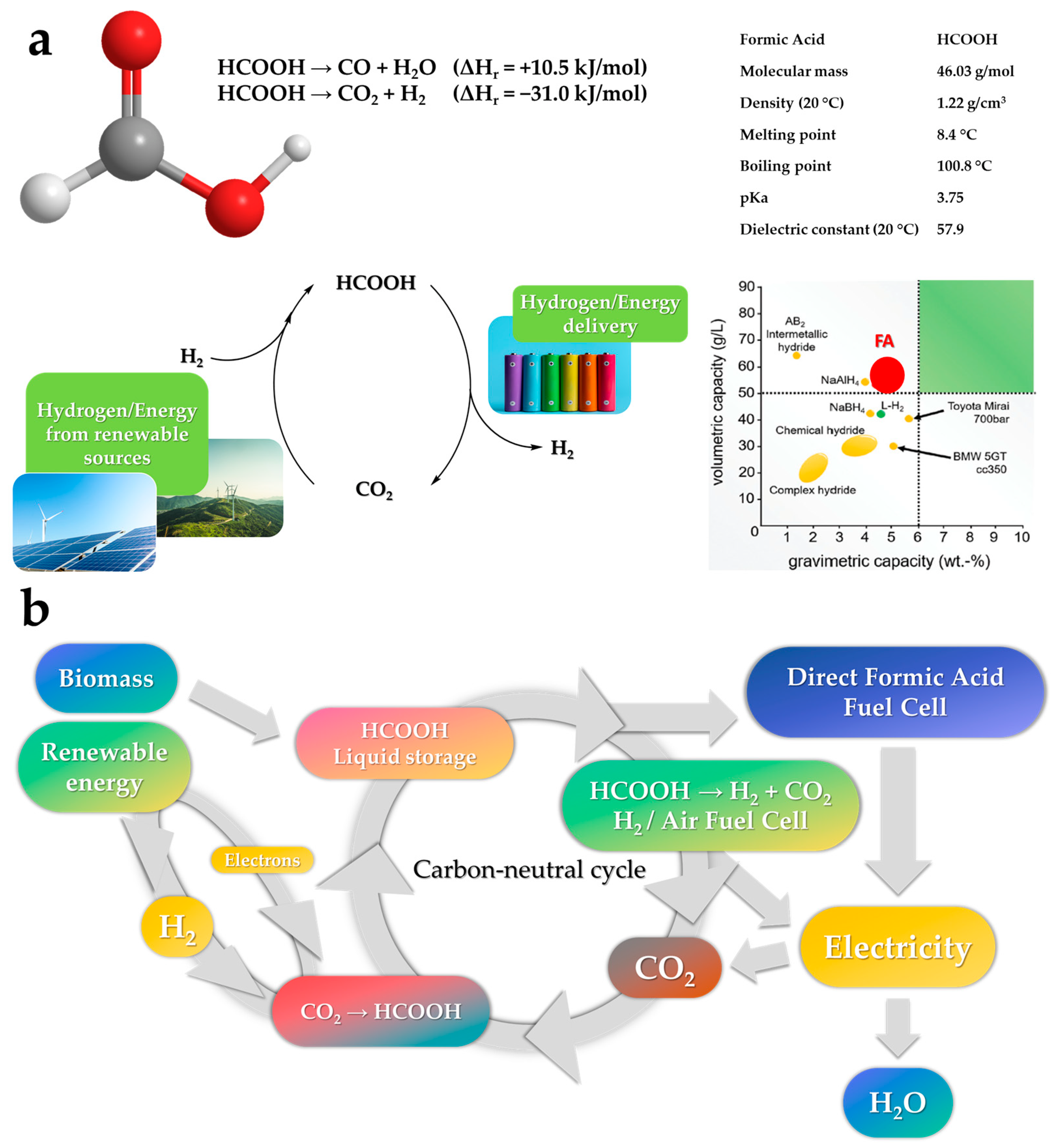
The development of low-carbon technologies that will facilitate the efficient use of hydrogen (H2) as an energy carrier is a critical requirement of contemporary society. To this end, it is anticipated that the cost of H2 production will become a key factor in tandem with production efficiency, process safety, and transport. Much effort has been made to create and develop new, reversible, and sustainable H2 storage systems. Among current techniques, formic acid (FA) has been identified as an efficient energy carrier for H2 storage. Numerous homogeneous catalysts based on transition metals with high activity and selectivity have been reported for selective FA dehydrogenation.
- hydrogen production
- cost analysis
- formic acid dehydrogenation
1. Introduction
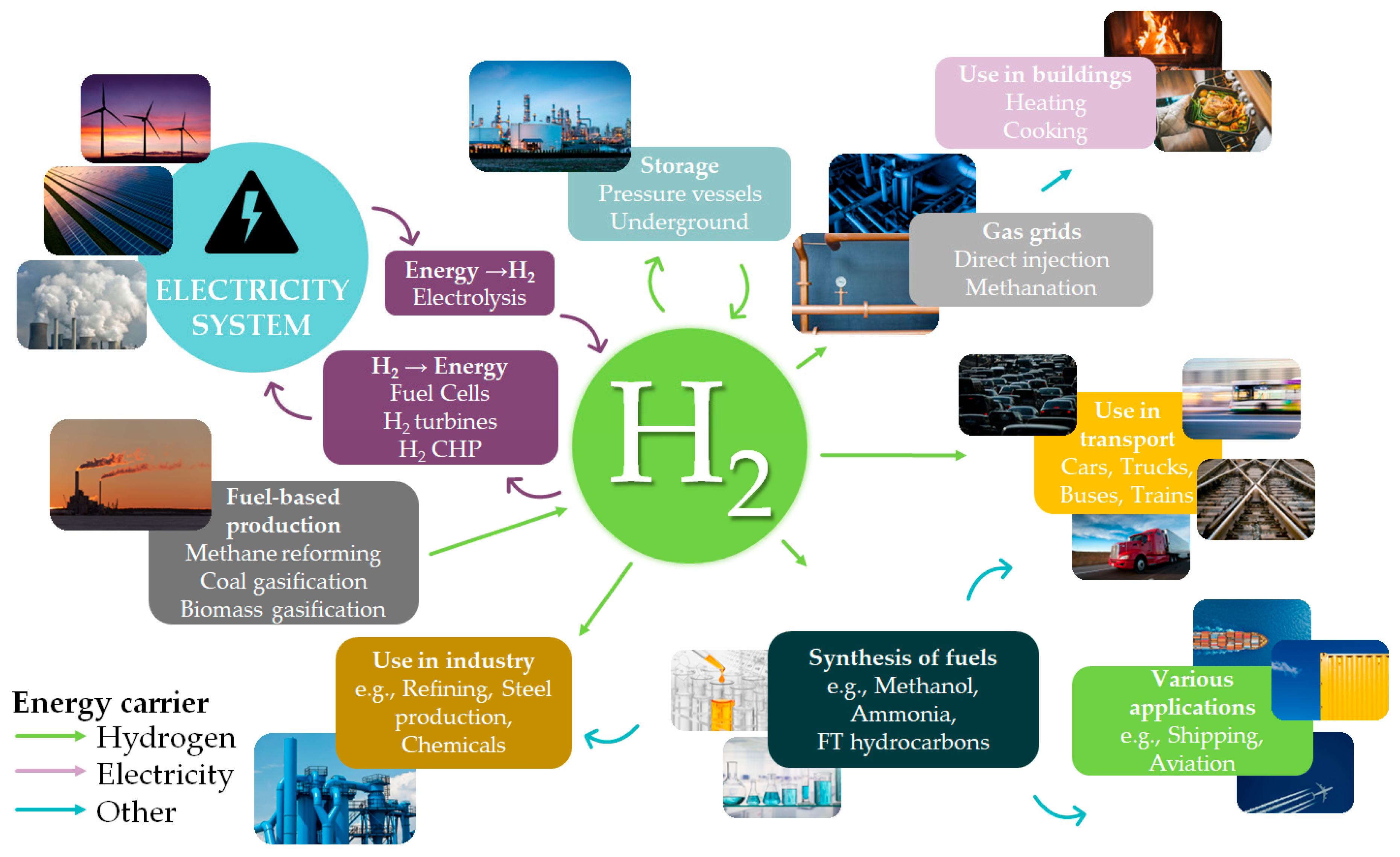
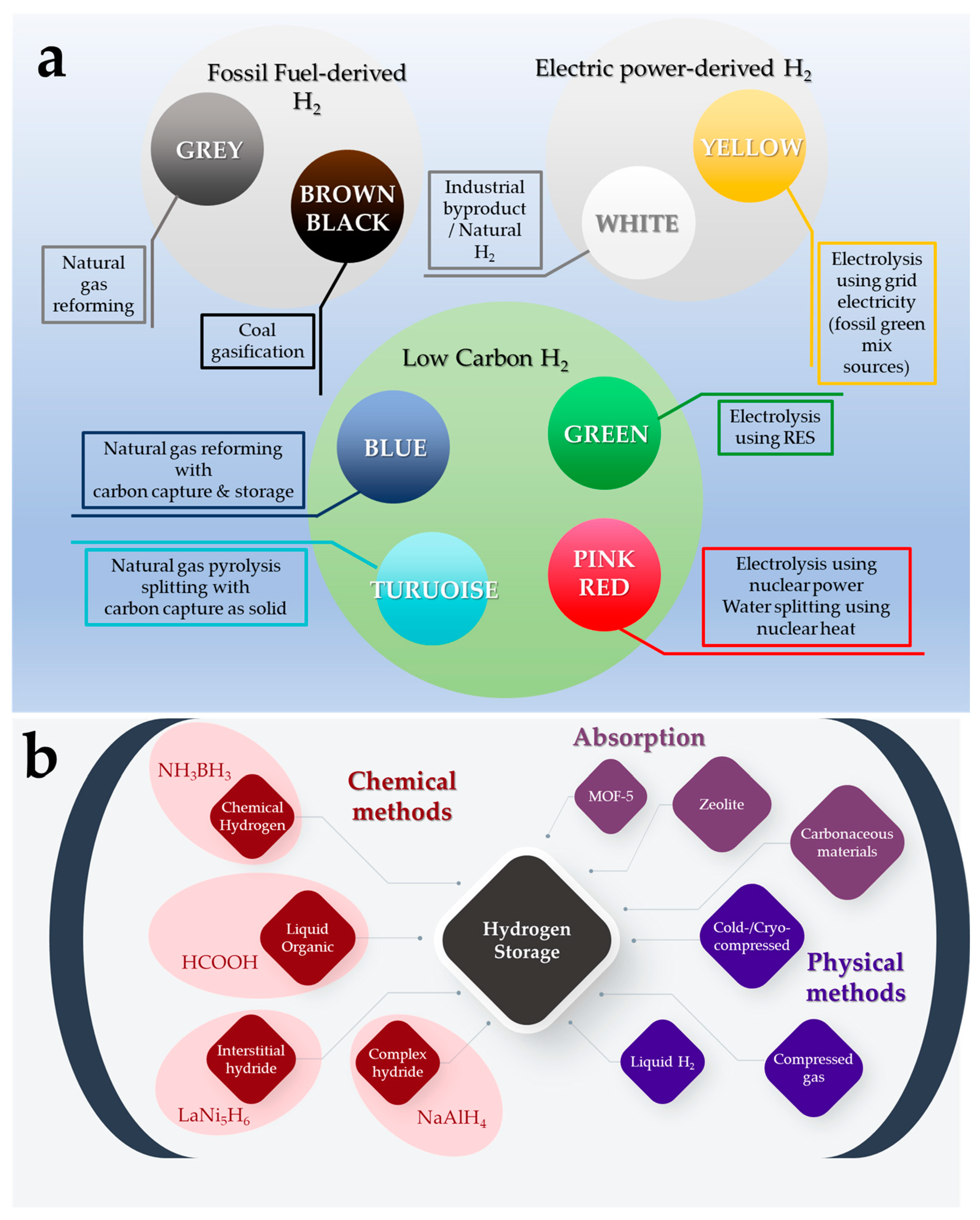
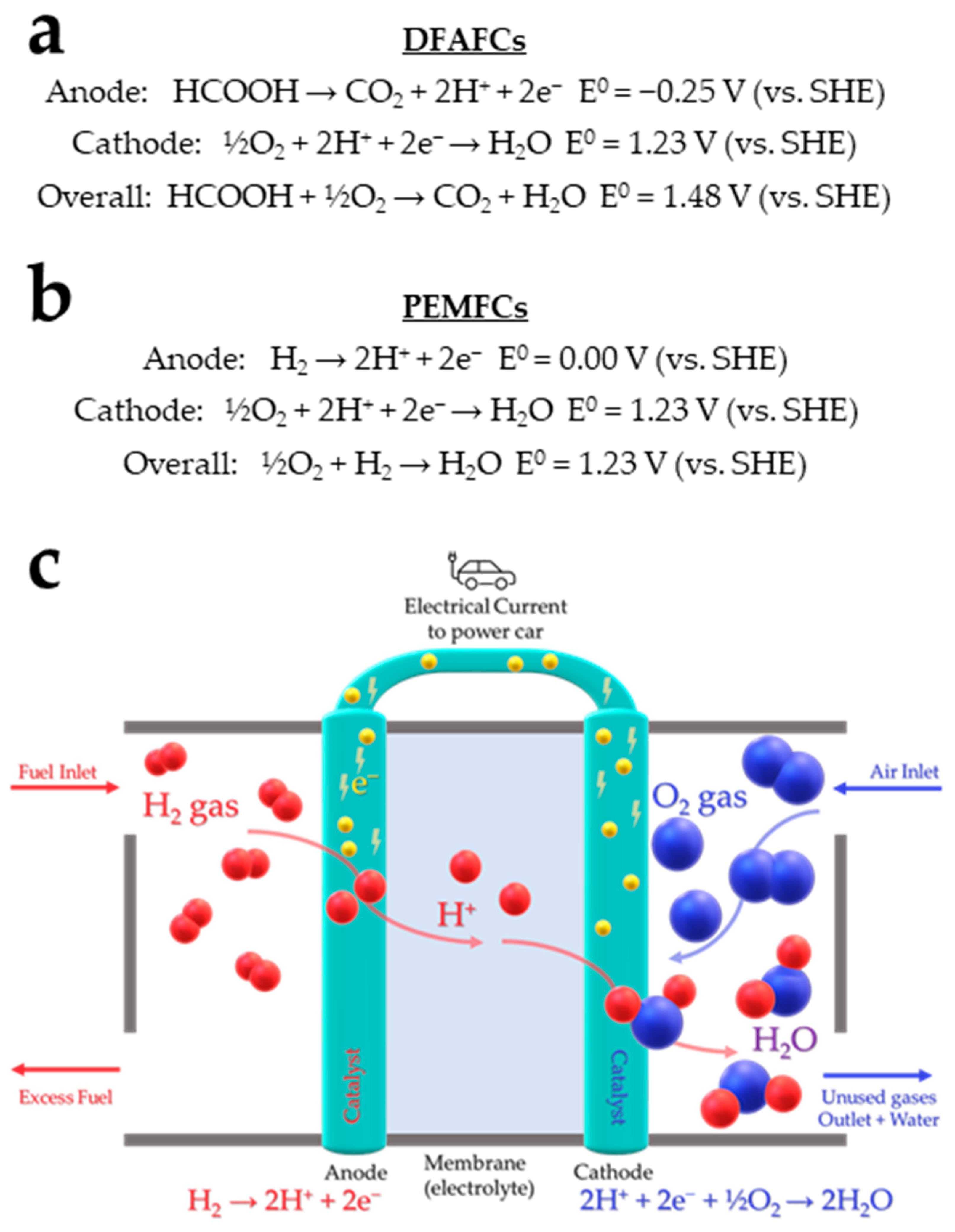
2. Formic Acid as An Efficient Liquid Carrier for H2 Storage
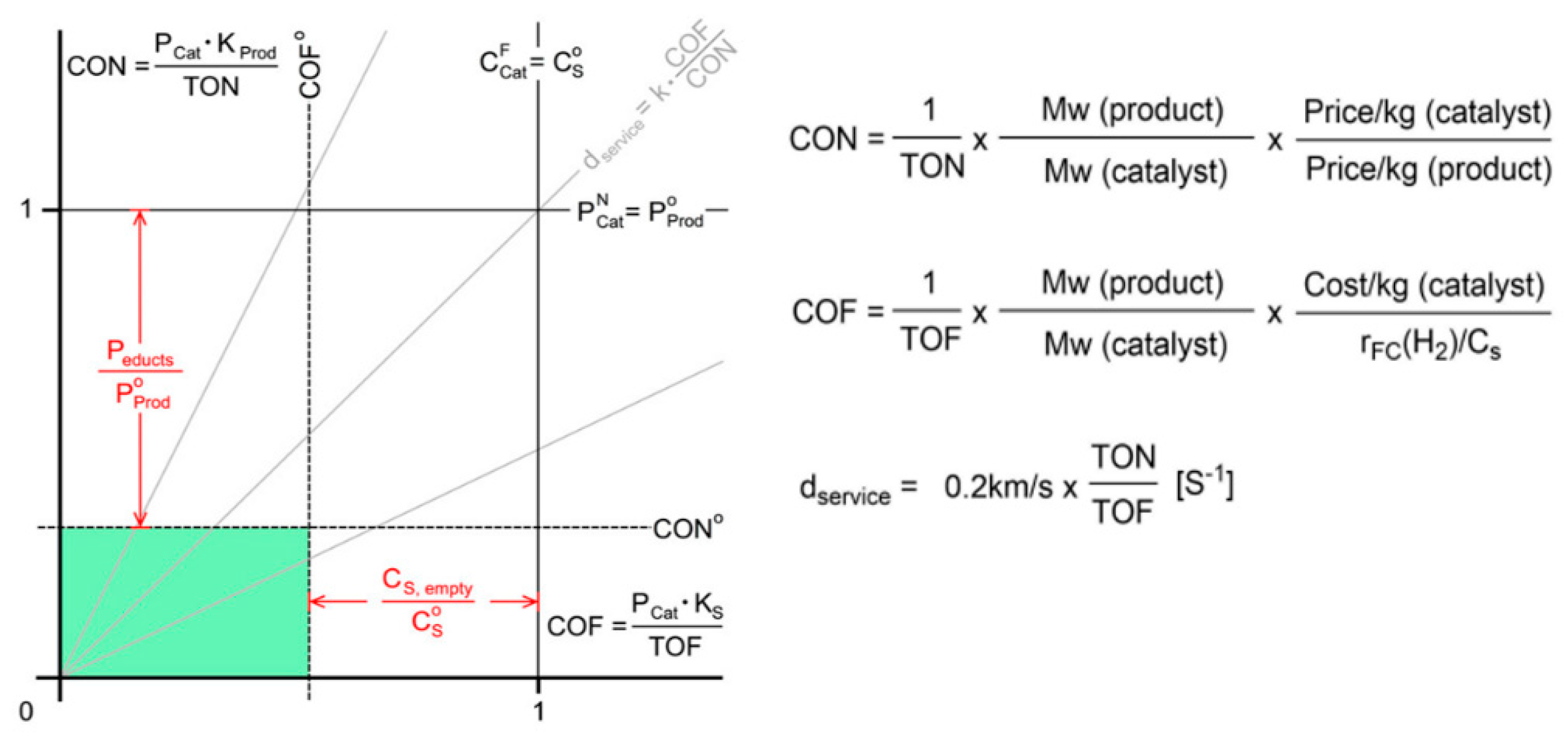
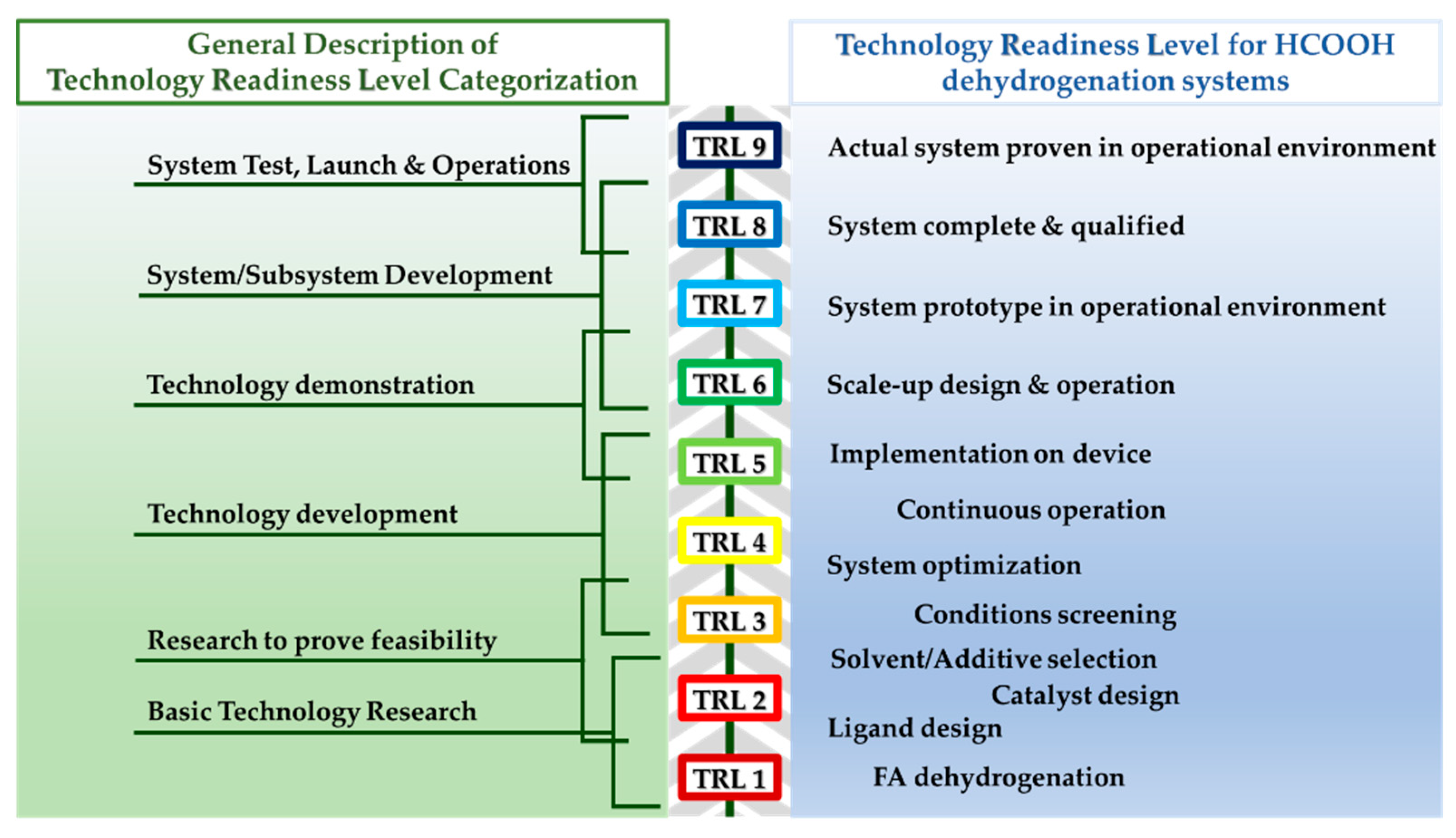
This entry is adapted from the peer-reviewed paper 10.3390/en16041723
References
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180.
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086.
- Anandarajah, G.; McDowall, W.; Ekins, P. Decarbonising road transport with hydrogen and electricity: Long term global technology learning scenarios. Int. J. Hydrogen Energy 2013, 38, 3419–3432.
- Ruffini, E.; Wei, M. Future costs of fuel cell electric vehicles in California using a learning rate approach. Energy 2018, 150, 329–341.
- Hydrogen Refuelling Stations Worldwide. Available online: https://www.h2stations.org/ (accessed on 21 December 2022).
- Hua, T.; Ahluwalia, R.; Eudy, L.; Singer, G.; Jermer, B.; Asselin-Miller, N.; Wessel, S.; Patterson, T.; Marcinkoski, J. Status of hydrogen fuel cell electric buses worldwide. J. Power Source 2014, 269, 975–993.
- Alstom Coradia iLint Distance Run. Available online: https://www.alstom.com/alstom-coradia-ilint-distance-run (accessed on 15 November 2022).
- Zhang, F.; Zhao, P.; Niu, M.; Maddy, J. The survey of key technologies in hydrogen energy storage. Int. J. Hydrogen Energy 2016, 41, 14535–14552.
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrogen Energy 2017, 42, 30470–30492.
- Quarton, C.J.; Samsatli, S. The value of hydrogen and carbon capture, storage and utilisation in decarbonising energy: Insights from integrated value chain optimisation. Appl. Energy 2020, 257, 113936.
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491.
- Toyota MIRAI, MIRAI Means ‘The Future’. Available online: https://h2.live/en/fuelcell-cars/toyota-mirai/ (accessed on 23 October 2022).
- Formic Acid Market—Growth, Trends, COVID-19 Impact, and Forecasts (2023–2028). Available online: https://www.mordorintelligence.com/industry-reports/formic-acid-market (accessed on 28 November 2022).
- Formic Acid Market by Product Types (Grades of 85%, 94%, 99%, and Others), Applications (Agriculture, Leather & Textile, Rubber, Chemical & Pharmaceuticals, and Others), and Regions (Asia Pacific, North America, Latin America, Europe, and Middle East & Africa)—Global Industry Analysis, Growth, Share, Size, Trends, and Forecast, 2021–2028. Available online: https://dataintelo.com/report/formic-acid-market/ (accessed on 29 November 2022).
- van Putten, R.; Wissink, T.; Swinkels, T.; Pidko, E.A. Fuelling the hydrogen economy: Scale-up of an integrated formic acid-to-power system. Int. J. Hydrogen Energy 2019, 44, 28533–28541.
- Singh, A.K.; Singh, S.; Kumar, A. Hydrogen energy future with formic acid: A renewable chemical hydrogen storage system. Catal. Sci. Technol. 2016, 6, 12–40.
- Czaun, M.; Goeppert, A.; Kothandaraman, J.; May, R.B.; Haiges, R.; Prakash, G.K.S.; Olah, G.A. Formic Acid as a Hydrogen Storage Medium: Ruthenium-Catalyzed Generation of Hydrogen from Formic Acid in Emulsions. ACS Catal. 2013, 4, 311–320.
- Dutta, I.; Chatterjee, S.; Cheng, H.; Parsapur, R.K.; Liu, Z.; Li, Z.; Ye, E.; Kawanami, H.; Low, J.S.C.; Lai, Z.; et al. Formic Acid to Power towards Low-Carbon Economy. Adv. Energy Mater. 2022, 12, 2103799.
- Sordakis, K.; Tang, C.; Vogt, L.K.; Junge, H.; Dyson, P.J.; Beller, M.; Laurenczy, G. Homogeneous Catalysis for Sustainable Hydrogen Storage in Formic Acid and Alcohols. Chem. Rev. 2018, 118, 372–433.
- Yu, X.; Pickup, P.G. Recent advances in direct formic acid fuel cells (DFAFC). J. Power Source 2008, 182, 124–132.
- Rejal, S.Z.; Masdar, M.S.; Kamarudin, S.K. A parametric study of the direct formic acid fuel cell (DFAFC) performance and fuel crossover. Int. J. Hydrogen Energy 2014, 39, 10267–10274.
- Han, D.; Tsipoaka, M.; Shanmugam, S. A modified cathode catalyst layer with optimum electrode exposure for high current density and durable proton exchange membrane fuel cell operation. J. Power Sources 2021, 496, 229816.
- Chang, J.; Feng, L.; Liu, C.; Xing, W.; Hu, X. An Effective Pd-Ni2P/C Anode Catalyst for Direct Formic Acid Fuel Cells. Angew. Chem. Int. Ed. 2014, 53, 122–126.
- Eppinger, J.; Huang, K.-W. Formic Acid as a Hydrogen Energy Carrier. ACS Energy Lett. 2017, 2, 188–195.
- Guan, C.; Pan, Y.; Zhang, T.; Ajitha, M.J.; Huang, K.-W. An Update on Formic Acid Dehydrogenation by Homogeneous Catalysis. Chem. Asian J. 2020, 15, 937–946.
- U.S. Department of Energy. Technology Readiness Assessment Guide. Available online: https://www.directives.doe.gov/directives-documents/400-series/0413.3-EGuide-04/@@images/file (accessed on 21 October 2022).
- What Are Technology Readiness Levels (TRL)? Available online: https://www.twi-global.com/technical-knowledge/faqs/technology-readiness-levels (accessed on 29 December 2022).
- Gabor, L.; Céline, F.; Paul, D. Hydrogen Production from Formic Acid. EP 1 918 247 A1. 7 May 2008. Available online: https://patentimages.storage.googleapis.com/02/4b/0e/db9b2f842c2658/EP1918247A1.pdf (accessed on 20 October 2022).
- Huang, K.-W.; Zheng, J. Electricity Generation Devices Using Formic Acid. WO 2017/103820 A1. 22 June 2017. Available online: https://repository.kaust.edu.sa/bitstream/handle/10754/625325/WO2017103820A1.pdf?sequence=1&isAllowed=y (accessed on 20 October 2022).
- The world’s First Formic Acid-Based Fuel Cell. Available online: https://actu.epfl.ch/news/the-world-s-first-formic-acid-based-fuel-cell/ (accessed on 12 November 2022).
