Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yiannis Deligiannakis | -- | 1498 | 2023-04-19 10:23:10 | | | |
| 2 | Lindsay Dong | Meta information modification | 1498 | 2023-04-20 07:24:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Solakidou, M.; Gemenetzi, A.; Koutsikou, G.; Theodorakopoulos, M.; Deligiannakis, Y.; Louloudi, M. Formic Acid as Liquid Carrier for H2 Storage. Encyclopedia. Available online: https://encyclopedia.pub/entry/43227 (accessed on 07 February 2026).
Solakidou M, Gemenetzi A, Koutsikou G, Theodorakopoulos M, Deligiannakis Y, Louloudi M. Formic Acid as Liquid Carrier for H2 Storage. Encyclopedia. Available at: https://encyclopedia.pub/entry/43227. Accessed February 07, 2026.
Solakidou, Maria, Aikaterini Gemenetzi, Georgia Koutsikou, Marinos Theodorakopoulos, Yiannis Deligiannakis, Maria Louloudi. "Formic Acid as Liquid Carrier for H2 Storage" Encyclopedia, https://encyclopedia.pub/entry/43227 (accessed February 07, 2026).
Solakidou, M., Gemenetzi, A., Koutsikou, G., Theodorakopoulos, M., Deligiannakis, Y., & Louloudi, M. (2023, April 19). Formic Acid as Liquid Carrier for H2 Storage. In Encyclopedia. https://encyclopedia.pub/entry/43227
Solakidou, Maria, et al. "Formic Acid as Liquid Carrier for H2 Storage." Encyclopedia. Web. 19 April, 2023.
Copy Citation
The development of low-carbon technologies that will facilitate the efficient use of hydrogen (H2) as an energy carrier is a critical requirement of contemporary society. To this end, it is anticipated that the cost of H2 production will become a key factor in tandem with production efficiency, process safety, and transport. Much effort has been made to create and develop new, reversible, and sustainable H2 storage systems. Among current techniques, formic acid (FA) has been identified as an efficient energy carrier for H2 storage. Numerous homogeneous catalysts based on transition metals with high activity and selectivity have been reported for selective FA dehydrogenation.
hydrogen production
cost analysis
formic acid dehydrogenation
1. Introduction
H2 may be used in industrial processes (synthesis of fuels, chemicals, etc.), transport, and building heating systems. So far, the major H2 usage pathways are summarized in Figure 1. H2 use presents many advantages. For example, it is not only the most plentiful chemical element in the universe, but its utilization also produces no greenhouse gas emissions and just water as a byproduct [1]. Its energy-storage capacity is an additional benefit, as 1 kg of H2 may produce about 120 MJ (=33.33 kWh) of energy, which is three times that of ordinary diesel, four times that of coking coal, and six times that of coal [2].
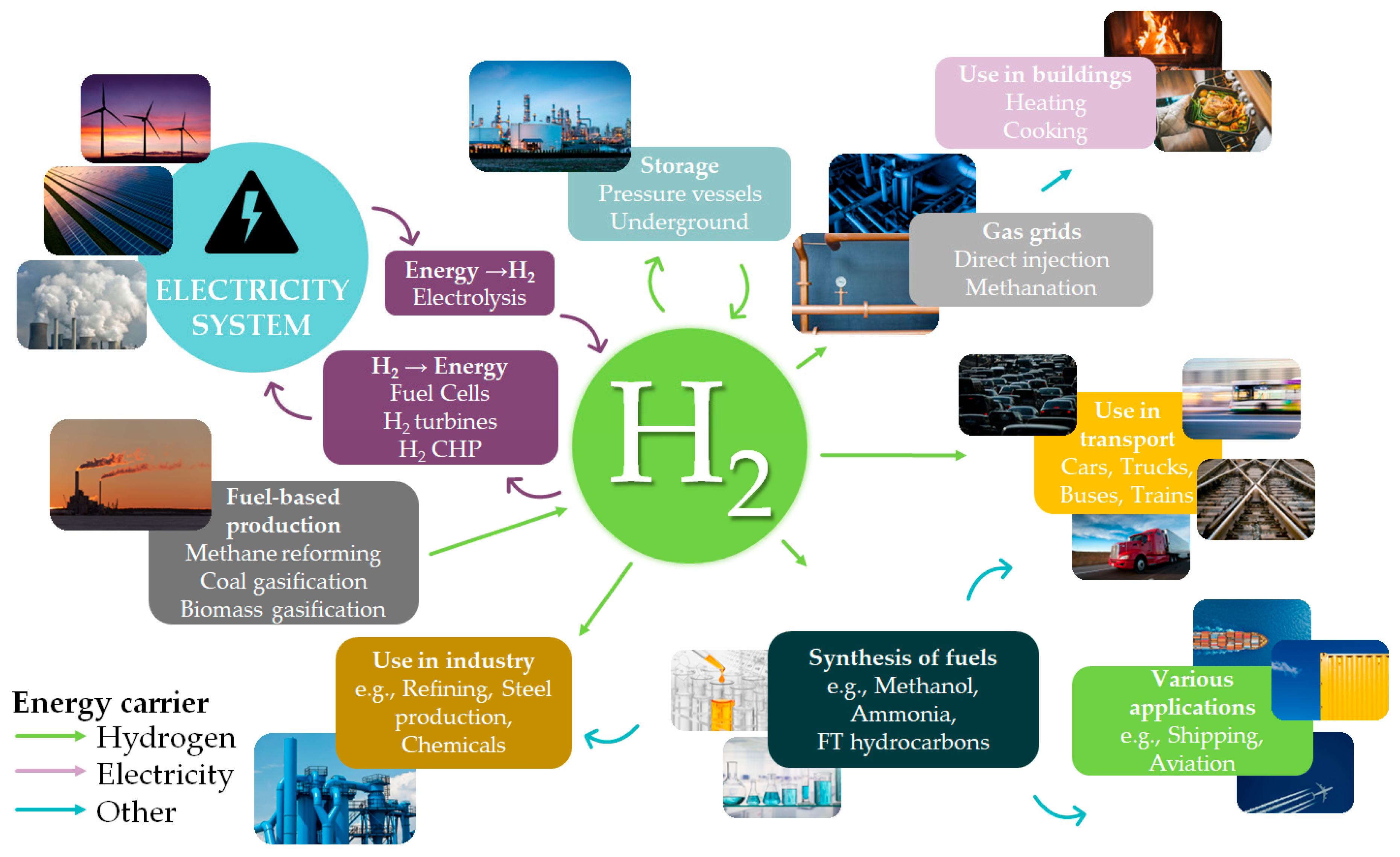
Figure 1. H2 production and usage pathways.
These characteristics make H2 an attractive transportation fuel. It can be used as fuel in an internal combustion engine or provide the electrical power for an electric car fuel cell. Within this context, H2 is viewed as a potential low-carbon fuel for long-distance transportation modes, such as trucks, rail, and shipping [3]. Compared to battery-powered electric vehicles, H2-powered passenger vehicles might cover longer driving ranges, with shorter refueling periods and, in certain circumstances, reduced ownership costs [4]. There are more than 350 H2 fueling stations in North America, Europe, Asia, and Oceania [5]. Numerous cities throughout the world, including those in the United States, Japan, China, and Europe, employ hydrogen buses [6]. Alstom has created a hydrogen-powered train, the first of which entered service in 2018 in Germany and this year has traveled without refueling 1175 km [7]. In the context of a forthcoming wide use of H2, the development of H2 infrastructure and technologies is often assessed taking into consideration larger economic development goals, too.
Up to now, the most common method for H2 production is from fossil fuels, such as natural gas reforming (Grey Hydrogen) and coal gasification (Black Hydrogen), (Figure 2a) [8]. Water electrolysis as a H2 source (Yellow Hydrogen) has been used in specific industrial applications for more than a century. Its popularity has increased in recent decades due to the need for environmentally friendly energy technologies using renewable energy sources (Green Hydrogen). However, the main limitation of its use remains its high cost of 3.6–5.1 $/kg H2 [9]. Natural gas reforming combined with CO2 capture and storage (Blue Hydrogen) is considered the most appealing alternative for a carbon-free technology at a reasonable price (Figure 2a) [10].
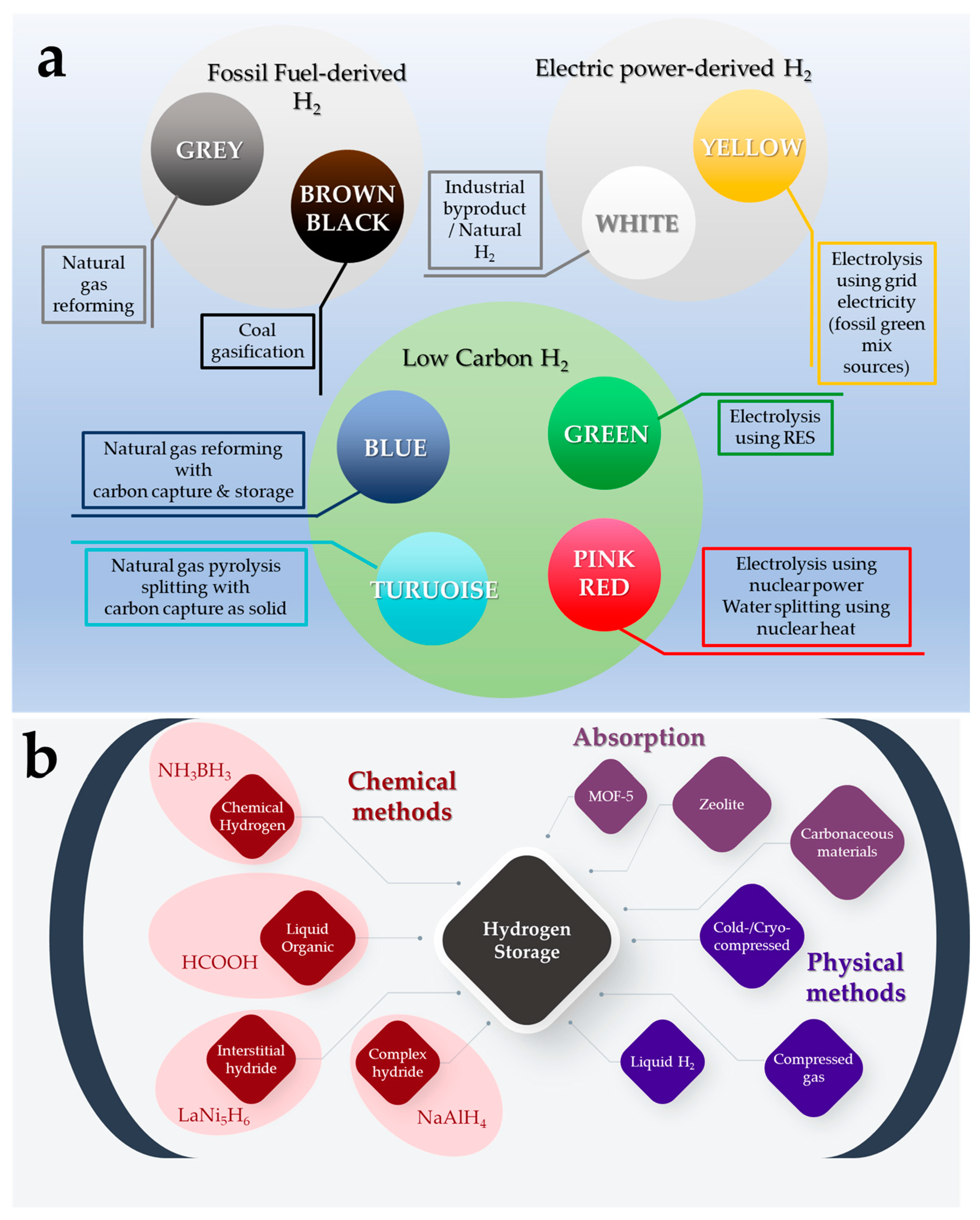
Figure 2. (a) Color classification of H2 based on the means of production (b) Hydrogen storage methods.
2. Formic Acid as An Efficient Liquid Carrier for H2 Storage
FA’s high density (1.22 g cm3) translates into a volumetric H2 concentration of 53 g H2/L, which is equivalent to 1.77 kWh L−1. This surpasses the industry standards of the Toyota Mirai (700 bar H2 storage tank with 1.4 kWh L−1), which consumes 0.76 kg H2/100 km [11][12]. Despite the advent of electricity-driven technologies and the manufacturing of FA from green feedstock, the FA market is still restricted until new breakthroughs impose its broader commercialization [13]. FA, a typical industrial reagent, is non-flammable, non-toxic, simple to handle at room temperature, and does not require high-pressure storage or a catalytic reforming unit. It is one of the most widely used organic chemical raw ingredients in the pesticide, leather, dyes, medicine, and rubber sectors [14]. FA supplied at a concentration of 85% is the worldwide industry standard, while 99% is required for particular applications [15]. The global output of FA ranged from 750,000 to 800,000 tons between 2014 and 2019, with the price being $400/ton. The Global FA Market was worth 756.5 million $ in 2018 and is projected to reach 828.1 million $ by the end of 2025, expanding at a CAGR (compound annual growth rate) of 1.3% between 2018 and 2025 [14]. The reduced labor and capital costs, along with the constantly expanding market, will enable China to boost its FA production capacity, making it the leading producer and exporter of FA [13].
Regarding the involvement of FA in energy issues, FA decomposition occurs via the two routes, as depicted in Figure 3a. The first equation describes HCOOH dehydrogenation into H2 and CO2, whereas in the second one, HCOOH dehydration provides H2O and CO, which is responsible for the inactivation of fuel cell electrodes. Both reactions are dependent on the employed catalysts, the temperature, and the concentration of FA [16]. Using an appropriate catalyst at low temperatures, both the dehydrogenation of FA (FADH) to CO2 and the hydrogenation of CO2 to FA (FAH) are possible, resulting in a “carbon-neutral cycle” (See Figure 3b) [17].
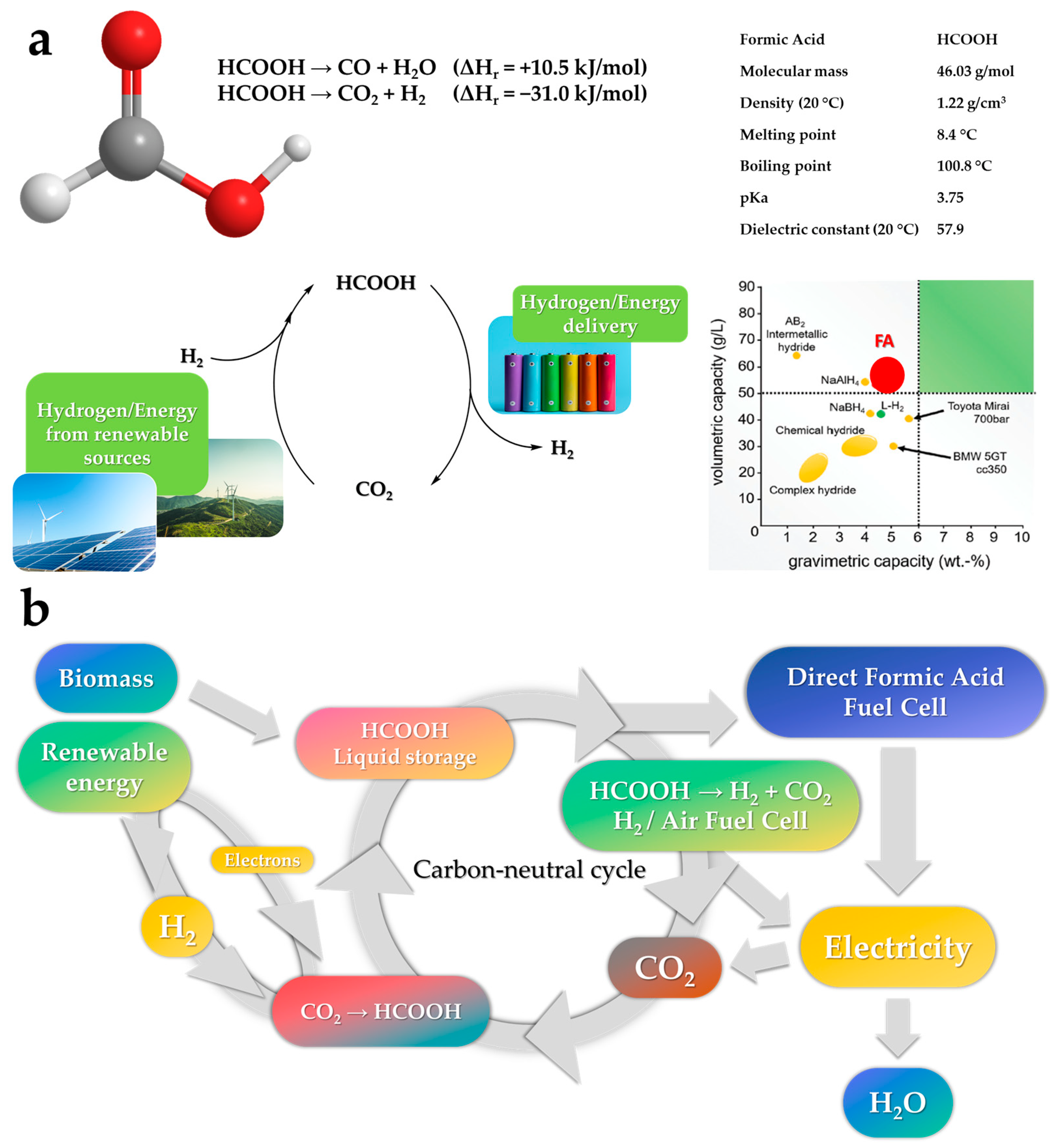
Figure 3. (a) FA properties and decomposition reactions (b) Cycle of FA dehydrogenation and regeneration by CO2 hydrogenation.
CO2 hydrogenation to FA is a crucial challenge targeting a double benefit for the planet since it prevents environmental pollution caused by CO2 emissions from industrial effluents and, at the same time, it regenerates a substantial organic feedstock. A carbon-neutral H2 storage/release cycle is feasible and it consists of the quantitative hydrogenation of CO2 to form FA, followed by its selective dehydrogenation [18]. Up-to-day, dehydrogenation of FA, using a molecular catalyst, has gained a lot of interest in the research community, due to its higher performance in mild conditions vs. the hydrogenation path [19]. Based on this potential, it is possible to envision automotive applications in which gasoline is substituted by FA and automobiles are equipped with technology based on fuel cells; either direct formic acid fuel cells (DFAFC) [20] or hydrogen fuel cells (HFC) such as PEMFCs (Proton-exchange membrane fuel cells) [11].
DFAFCs are advantageous for compact portable HFC applications and promising for automotive batteries of electric vehicles. They offer the potential for a carbon-neutral cycle in which CO2 is collected and subsequently electrolyzed to produce FA. Although DFAFCs conversion of FA to electricity seems to be simple, their performance is restricted by fuel crossover and anode catalyst poisoning [21]. PEMFCs, on the other hand, are well-established for use in electric vehicles, with maximum power density higher than DFAFCS, i.e., 1400–2000 mW/cm−2 [22] vs. 550 mW/cm−2 [23] (Figure 4).
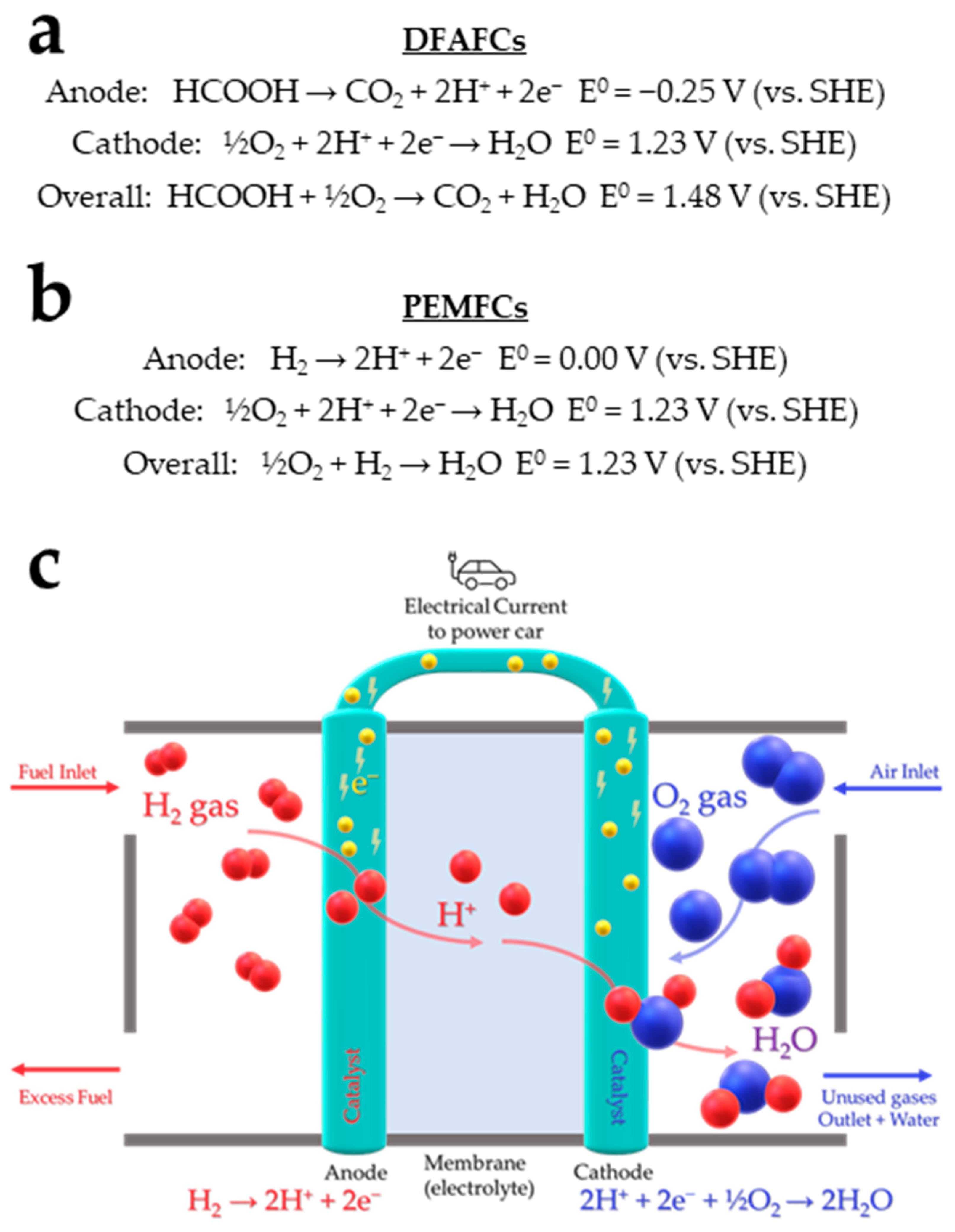
Figure 4. The reactions that occur at the anode and cathode of (a) Direct Formic Acid Fuel Cells (DFAFC), (b) Proton-exchange membrane fuel cells (PEMFC), (c) Schematic representation of a PEMFC.
An efficient catalyst for H2 generation to be used in PEMFC must meet the following requirements: selectivity, high stability (TON), high activity (TOF), and low cost [24]. In order to obtain customer acceptance of a new technology, economic factors are of paramount significance. Huang recently evaluated eight homogenous systems which rely on the use of two dimension-free equations, CON (Catalyst cost normalized to the TON value) and COF (Catalyst cost normalized to the TOF value) [24] (Figure 5). In this methodology, it is proposed that for a catalyst to be considered valuable for use in automobile applications, it should have a maximum CON and COF value of 0.35 [24], a range of TOF between 5000 to 10,000 h−1, and TON in the order of multi-millions [25]. The same research group, in another perspective, enrich their methodology with a life cycle assessment (LCA) data analysis of FA with CO2 capture and storage (CCS) and CO2 capture and utilize prospects (CCU) [18]. As depicted in Figure 5, the set thresholds for a product (H2 in this example) and the overall reactor system (tank, converter, controller) allow for a comparison of the economic viability of new technologies to those now in use. For each process, there exists a region in the CON/COF diagram where catalyst costs satisfy the corresponding cost threshold requirements. Catalysts with greater CON or COF values result in a higher-than-desired product cost (CON > CON0) or system cost (COF > COF0). Consequently, the green region in the diagram denotes the aim for catalysts that economically meet all the required criteria.
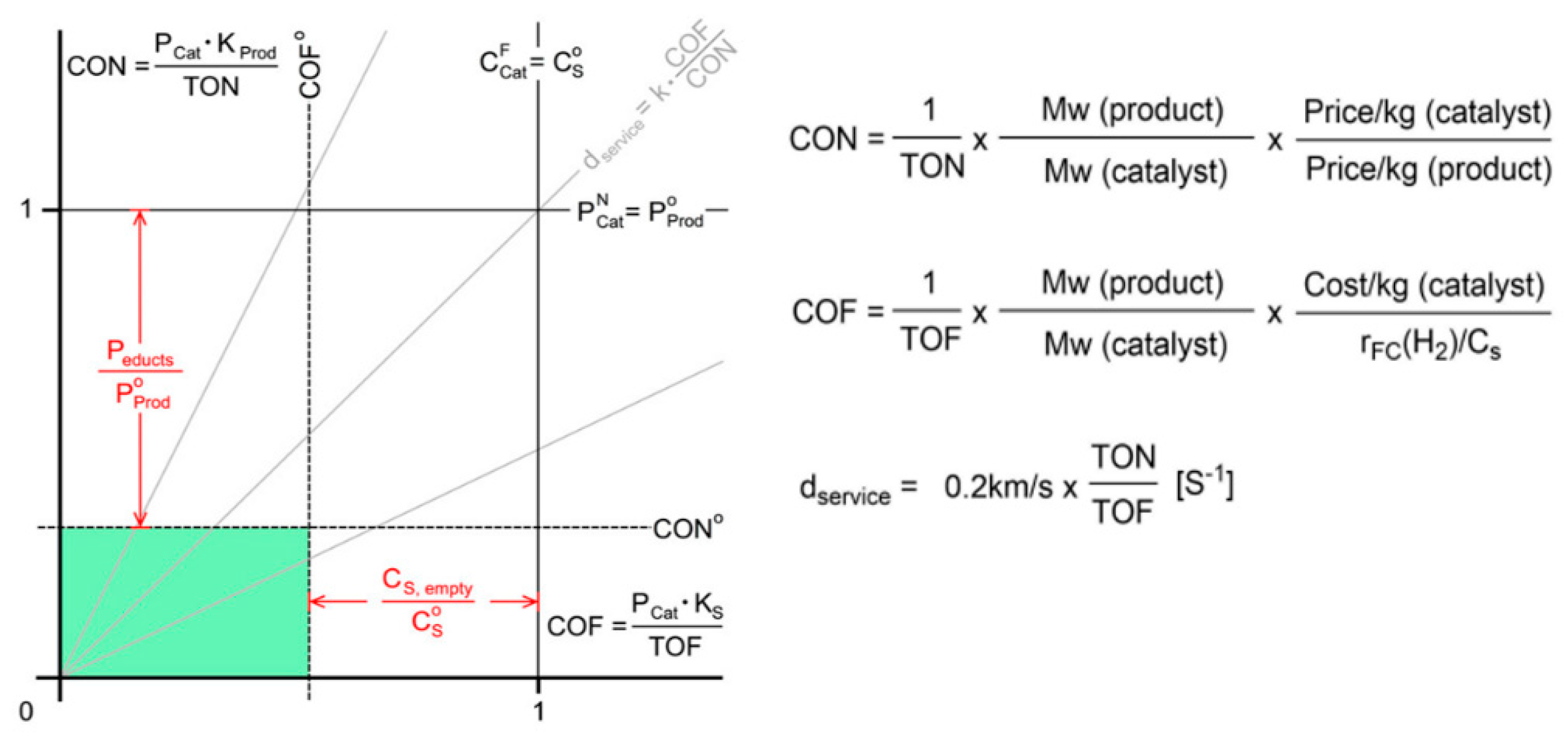
Figure 5. Μargins of catalyst price normalized vs. TON (CON) or TOF (COF). According to cost-analysis methodology, “product” refers to the total cost of all raw materials, while Cs is the system expenses without a catalyst (e.g., fuel tank, pumps, FA converter, and controller). Adapted from [24] with permission.
Moreover, to determine how close a technology is to operational deployment and to evaluate the development status of emerging technologies, the U.S. Department of Defense and Horizon 2020 proposed the TRL definition (see Figure 6) [26][27]. Based on this technological assessment, the scale-up implementation of some homogeneous catalysts for the majority of FA-to-power technologies ranges between TRL 7 and TRL 8 [15][28][29][30]. However, for these systems to be applied in a real operational environment [TRL 9], a lot of further improvement is needed as the requirements for this level take into consideration the catalytic activity in tandem with the decrease of the cost.
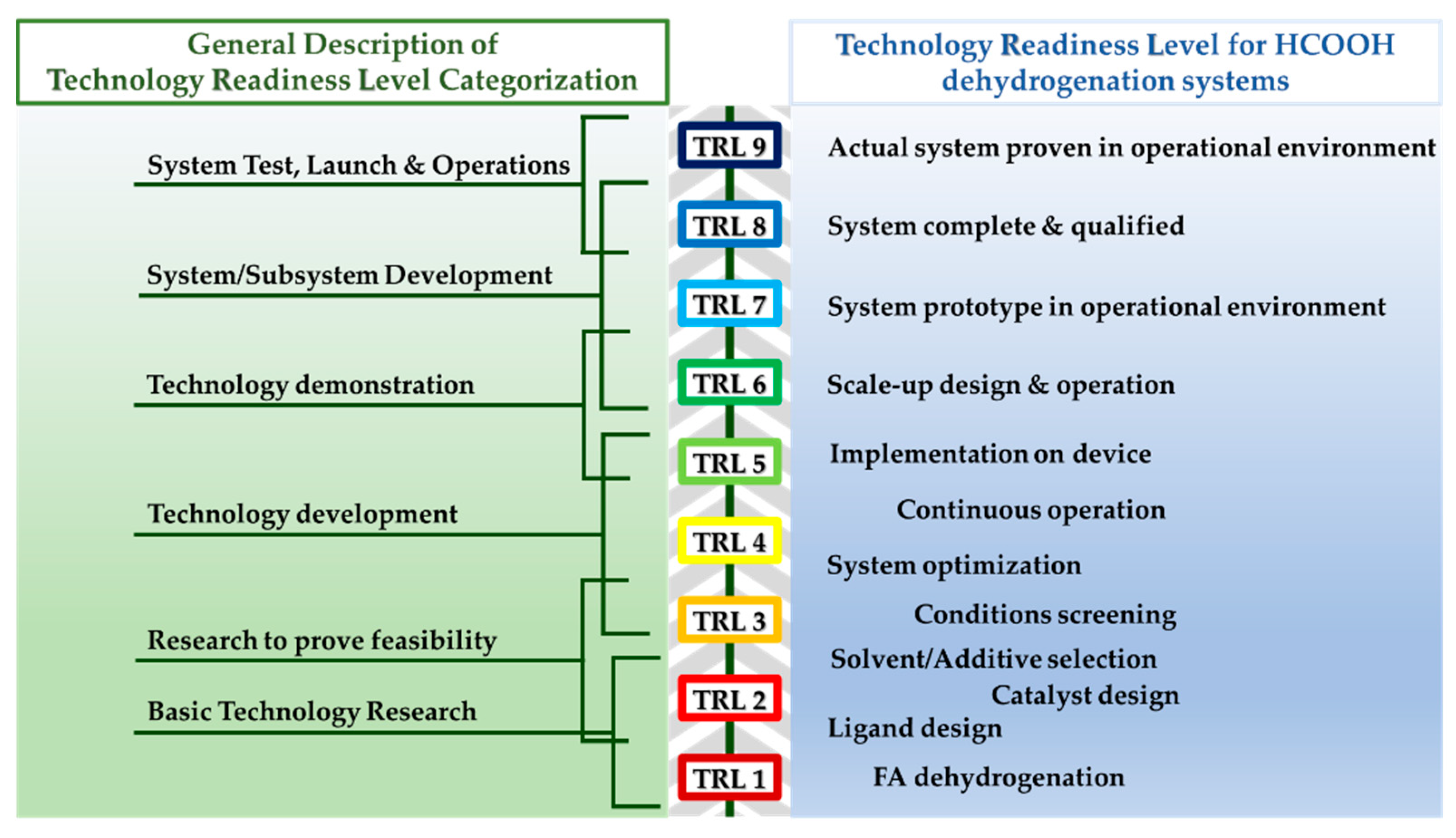
Figure 6. Technology Readiness Levels (TRL) for FA dehydrogenation catalytic systems.
References
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180.
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086.
- Anandarajah, G.; McDowall, W.; Ekins, P. Decarbonising road transport with hydrogen and electricity: Long term global technology learning scenarios. Int. J. Hydrogen Energy 2013, 38, 3419–3432.
- Ruffini, E.; Wei, M. Future costs of fuel cell electric vehicles in California using a learning rate approach. Energy 2018, 150, 329–341.
- Hydrogen Refuelling Stations Worldwide. Available online: https://www.h2stations.org/ (accessed on 21 December 2022).
- Hua, T.; Ahluwalia, R.; Eudy, L.; Singer, G.; Jermer, B.; Asselin-Miller, N.; Wessel, S.; Patterson, T.; Marcinkoski, J. Status of hydrogen fuel cell electric buses worldwide. J. Power Source 2014, 269, 975–993.
- Alstom Coradia iLint Distance Run. Available online: https://www.alstom.com/alstom-coradia-ilint-distance-run (accessed on 15 November 2022).
- Zhang, F.; Zhao, P.; Niu, M.; Maddy, J. The survey of key technologies in hydrogen energy storage. Int. J. Hydrogen Energy 2016, 41, 14535–14552.
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrogen Energy 2017, 42, 30470–30492.
- Quarton, C.J.; Samsatli, S. The value of hydrogen and carbon capture, storage and utilisation in decarbonising energy: Insights from integrated value chain optimisation. Appl. Energy 2020, 257, 113936.
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491.
- Toyota MIRAI, MIRAI Means ‘The Future’. Available online: https://h2.live/en/fuelcell-cars/toyota-mirai/ (accessed on 23 October 2022).
- Formic Acid Market—Growth, Trends, COVID-19 Impact, and Forecasts (2023–2028). Available online: https://www.mordorintelligence.com/industry-reports/formic-acid-market (accessed on 28 November 2022).
- Formic Acid Market by Product Types (Grades of 85%, 94%, 99%, and Others), Applications (Agriculture, Leather & Textile, Rubber, Chemical & Pharmaceuticals, and Others), and Regions (Asia Pacific, North America, Latin America, Europe, and Middle East & Africa)—Global Industry Analysis, Growth, Share, Size, Trends, and Forecast, 2021–2028. Available online: https://dataintelo.com/report/formic-acid-market/ (accessed on 29 November 2022).
- van Putten, R.; Wissink, T.; Swinkels, T.; Pidko, E.A. Fuelling the hydrogen economy: Scale-up of an integrated formic acid-to-power system. Int. J. Hydrogen Energy 2019, 44, 28533–28541.
- Singh, A.K.; Singh, S.; Kumar, A. Hydrogen energy future with formic acid: A renewable chemical hydrogen storage system. Catal. Sci. Technol. 2016, 6, 12–40.
- Czaun, M.; Goeppert, A.; Kothandaraman, J.; May, R.B.; Haiges, R.; Prakash, G.K.S.; Olah, G.A. Formic Acid as a Hydrogen Storage Medium: Ruthenium-Catalyzed Generation of Hydrogen from Formic Acid in Emulsions. ACS Catal. 2013, 4, 311–320.
- Dutta, I.; Chatterjee, S.; Cheng, H.; Parsapur, R.K.; Liu, Z.; Li, Z.; Ye, E.; Kawanami, H.; Low, J.S.C.; Lai, Z.; et al. Formic Acid to Power towards Low-Carbon Economy. Adv. Energy Mater. 2022, 12, 2103799.
- Sordakis, K.; Tang, C.; Vogt, L.K.; Junge, H.; Dyson, P.J.; Beller, M.; Laurenczy, G. Homogeneous Catalysis for Sustainable Hydrogen Storage in Formic Acid and Alcohols. Chem. Rev. 2018, 118, 372–433.
- Yu, X.; Pickup, P.G. Recent advances in direct formic acid fuel cells (DFAFC). J. Power Source 2008, 182, 124–132.
- Rejal, S.Z.; Masdar, M.S.; Kamarudin, S.K. A parametric study of the direct formic acid fuel cell (DFAFC) performance and fuel crossover. Int. J. Hydrogen Energy 2014, 39, 10267–10274.
- Han, D.; Tsipoaka, M.; Shanmugam, S. A modified cathode catalyst layer with optimum electrode exposure for high current density and durable proton exchange membrane fuel cell operation. J. Power Sources 2021, 496, 229816.
- Chang, J.; Feng, L.; Liu, C.; Xing, W.; Hu, X. An Effective Pd-Ni2P/C Anode Catalyst for Direct Formic Acid Fuel Cells. Angew. Chem. Int. Ed. 2014, 53, 122–126.
- Eppinger, J.; Huang, K.-W. Formic Acid as a Hydrogen Energy Carrier. ACS Energy Lett. 2017, 2, 188–195.
- Guan, C.; Pan, Y.; Zhang, T.; Ajitha, M.J.; Huang, K.-W. An Update on Formic Acid Dehydrogenation by Homogeneous Catalysis. Chem. Asian J. 2020, 15, 937–946.
- U.S. Department of Energy. Technology Readiness Assessment Guide. Available online: https://www.directives.doe.gov/directives-documents/400-series/0413.3-EGuide-04/@@images/file (accessed on 21 October 2022).
- What Are Technology Readiness Levels (TRL)? Available online: https://www.twi-global.com/technical-knowledge/faqs/technology-readiness-levels (accessed on 29 December 2022).
- Gabor, L.; Céline, F.; Paul, D. Hydrogen Production from Formic Acid. EP 1 918 247 A1. 7 May 2008. Available online: https://patentimages.storage.googleapis.com/02/4b/0e/db9b2f842c2658/EP1918247A1.pdf (accessed on 20 October 2022).
- Huang, K.-W.; Zheng, J. Electricity Generation Devices Using Formic Acid. WO 2017/103820 A1. 22 June 2017. Available online: https://repository.kaust.edu.sa/bitstream/handle/10754/625325/WO2017103820A1.pdf?sequence=1&isAllowed=y (accessed on 20 October 2022).
- The world’s First Formic Acid-Based Fuel Cell. Available online: https://actu.epfl.ch/news/the-world-s-first-formic-acid-based-fuel-cell/ (accessed on 12 November 2022).
More
Information
Subjects:
Energy & Fuels
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
2 times
(View History)
Update Date:
20 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




