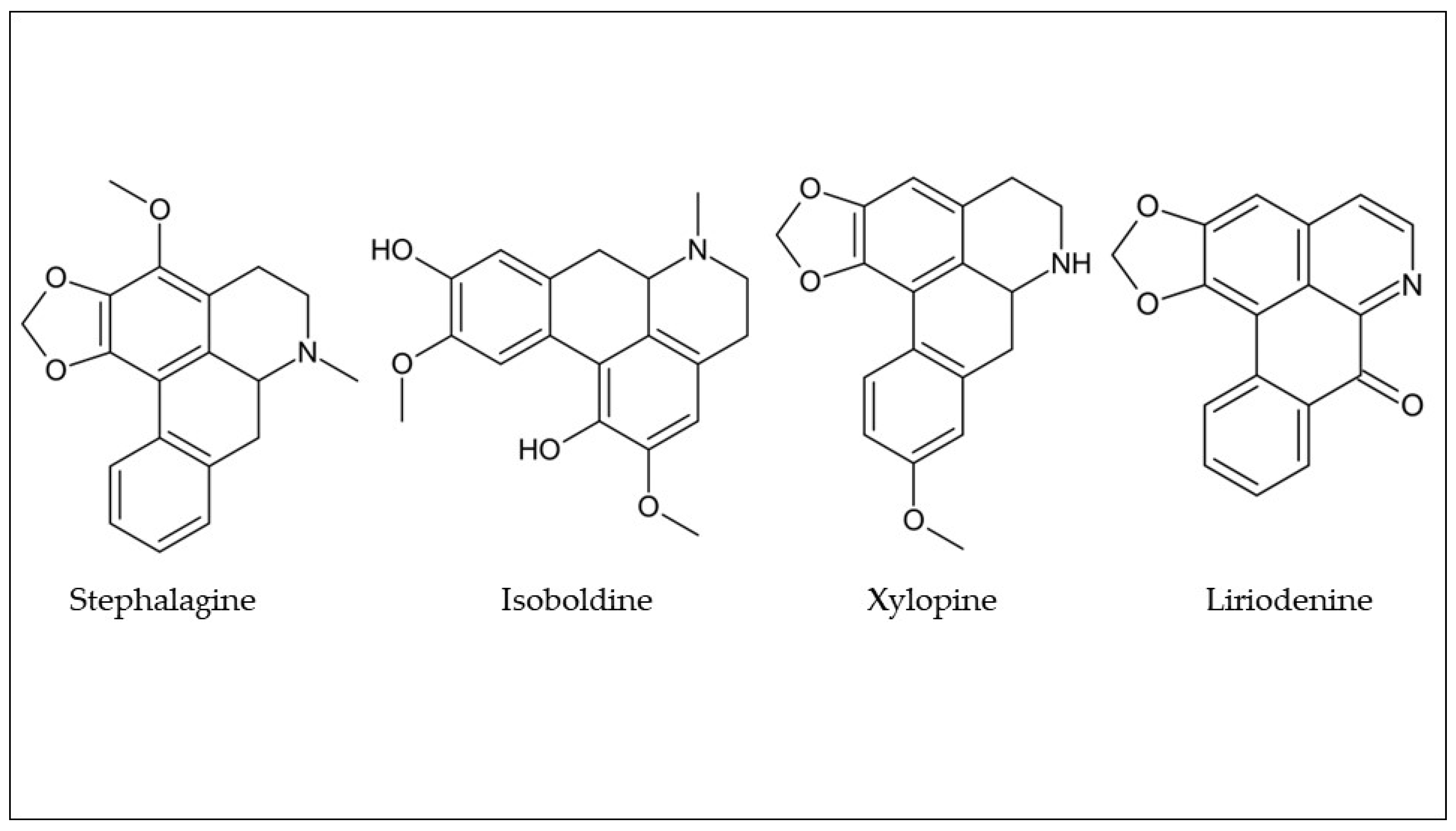
Table 1, several alkaloids have been found in araticum pulp and peel. However, the most frequently found alkaloids were stephalagine, isoboldine, xylopine, and liriodenine (
Figure 3). The stephalagine was purified by semi-preparative HPLC on RP-C
18 and structurally characterized by high-resolution mass spectrometry with electrospray ionization (HR-ESI-MS) and nuclear magnetic resonance (NMR) techniques, for the first time by Pereira et al.
[28]. Additionally, recent studies have reported the presence of stephalagine in araticum peel and pulp
[13][25][26][27][29], indicating that this compound is present in more than a fraction of the fruit. Stephalagine, an aporphine alkaloid, has demonstrated anti-obesity, antinociceptive, and anti-edematogenic effects, suggesting its use as a new potential drug
[27][28]. In addition to stephalagine, other alkaloids, such as xylopine, romucosine, asimilobine, roemerine, nornuciferine,
N-methylcoclaurine, guattescidine, actinodaphnine, isoboldine, and
N-methyllaurotetanine, have been identified in araticum pulp
[13][21]. Several studies demonstrated the presence of liriodenine, atherospermidine, isopiline, isoboldine, isocorydine, anonaine, xylopine, nuciferine, liriodenine, atherospermidine, reticuline, roemerine, nornuciferine,
N-methylcoclaurine, guattescidine, actinodaphnine, and
N-methyllaurotetanine in the araticum peel
[21][25][29]. As mentioned above, there is a significant interest in isolating and purifying alkaloids from araticum pulp and its byproduct (peel), which can serve as potential sources for obtaining alkaloids for the pharmaceutical industry.
Figure 3. Chemical structures of the most common alkaloids found in araticum fruit. Own authorship created by ChemSketch software.
2.3. Annonaceous Acetogenins
Annonaceous acetogenins constitute a group of powerful bioactive substances derived from long-chain fatty acids (C35-C37) by polyketide pathways found exclusively in plants of the Annonaceae family. They are commonly characterized by a combination of fatty acids, with a 2-propanol unit at C-2 that forms a methyl-substituted α,β-unsaturated γ-lactone
[41][42]. Annonaceous acetogenins have attracted significant scientific interest for the last decade because of their biological activities such as antiparasitic, immunosuppressive, neurotoxic, and pesticidal properties
[42]. Beyond that, these compounds appear to be powerful against cancer
[6][42][43]. However, chronic exposure to annonaceous acetogenins can potentiate neural damage in the body; thus, moderate consumption of these compounds is recommended
[43].
Despite their biological benefits, only five studies have reported annonaceous acetogenins composition in araticum fruits (
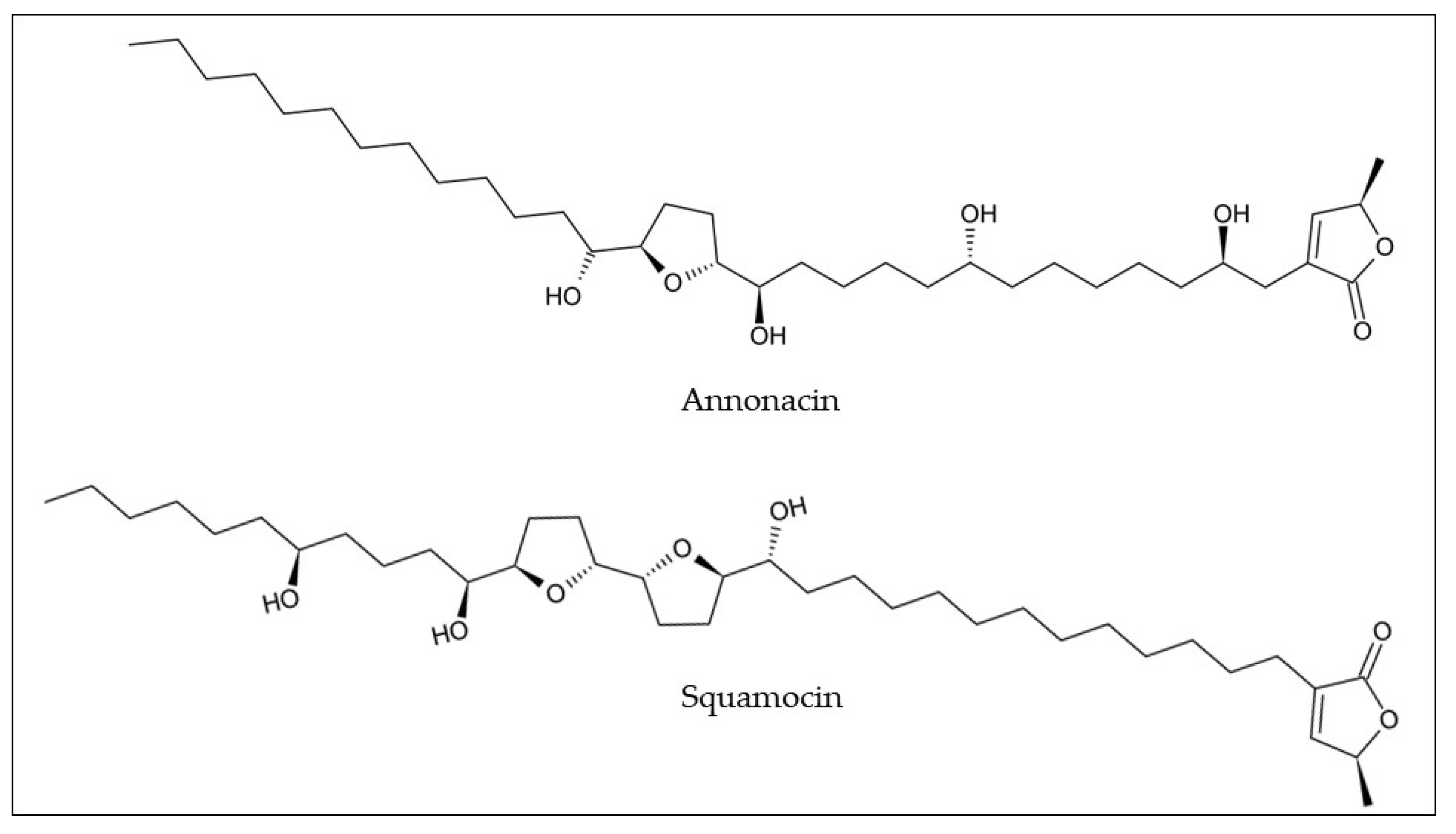
Table 1). The methanolic extract, from araticum pulp and seeds, revealed the presence of two annonaceous acetogenins, namely annonacin (0.33 and 5.90 mg/g dw) and squamocin (0.40 and 142 mg/g dw), respectively (
Figure 4)
[30]. The extraction procedure and chromatographic analyses were different for each study, which allowed for the identification of different compounds from the pulp, peel, and seed fractions of the araticum fruit. Arruda et al.
[15] investigated the profile of hydroethanolic extract from araticum peel, by high-performance liquid chromatography coupled with mass spectrometry with an electrospray ionization source (HPLC-ESI-MS), and found 21 annonaceous acetogenins. Ramos et al.
[21] tentatively identified 17, 9, and 22 annonaceous acetogenins in the ethanolic extracts from pulp, peel, and seeds, respectively, by paper spray ionization mass spectrometry analysis (PS-MS). As reported above, in recent years, there has been an increase in interest in discovering annonaceous acetogenins present in araticum fruit because these metabolites possess a broad spectrum of biological activities and are recognized as anticancer agents. However, further studies are needed regarding their quantification and identification in araticum fruit parts.
Figure 4. Chemical structures of the most common annonaceous acetogenins found in araticum fruit. Own authorship created by ChemSketch software.
2.4. Carotenoids
Carotenoids are considered a very diverse group of natural liposoluble pigments responsible for the red, orange, and yellow colors synthesized by plants, microorganisms, and vertebrates
[44]. This compound group is comprised of a large class of isoprenoid compounds characterized by a backbone with 40 carbon atoms, the possession of a centrally located conjugated double-bond system, and an ability to carry cyclic or acyclic end groups. This system serves as the chromophore and allows these compounds to absorb wavelengths in the visible spectrum (400–550 nm)
[44][45]. Carotenoids are involved in a series of biochemical activities with high antioxidant activity, can display a prebiotic-like effect, and some of them present pro-vitamin A activities. Additionally, there is an association between carotenoid intake and reduced risk of several diseases, especially chronic non-communicable diseases
[46].
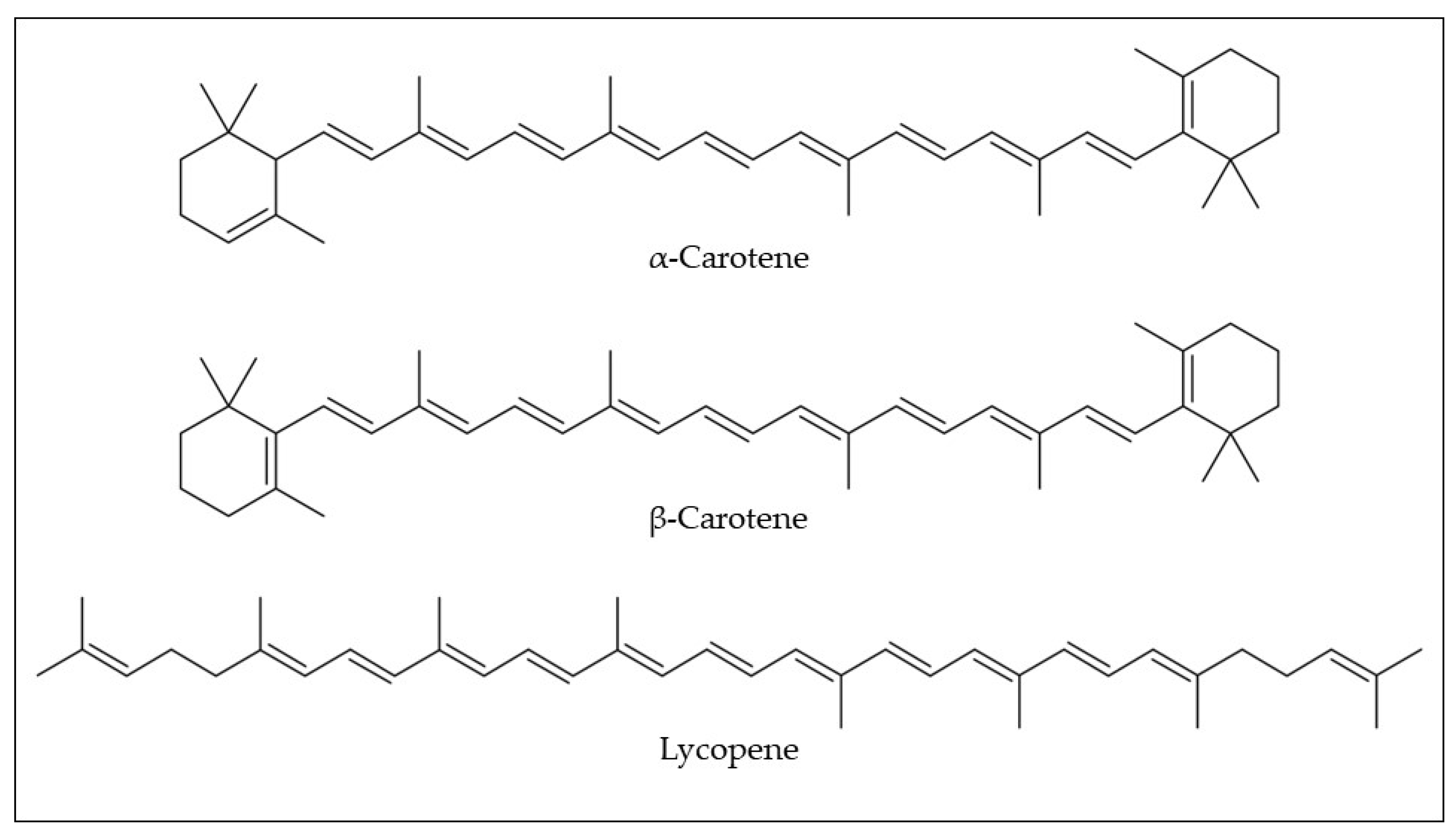
Carotenoids have been identified and quantified in araticum pulp by high-performance liquid chromatography coupled with the diode array detector method (HPLC-DAD) (
Table 1). In the araticum pulp, a low quantity of lycopene (0.02 mg/100 g fw) could be found, while α and β-carotene (2.98 and 1.97 mg/100 g) were its major carotenoids (
Figure 5)
[31]. Silva et al.
[32] found different isoforms of carotenes in araticum pulp, namely all-trans-α-carotene (1.55–1.98 mg/100 g fw) and all-trans-β-carotene (0.86–1.58 mg/100 g fw). As mentioned above, carotenoids exert an important nutritional role, as they present pro-vitamin A activity (e.g., α and β-carotene). Additionally, these compounds are essential for eye health because some pro-vitamin A carotenoids (e.g., α-carotene, β-carotene, γ-carotene, and β-cryptoxanthin) are endogenously converted into retinoids
[44]. Although there are few reports on the content and composition of carotenoids in araticum fruit, the literature is consistent in mentioning that the consumption of 100 g of the araticum pulp is enough to reach the recommended daily intake of vitamin A
[6]. Thus,
in natura consumption of this fruit could be an important tool to reduce food insecurity, malnutrition, and risk for several diseases.
Figure 5. Chemical structures of the most common carotenoids found in araticum fruit. Own authorship created by ChemSketch software.
2.5. Phytosterols
Phytosterols are plant-derived sterols that are structurally similar to cholesterol; however, they differ by the presence of one or two methyl or ethyl groups in the molecule’s side chain
[47]. These bioactive compounds can be found in vegetable oils, nuts, and seeds, mainly in the form of sitosterol, campesterol, and stigmasterol
[48]. Phytosterols have important roles in the pharmaceutical, medicine, food, and nutrition areas due to their high nutritional value, potent bioactivity, and multiple medicinal effects, such as lowering blood cholesterol levels, anti-inflammatory, antitumoral, antimicrobial, and antioxidant properties, as well as others.
[6][48].
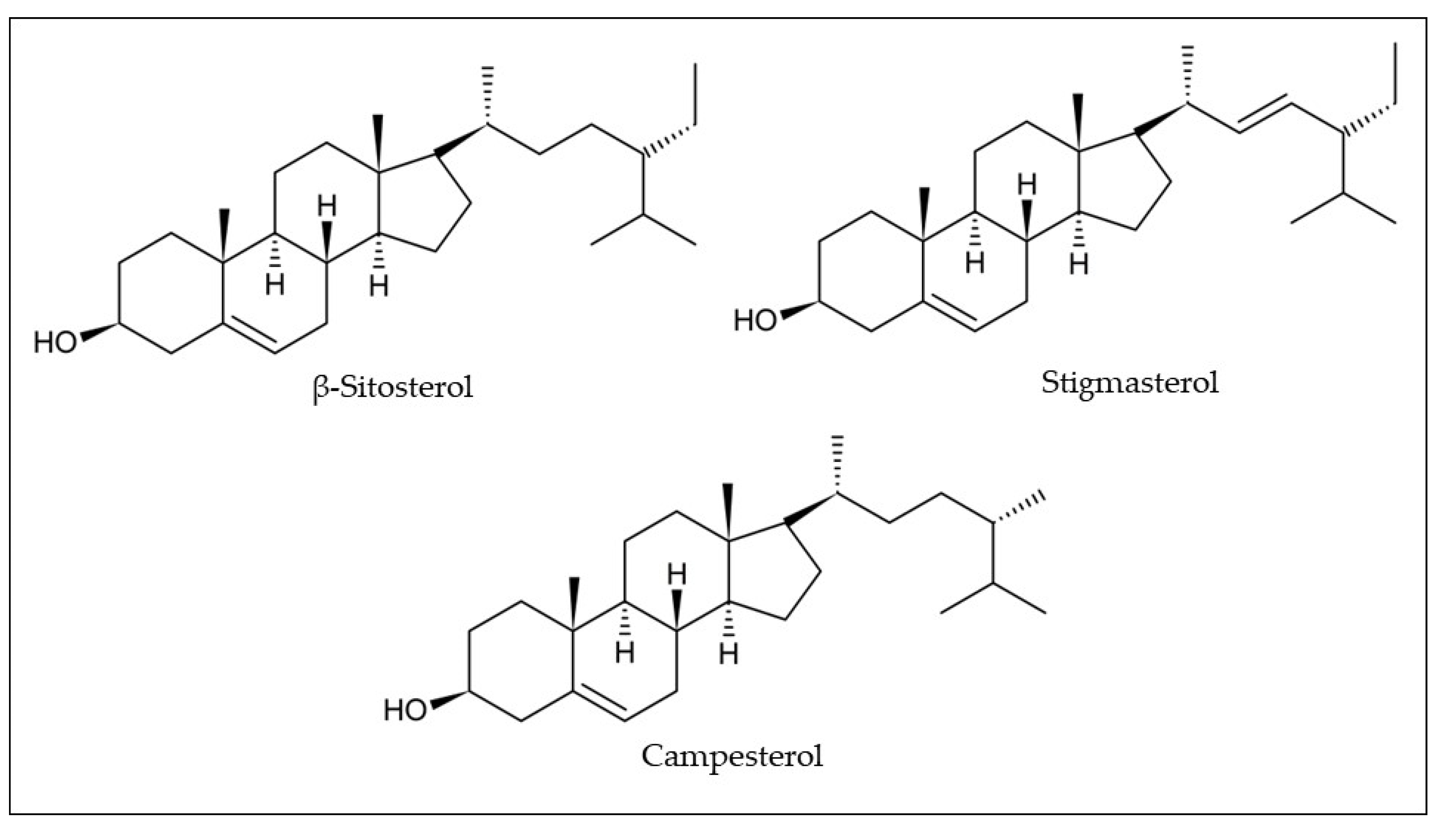
Phytosterols have been identified and quantified in araticum seeds by gas chromatography with the flame ionization detection method (GC-FID) (
Table 1). Luzia and Jorge
[33] showed that the seed lipid fraction contains 683.59 mg/kg of total phytosterols, namely β-sitosterol (300.02 mg/kg), campesterol (204.32 mg/kg), and stigmasterol (179.25 mg/kg) (
Figure 6)
[33]. Although the araticum seeds showed to be a promising source of obtaining phytosterols, little is still known about their phytosterol composition and their real contributions to health benefits.
Figure 6. Chemical structures of the most common phytosterols found in araticum fruit. Own authorship created by ChemSketch software.
2.6. Tocols
Tocols are a class of bioactive compounds present in various foods, predominantly in fruits and plant seeds
[49]. Tocols can be divided into two major groups: tocopherols and tocotrienols, which are distinguished by their chemical structure. Tocopherols (α, β, γ, and δ) contain a chromanol ring and a 16-carbon phytyl side chain in their structure, differing one from the other by position and number of methylation, while tocotrienols differ from tocopherols by possessing double bonds in the 16-carbon side chain at the positions of 3′-, 7′-, and 11′
[49][50]. These bioactive compounds are vitamin E homologs and, therefore, are considered beneficial in the prevention of different pathological conditions, acting as lipid-soluble antioxidants, antihypertensive, neuroprotective, hypolipidemic, antitumoral, and anti-inflammatory materials
[6][50].
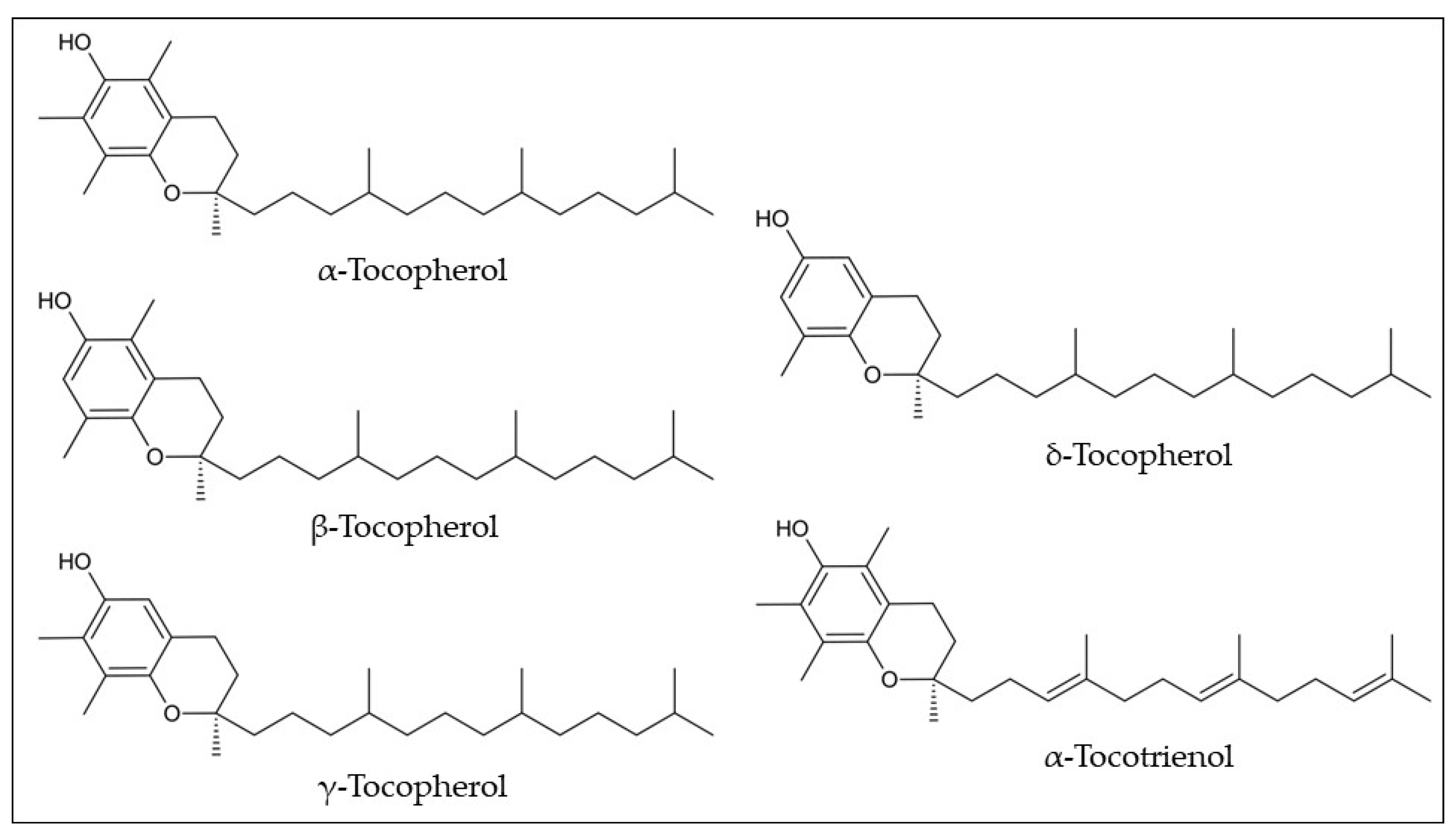
Despite their biological benefits, only two studies have reported the tocols’ composition in araticum fruits in the last decade (
Table 1). The tocopherol isomers were quantified in the araticum seeds by high-performance liquid chromatography coupled with a fluorescence detector (HPLC-FLD), totalizing 138.90 mg/kg, namely α-tocopherol (12.02 mg/kg dw), β-tocopherol (3.30 mg/kg dw), γ-tocopherol (123.42 mg/kg dw), and δ-tocopherol (0.16 mg/kg dw) (
Figure 7)
[33]. Cardoso et al.
[31] found two tocols in the pulp, with α-tocotrienol being the major tocol (332.94 μg/100 g) followed by α-tocopherol (163.11 μg/100 g), resulting in a total amount of 494.04 μg/100 g of tocols. The recommended daily intake of vitamin E is 15 mg. Therefore, neither the seeds nor the pulp can be considered a source of vitamin E. α-Tocopherol is the only one that meets human requirements for vitamin E, based on the concept that this form is the preferable retained form by the body, and reverses the symptoms of human deficiency. However, the other isomers may contribute to the liposoluble antioxidant function
[49]. Thus, the higher content of the γ-tocopherol isomer in the seed oil and α-tocotrienol in the pulp could contribute significantly to this activity.
Figure 7. Chemical structures of the most common tocols found in araticum fruit. Own authorship created by ChemSketch software.
3. Concluding Remarks
The use of advanced analytical tools (e.g., HPLC, GC, MS, among others, and their combinations) has allowed the identification and quantification of several bioactive compounds in the araticum fruit over the last decade. Phenolic compounds have been the most studied and frequently reported class of bioactive compounds in the araticum fruit. However, other classes of bioactive compounds of interest have also been found in the different parts of this fruit, including alkaloids, annonaceous acetogenins, carotenoids, phytosterols, and tocols. The presence of these bioactive compounds can explain the biological effects of araticum fruit obtained from in vitro assays and animal trials, as well as its effectiveness in folk medicine, demonstrating its promising potential for drug development to treat/manage various pathological conditions.