
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yagmur Kan | -- | 3285 | 2023-04-17 17:36:23 | | | |
| 2 | Peter Tang | Meta information modification | 3285 | 2023-04-18 03:25:39 | | |
Video Upload Options
The nonreceptor tyrosine kinase (NRTK) Ack1 comprises a distinct arrangement of non-catalytic modules. Its SH3 domain has a C-terminal to the kinase domain (SH1), in contrast to the typical SH3-SH2-SH1 layout in NRTKs. The Ack1 is the only protein that shares a region of high homology to the tumor suppressor protein Mig6, a modulator of EGFR. The vertebrate Acks make up the only tyrosine kinase (TK) family known to carry a UBA domain. The GTPase binding and SAM domains are also uncommon in the NRTKs. In addition to being a downstream effector of receptor tyrosine kinases (RTKs) and integrins, Ack1 can act as an epigenetic regulator, modulate the degradation of the epidermal growth factor receptor (EGFR), confer drug resistance, and mediate the progression of hormone-sensitive tumors.
1. Introduction
2. Ack Family Kinases
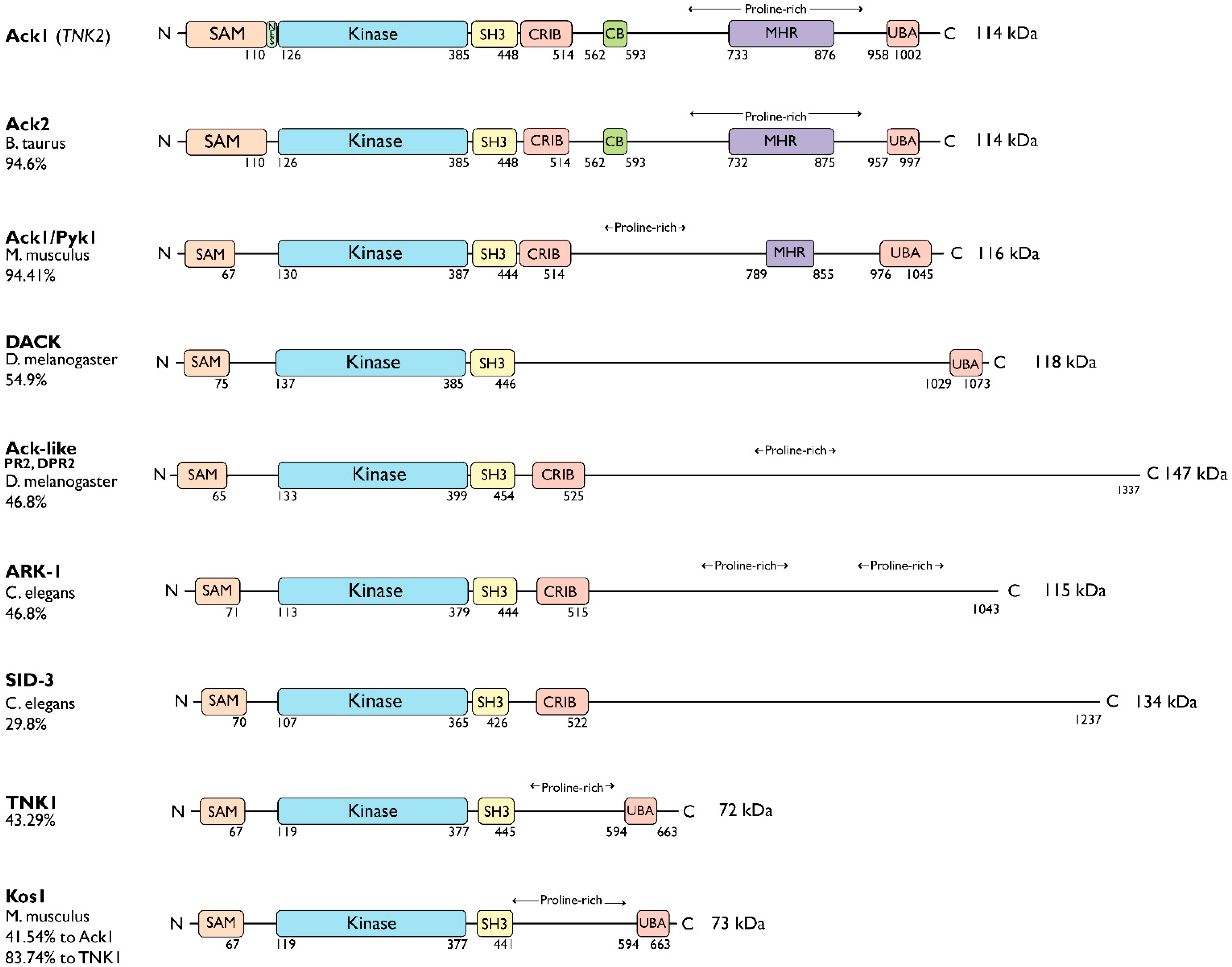
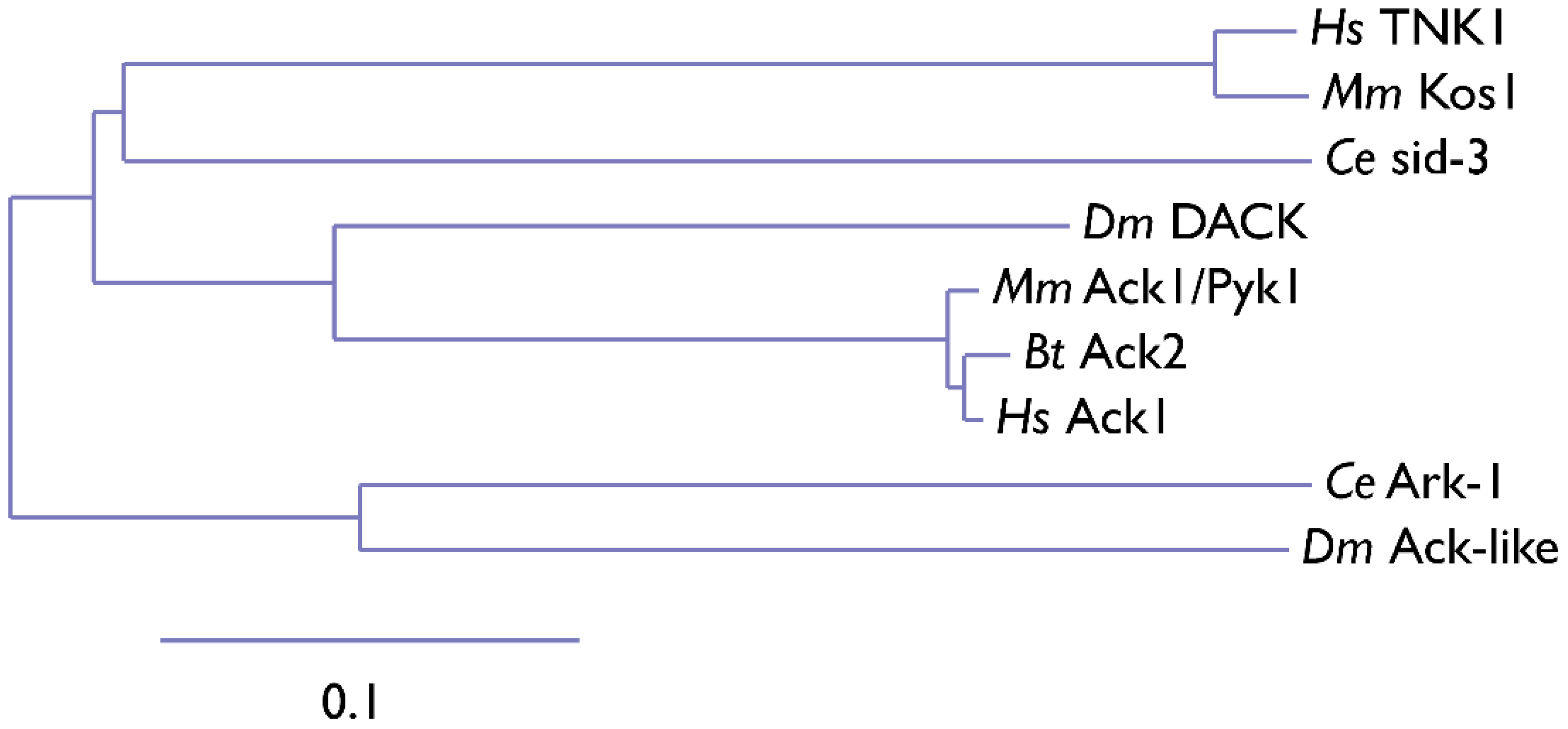
3. Mechanism of Action
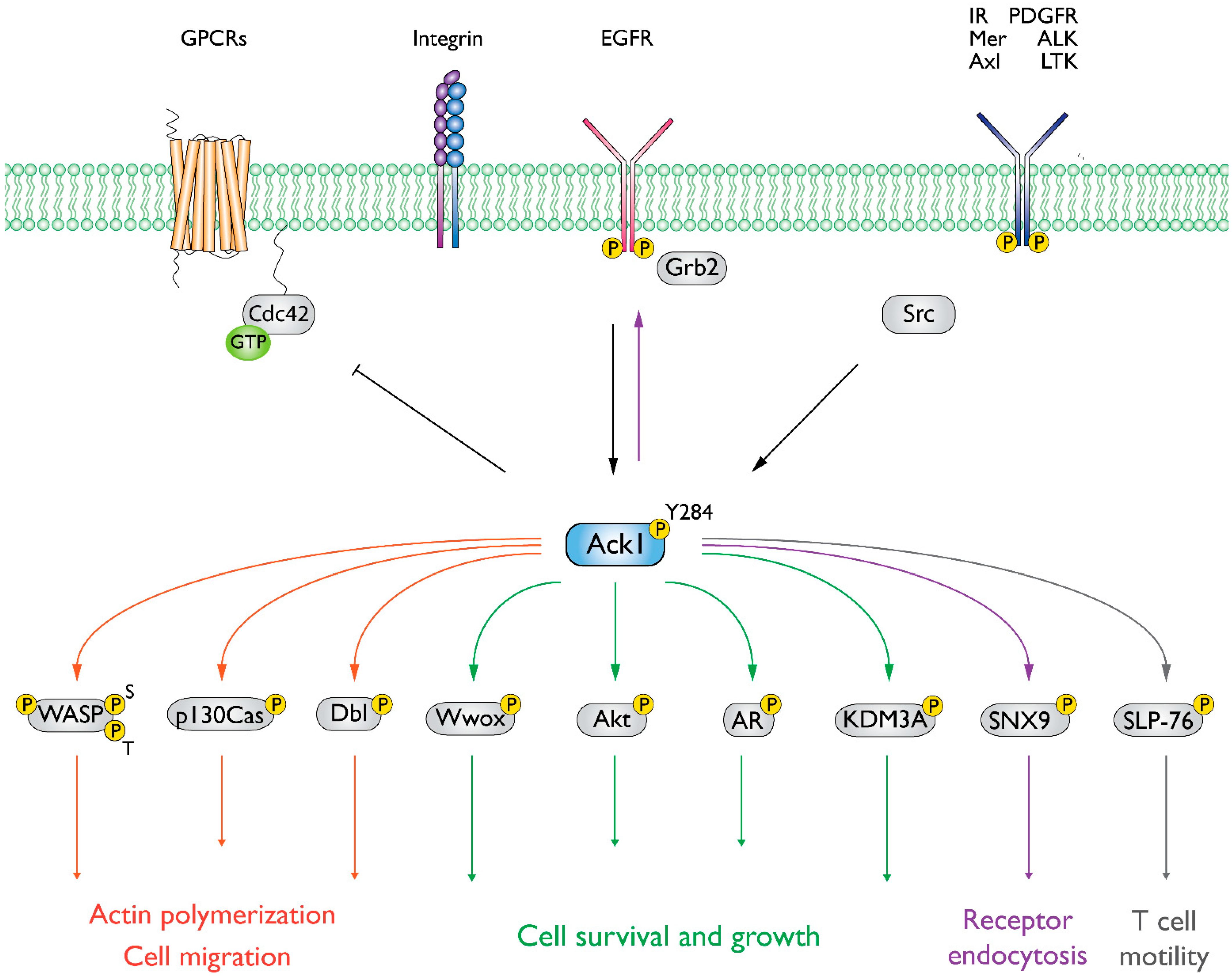
4. Emerging Roles in Signaling Pathways
4.1. Role of Ack1 in Neural Signaling Pathways
4.2. Role of Ack1 in Immune Signaling Pathways
5. Ack1 Substrates
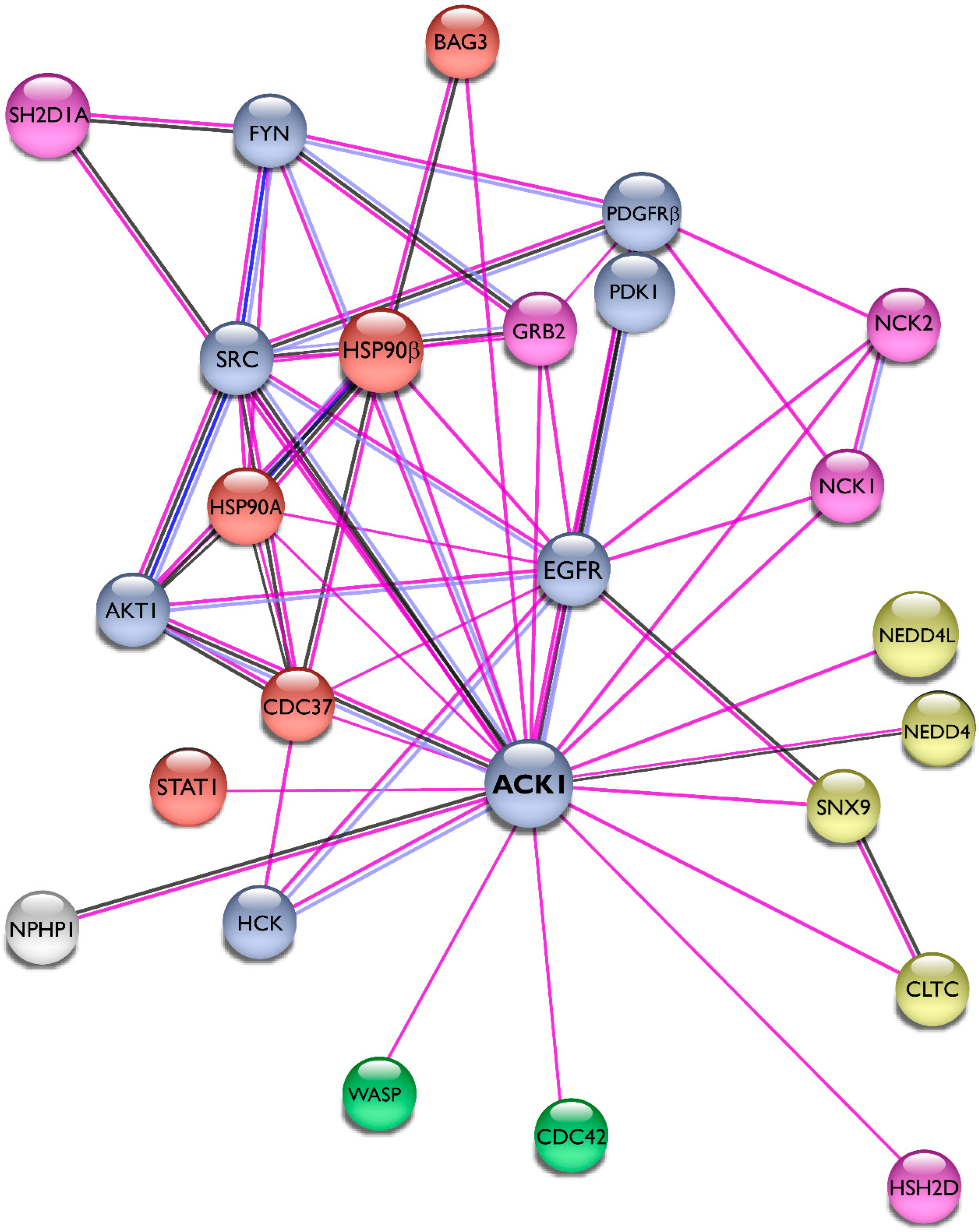
6. Ack1 Involvement in Disease
6.1. Genomic Level
6.2. Transcriptional Level
6.3. Post-Transcriptional Level
7. Ack1-Mediated Drug Resistance
8. Domain Localization of Cancer-Associated Mutations
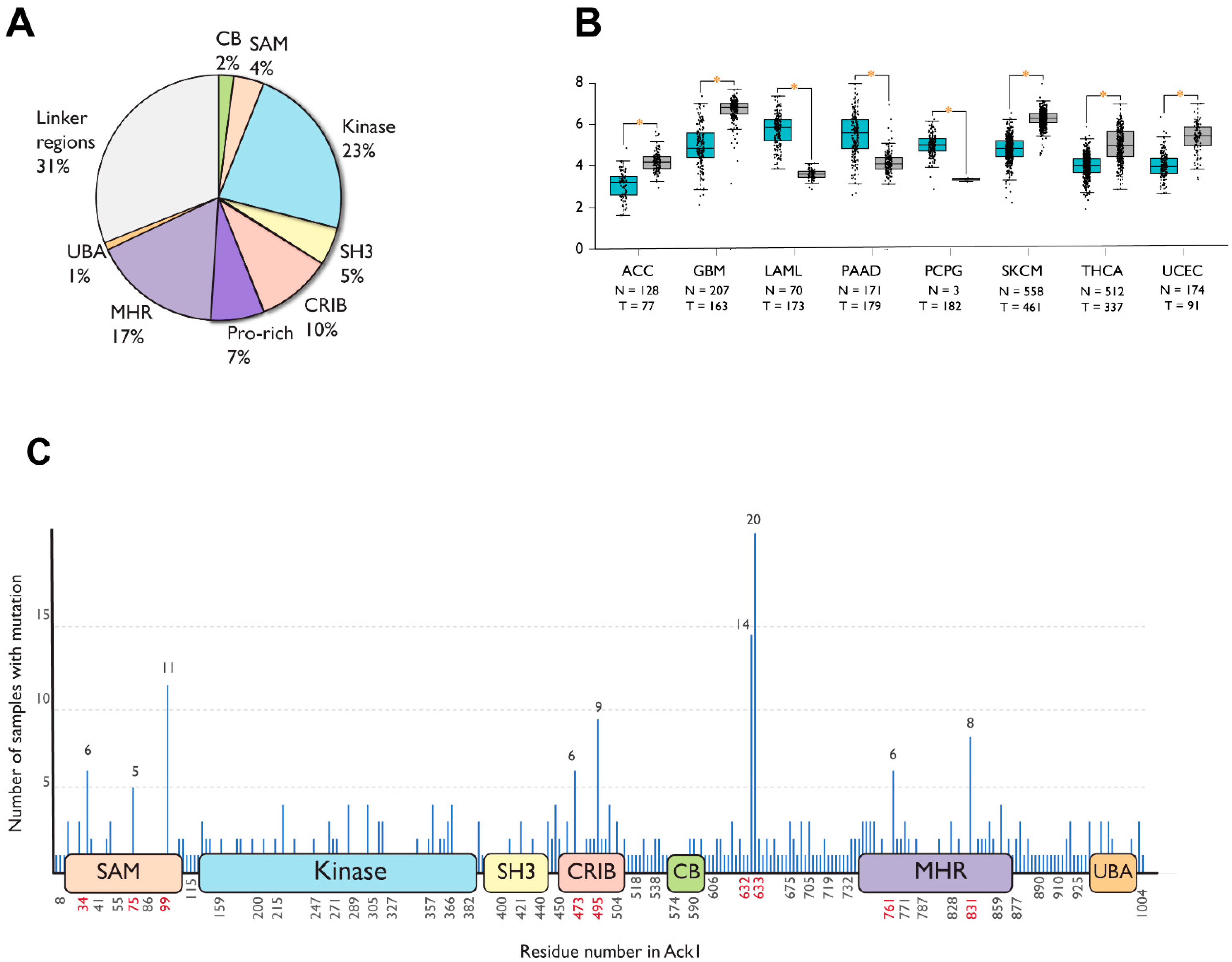
9. Ack1 Drug Development Efforts
References
- Yang, W.; Lin, Q.; Guan, J.L.; Cerione, R.A. Activation of the Cdc42-associated tyrosine kinase-2 (ACK-2) by cell adhesion via integrin beta1. J. Biol. Chem. 1999, 274, 8524–8530.
- Galisteo, M.L.; Yang, Y.; Ureña, J.; Schlessinger, J. Activation of the nonreceptor protein tyrosine kinase Ack by multiple extracellular stimuli. Proc. Natl. Acad. Sci. USA 2006, 103, 9796–9801.
- Mahajan, N.P.; Whang, Y.E.; Mohler, J.L.; Earp, H.S. Activated tyrosine kinase Ack1 promotes prostate tumorigenesis: Role of Ack1 in polyubiquitination of tumor suppressor Wwox. Cancer Res. 2005, 65, 10514–10523.
- Mahajan, K.; Lawrence, H.R.; Lawrence, N.J.; Mahajan, N.P. ACK1 tyrosine kinase interacts with histone demethylase KDM3A to regulate the mammary tumor oncogene HOXA1. J. Biol. Chem. 2014, 289, 28179–28191.
- Mott, H.R.; Owen, D.; Nietlispach, D.; Lowe, P.N.; Manser, E.; Lim, L.; Laue, E.D. Structure of the small G protein Cdc42 bound to the GTPase-binding domain of ACK. Nature 1999, 399, 384–388.
- Teo, M.; Tan, L.; Lim, L.; Manser, E. The tyrosine kinase ACK1 associates with clathrin-coated vesicles through a binding motif shared by arrestin and other adaptors. J. Biol. Chem. 2001, 276, 18392–18398.
- Consortium, T.U. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2022, 51, D523–D531.
- Hoehn, G.T.; Stokland, T.; Amin, S.; Ramírez, M.; Hawkins, A.L.; Griffin, C.A.; Small, D.; Civin, C.I. Tnk1: A novel intracellular tyrosine kinase gene isolated from human umbilical cord blood CD34+/Lin-/CD38- stem/progenitor cells. Oncogene 1996, 12, 903–913.
- Chan, T.Y.; Egbert, C.M.; Maxson, J.E.; Siddiqui, A.; Larsen, L.J.; Kohler, K.; Balasooriya, E.R.; Pennington, K.L.; Tsang, T.-M.; Frey, M.; et al. TNK1 is a ubiquitin-binding and 14-3-3-regulated kinase that can be targeted to block tumor growth. Nat. Commun. 2021, 12, 17.
- Hoare, K.; Hoare, S.; Smith, O.M.; Kalmaz, G.; Small, D.; May, W.S. Kos1, a nonreceptor tyrosine kinase that suppresses Ras signaling. Oncogene 2003, 22, 3562–3577.
- Nawarathnage, S.S.; Moody, J.; Bunn, D.; Towne, N.; Duokov, T. ELSAM accelerates crystallization of fused target proteins by stabilizing weak crystal contacts. Acta Crystallogr. Sect. A Found. Adv. 2021, 77, a46.
- Hoare, S.; Hoare, K.; Reinhard, M.K.; Lee, Y.J.; Oh, S.P.; May, W.S. Tnk1/Kos1 knockout mice develop spontaneous tumors. Cancer Res. 2008, 68, 8723–8732.
- Hopper, N.A.; Lee, J.H.; Sternberg, P.W. ARK-1 inhibits EGFR signaling in C. elegans. Mol. Cell 2000, 6, 65–75.
- Dereeper, A.; Guignon, V.; Blanc, G.; Audic, S.; Buffet, S.; Chevenet, F.; Dufayard, J.-F.; Guindon, S.; Lefort, V.; Lescot, M.; et al. Phylogeny.fr: Robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008, 36, W465–W469.
- Urena, J.M.; La Torre, A.; Martínez, A.; Lowenstein, E.; Franco, N.; Winsky-Sommerer, R.; Fontana, X.; Casaroli-Marano, R.; Ibáñez-Sabio, M.A.; Pascual, M.; et al. Expression, synaptic localization, and developmental regulation of Ack1/Pyk1, a cytoplasmic tyrosine kinase highly expressed in the developing and adult brain. J. Comp. Neurol. 2005, 490, 119–132.
- Grovdal, L.M.; Johannessen, L.E.; Rødland, M.S.; Madshus, I.H.; Stang, E. Dysregulation of Ack1 inhibits down-regulation of the EGF receptor. Exp. Cell Res. 2008, 314, 1292–1300.
- Yang, W.N.; Lo, C.G.; Dispenza, T.; Cerione, R.A. The Cdc42 target ACK2 directly interacts with clathrin and influences clathrin assembly. J. Biol. Chem. 2001, 276, 17468–17473.
- Burbelo, P.D.; Drechsel, D.; Hall, A. A conserved binding motif defines numerous candidate target proteins for both Cdc42 and Rac GTPases. J. Biol. Chem. 1995, 270, 29071–29074.
- Sem, K.P.; Zahedi, B.; Tan, I.; Deak, M.; Lim, L.; Harden, N. ACK family tyrosine kinase activity is a component of Dcdc42 signaling during dorsal closure in Drosophila melanogaster. Mol. Cell. Biol. 2002, 22, 3685–3697.
- Abdallah, A.M. Dissecting the Function of Ack Family Kinases in Drosophila through Understanding Their Interactions with Dock and Cdc42. Ph.D. Thesis, Purdue University, West Lafayetta, IN, USA, 2014.
- Prieto-Echague, V.; Miller, W.T. Regulation of ack-family nonreceptor tyrosine kinases. J. Signal Transduct. 2011, 2011, 742372.
- Shen, F.; Lin, Q.; Gu, Y.; Childress, C.; Yang, W. Activated Cdc42-associated kinase 1 is a component of EGF receptor signaling complex and regulates EGF receptor degradation. Mol. Biol. Cell 2007, 18, 732–742.
- Jose, A.M.; Kim, Y.A.; Leal-Ekman, S.; Hunter, C.P. Conserved tyrosine kinase promotes the import of silencing RNA into Caenorhabditis elegans cells. Proc. Natl. Acad. Sci. USA 2012, 109, 14520–14525.
- Linseman, D.A.; Heidenreich, K.A.; Fisher, S.K. Stimulation of M3 muscarinic receptors induces phosphorylation of the Cdc42 effector activated Cdc42Hs-associated kinase-1 via a Fyn tyrosine kinase signaling pathway. J. Biol. Chem. 2001, 276, 5622–5628.
- Pao-Chun, L.; Chan, P.M.; Chan, W.; Manser, E.D. Cytoplasmic ACK1 interaction with multiple receptor tyrosine kinases is mediated by Grb2: An analysis of ACK1 effects on Axl signaling. J. Biol. Chem. 2009, 284, 34954–34963.
- La Torre, A.; Masdeu, M.D.M.; Cotrufo, T.; Moubarak, R.S.; del Río, J.A.; Comella, J.X.; Soriano, E.; Ureña, J.M. A role for the tyrosine kinase ACK1 in neurotrophin signaling and neuronal extension and branching. Cell Death Dis. 2013, 4, e602.
- Lougheed, J.C.; Chen, R.-H.; Mak, P.; Stout, T.J. Crystal structures of the phosphorylated and unphosphorylated kinase domains of the Cdc42-associated tyrosine kinase ACK1. J. Biol. Chem. 2004, 279, 44039–44045.
- Howlin, J.; Rosenkvist, J.; Andersson, T. TNK2 preserves epidermal growth factor receptor expression on the cell surface and enhances migration and invasion of human breast cancer cells. Breast Cancer Res. 2008, 10, R36.
- Fox, M.; Crafter, C.; Owen, D. The non-receptor tyrosine kinase ACK: Regulatory mechanisms, signalling pathways and opportunities for attACKing cancer. Biochem. Soc. Trans. 2019, 47, 1715–1731.
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242.
- Chan, W.; Sit, S.T.; Manser, E. The Cdc42-associated kinase ACK1 is not autoinhibited but requires Src for activation. Biochem. J. 2011, 435, 355–364.
- Kiyono, M.; Kato, J.; Kataoka, T.; Kaziro, Y.; Satoh, T. Stimulation of Ras guanine nucleotide exchange activity of Ras-GRF1/CDC25(Mm) upon tyrosine phosphorylation by the Cdc42-regulated kinase ACK1. J. Biol. Chem. 2000, 275, 29788–29793.
- Syed, E.C.; Grima, L.L.; Magill, P.J.; Bogacz, R.; Brown, P.; Walton, M.E. Action initiation shapes mesolimbic dopamine encoding of future rewards. Nat. Neurosci. 2016, 19, 34–36.
- Wu, S.; Bellve, K.D.; Fogarty, K.E.; Melikian, H.E. Ack1 is a dopamine transporter endocytic brake that rescues a trafficking-dysregulated ADHD coding variant. Proc. Natl. Acad. Sci. USA 2015, 112, 15480–15485.
- Zhu, J.; Liu, Y.; Zhao, M.; Cao, K.; Ma, J.; Peng, S. Identification of downstream signaling cascades of ACK1 and prognostic classifiers in non-small cell lung cancer. Aging 2021, 13, 4482–4502.
- Zhao, X.; Lv, C.; Chen, S.; Zhi, F. A role for the non-receptor tyrosine kinase ACK1 in TNF-alpha-mediated apoptosis and proliferation in human intestinal epithelial caco-2 cells. Cell Biol. Int. 2018, 42, 1097–1105.
- Jing, L.; Zhang, X.; Liu, D.; Yang, Y.; Xiong, H.; Dong, G. ACK1 Contributes to the Pathogenesis of Inflammation and Autoimmunity by Promoting the Activation of TLR Signaling Pathways. Front. Immunol. 2022, 13, 864995.
- Thaker, Y.R.; Recino, A.; Raab, M.; Jabeen, A.; Wallberg, M.; Fernandez, N.; Rudd, C.E. Activated Cdc42-associated kinase 1 (ACK1) binds the sterile alpha motif (SAM) domain of the adaptor SLP-76 and phosphorylates proximal tyrosines. J. Biol. Chem. 2017, 292, 6281–6290.
- Shim, E.K.; Jung, S.H.; Lee, J.R. Role of two adaptor molecules SLP-76 and LAT in the PI3K signaling pathway in activated T cells. J. Immunol. 2011, 186, 2926–2935.
- Fang, N.; Motto, D.G.; Ross, S.E.; Koretzky, G.A. Tyrosines 113, 128, and 145 of SLP-76 are required for optimal augmentation of NFAT promoter activity. J. Immunol. 1996, 157, 3769–3773.
- Szklarczyk, D.; Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613.
- Mahajan, K.; Mahajan, N.P. Shepherding AKT and androgen receptor by Ack1 tyrosine kinase. J. Cell. Physiol. 2010, 224, 327–333.
- Mahajan, K.; Mahajan, N.P. ACK1/TNK2 tyrosine kinase: Molecular signaling and evolving role in cancers. Oncogene 2015, 34, 4162–4167.
- van der Horst, E.H.; Degenhardt, Y.Y.; Strelow, A.; Slavin, A.; Chinn, L.; Orf, J.; Rong, M.; Li, S.; See, L.-H.; Nguyen, K.Q.C.; et al. Metastatic properties and genomic amplification of the tyrosine kinase gene ACK1. Proc. Natl. Acad. Sci. USA 2005, 102, 15901–15906.
- Liu, X.; Wang, X.; Li, L.; Han, B. Research Progress of the Functional Role of ACK1 in Breast Cancer. BioMed Res. Int. 2019, 2019, 1018034.
- Kong, D.; Li, G.; Yang, Z.; Cheng, S.; Zhang, W.; Feng, L.; Zhang, K. Identification of an ACK1/TNK2-based prognostic signature for colon cancer to predict survival and inflammatory landscapes. BMC Cancer 2022, 22, 84.
- Ling, S.; He, Y.; Li, X.; Ma, Y.; Li, Y.; Kong, B.; Huang, P. Significant Gene Biomarker Tyrosine Kinase Non-receptor 2 Mediated Cell Proliferation and Invasion in Colon Cancer. Front. Genet. 2021, 12, 653657.
- Xu, S.H.; Huang, J.-Z.; Chen, M.; Zeng, M.; Zou, F.-Y.; Chen, D.; Yan, G.-R. Amplification of ACK1 promotes gastric tumorigenesis via ECD-dependent p53 ubiquitination degradation. Oncotarget 2017, 8, 12705–12716.
- Xu, S.H.; Huang, J.-Z.; Xu, M.-L.; Yu, G.; Yin, X.-F.; Chen, D.; Yan, G.-R. ACK1 promotes gastric cancer epithelial-mesenchymal transition and metastasis through AKT-POU2F1-ECD signalling. J. Pathol. 2015, 236, 175–185.
- Prieto-Echague, V.; Gucwa, A.; Craddock, B.P.; Brown, D.A.; Miller, W.T. Cancer-associated mutations activate the nonreceptor tyrosine kinase Ack1. J. Biol. Chem. 2010, 285, 10605–10615.
- Xie, B.; Zen, Q.; Wang, X.; He, X.; Xie, Y.; Zhang, Z.; Li, H. ACK1 promotes hepatocellular carcinoma progression via downregulating WWOX and activating AKT signaling. Int. J. Oncol. 2015, 46, 2057–2066.
- Mahajan, K.; Coppola, D.; Challa, S.; Fang, B.; Chen, Y.; Zhu, W.; Lopez, A.S.; Koomen, J.; Engelman, R.W.; Rivera, C.; et al. Ack1 mediated AKT/PKB tyrosine 176 phosphorylation regulates its activation. PLoS ONE 2010, 5, e9646.
- Lv, C.; Gu, H.; Zhao, X.; Huang, L.; Zhou, S.; Zhi, F. Involvement of Activated Cdc42 Kinase1 in Colitis and Colorectal Neoplasms. Med. Sci. Monit. 2016, 22, 4794–4802.
- Chen, Z.L.; Chang, S.; Li, B.-Z.; Zhou, F.; Tan, X.-G.; Shi, S.-S.; Feng, X.-L.; He, J. Correlation study of the overexpression of activated Cdc42-associated kinase 1 and the stage and prognosis of esophageal squamous cell carcinoma. Zhonghua Yi Xue Za Zhi 2011, 91, 166–170.
- Hu, F.; Liu, H.; Xie, X.; Mei, J.; Wang, M. Activated cdc42-associated kinase is up-regulated in non-small-cell lung cancer and necessary for FGFR-mediated AKT activation. Mol. Carcinog. 2016, 55, 853–863.
- Liu, Z.; Liu, Z.; Zhang, Y.; Li, Y.; Liu, B.; Zhang, K. miR-24 represses metastasis of human osteosarcoma cells by targeting Ack1 via AKT/MMPs pathway. Biochem. Biophys. Res. Commun. 2017, 486, 211–217.
- Maxson, J.E.; Abel, M.L.; Wang, J.; Deng, X.; Reckel, S.; Luty, S.B.; Sun, H.; Gorenstein, J.; Hughes, S.B.; Bottomly, D.; et al. Identification and Characterization of Tyrosine Kinase Nonreceptor 2 Mutations in Leukemia through Integration of Kinase Inhibitor Screening and Genomic Analysis. Cancer Res. 2016, 76, 127–138.
- Wu, X.; Saddiq, M.Z.; Renuse, S.; Kelkar, D.S.; Barbhuiya, M.A.; Rojas, P.L.; Stearns, V.; Gabrielson, E.; Malla, P.; Sukumae, S.; et al. The non-receptor tyrosine kinase TNK2/ACK1 is a novel therapeutic target in triple negative breast cancer. Oncotarget 2017, 8, 2971–2983.
- Wang, B.; Xu, T.; Liu, J.; Zang, S.; Gao, L.; Huang, A. Overexpression of activated Cdc42-associated kinase1 (Ack1) predicts tumor recurrence and poor survival in human hepatocellular carcinoma. Pathol. Res. Pract. 2014, 210, 787–792.
- Hitomi, Y.; Heinzen, E.L.; Donatello, S.; Dahl, H.-H.; Damiano, J.A.; BSc, J.M.M.; Berkovic, S.F.; Scheffer, I.E.; Legros, B.; Rai, M.; et al. Mutations in TNK2 in severe autosomal recessive infantile onset epilepsy. Ann. Neurol. 2013, 74, 496–501.
- Zhang, J.; Chen, T.; Mao, Q.; Lin, J.; Jia, J.; Li, S.; Xiong, W.; Lin, Y.; Liu, Z.; Liu, X.; et al. PDGFR-beta-activated ACK1-AKT signaling promotes glioma tumorigenesis. Int. J. Cancer 2015, 136, 1769–1780.
- Mahajan, K.; Coppola, D.; Rawal, B.; Chen, Y.A.; Lawrence, H.R.; Engelman, R.W.; Lawrence, N.J.; Mahajan, N.P. Ack1-mediated androgen receptor phosphorylation modulates radiation resistance in castration-resistant prostate cancer. J. Biol. Chem. 2012, 287, 22112–22122.
- Gelman, I.H. Androgen receptor activation in castration-recurrent prostate cancer: The role of Src-family and Ack1 tyrosine kinases. Int. J. Biol. Sci. 2014, 10, 620–626.
- Mahajan, K.; Malla, P.; Lawrence, H.R.; Chen, Z.; Kumar-Sinha, C.; Malik, R.; Shukla, S.; Kim, J.; Coppola, D.; Lawrence, N.J.; et al. ACK1/TNK2 Regulates Histone H4 Tyr88-phosphorylation and AR Gene Expression in Castration-Resistant Prostate Cancer. Cancer Cell 2017, 31, 790–803.e8.
- Mahajan, N.P.; Coppola, D.; Kim, J.; Lawrence, H.R.; Lawrence, N.J.; Mahajan, K. Blockade of ACK1/TNK2 To Squelch the Survival of Prostate Cancer Stem-like Cells. Sci. Rep. 2018, 8, 1954.
- Kim, E.H.; Cao, D.; Mahajan, N.P.; Andriole, G.L.; Mahajan, K. ACK1-AR and AR-HOXB13 signaling axes: Epigenetic regulation of lethal prostate cancers. NAR Cancer 2020, 2, zcaa018.
- Furlow, B. Tyrosine kinase ACK1 promotes prostate tumorigenesis. Lancet Oncol. 2006, 7, 17.
- Mahajan, N.P.; Liu, Y.; Majumder, S.; Warren, M.R.; Parker, C.E.; Mohler, J.L.; Earp, H.S.; Whang, Y.E. Activated Cdc42-associated kinase Ack1 promotes prostate cancer progression via androgen receptor tyrosine phosphorylation. Proc. Natl. Acad. Sci. USA 2007, 104, 8438–8443.
- Mahajan, K.; Challa, S.; Coppola, D.; Lawrence, H.; Luo, Y.; Gevariya, H.; Zhu, W.; Chen, Y.; Lawrence, N.J.; Mahajan, N.P. Effect of Ack1 tyrosine kinase inhibitor on ligand-independent androgen receptor activity. Prostate 2010, 70, 1274–1285.
- Ji, Z.; Njauw, C.-N.; Guhan, S.; Kumar, R.; Reddy, B.; Rajadurai, A.; Flaherty, K.; Tsao, H. Loss of ACK1 Upregulates EGFR and Mediates Resistance to BRAF Inhibition. J. Investig. Dermatol. 2021, 141, 1317–1324.e1.
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2018, 47, D941–D947.
- Wellcome Sanger Institute. COSMIC, the Catalogue of Somatic Mutations in Cancer. Available online: https://cancer.sanger.ac.uk/ (accessed on 6 January 2023).
- Park, S.J.; Yoon, B.-H.; Kim, S.-Y. GENT2: An updated gene expression database for normal and tumor tissues. BMC Med. Genom. 2019, 12 (Suppl. 5), 101.
- Maxson, J.E.; Gotlib, J.; Pollyea, D.A.; Fleischman, A.G.; Agarwal, A.; Eide, C.A.; Bottomly, D.; Wilmort, B.; McWeenay, S.K.; Tognon, C.E.; et al. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N. Engl. J. Med. 2013, 368, 1781–1790.
- Nonami, A.; Sattler, M.; Weisberg, E.; Liu, Q.; Zhang, J.; Patricelli, M.P.; Christie, A.L.; Saur, A.M.; Kohl, N.E.; Kung, A.L.; et al. Identification of novel therapeutic targets in acute leukemias with NRAS mutations using a pharmacologic approach. Blood 2015, 125, 3133–3143.
- Cho, H.; Shin, I.; Ju, E.; Choi, S.; Hur, W.; Kim, H.; Hong, E.; Kim, N.D.; Choi, H.G.; Gray, N.S.; et al. First SAR Study for Overriding NRAS Mutant Driven Acute Myeloid Leukemia. J. Med. Chem. 2018, 61, 8353–8373.
- Jenkins, C.; Luty, S.B.; Maxson, J.E.; Eide, C.A.; Abel, M.L.; Togiai, C.; Nemecek, E.R.; Bottomly, D.; McWeeney, S.K.; Wilmot, B.; et al. Synthetic lethality of TNK2 inhibition in PTPN11-mutant leukemia. Sci. Signal 2018, 11, eaao5617.
- Lawrence, H.R.; Mahajan, K.; Luo, Y.; Zhang, D.; Tindall, N.; Huseyin, M.; Gevariya, H.; Kazi†, S.; Ozcan, S.; Mahajan, N.P.; et al. Development of novel ACK1/TNK2 inhibitors using a fragment-based approach. J. Med. Chem. 2015, 58, 2746–2763.
- Wang, A.; Pei, J.; Shuai, W.; Lin, C.; Feng, L.; Wang, Y.; Lin, F.; Ouyang, L.; Wang, G. Small Molecules Targeting Activated Cdc42-Associated Kinase 1 (ACK1/TNK2) for the Treatment of Cancers. J. Med. Chem. 2021, 64, 16328–16348.
- Jin, M.; Wang, J.; Kleinberg, A.; Kadalbajoo, M.; Siu, K.W.; Cooke, A.; Bittner, M.A.; Yao, Y.; Thelemann, A.; Ji, Q.; et al. Discovery of potent, selective and orally bioavailable imidazo pyrazine derived ACK1 inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 979–984.
- Phatak, S.S.; Zhang, S. A novel multi-modal drug repurposing approach for identification of potent ACK1 inhibitors. Biocomputing 2013, 2013, 29–40.




