1. Introduction
Activated Cdc42-associated kinase (Ack1, TNK2) is a nonreceptor tyrosine kinase (NRTK) that belongs to the Ack family of kinases. The gene encoding Ack1, TNK2, is located on chromosome 3q29 in humans and is activated in response to growth factors and integrin-mediated signaling pathways [
1]. Activated Ack1 is recruited to receptor tyrosine kinases (RTKs) such as platelet-derived growth factor receptor (PDGFR) [
2], insulin receptor (IR), Mer [
3], and epidermal growth factor receptor (EGFR) [
4]. The Ack1 stands out among the other NRTKs because of its unusual domain composition. The Ack1 comprises an N-terminal sterile alpha motif (SAM) domain [
2], a catalytic domain, followed by a Src homology 3 (SH3) domain, and a Cdc42/Rac-interactive domain (CRIB) [
5]. The Ack1 is the only tyrosine kinase (TK) with a CRIB domain that specifically binds to the small GTPase Cdc42. The C-terminal region of Ack1 has other domains atypical for tyrosine kinases, such as a clathrin-binding (CB) motif [
6], a proline-rich carboxyl-tail, a region that shares high homology with Mig6 protein (MHR) [
2], and a ubiquitin association (UBA) domain.
2. Ack Family Kinases
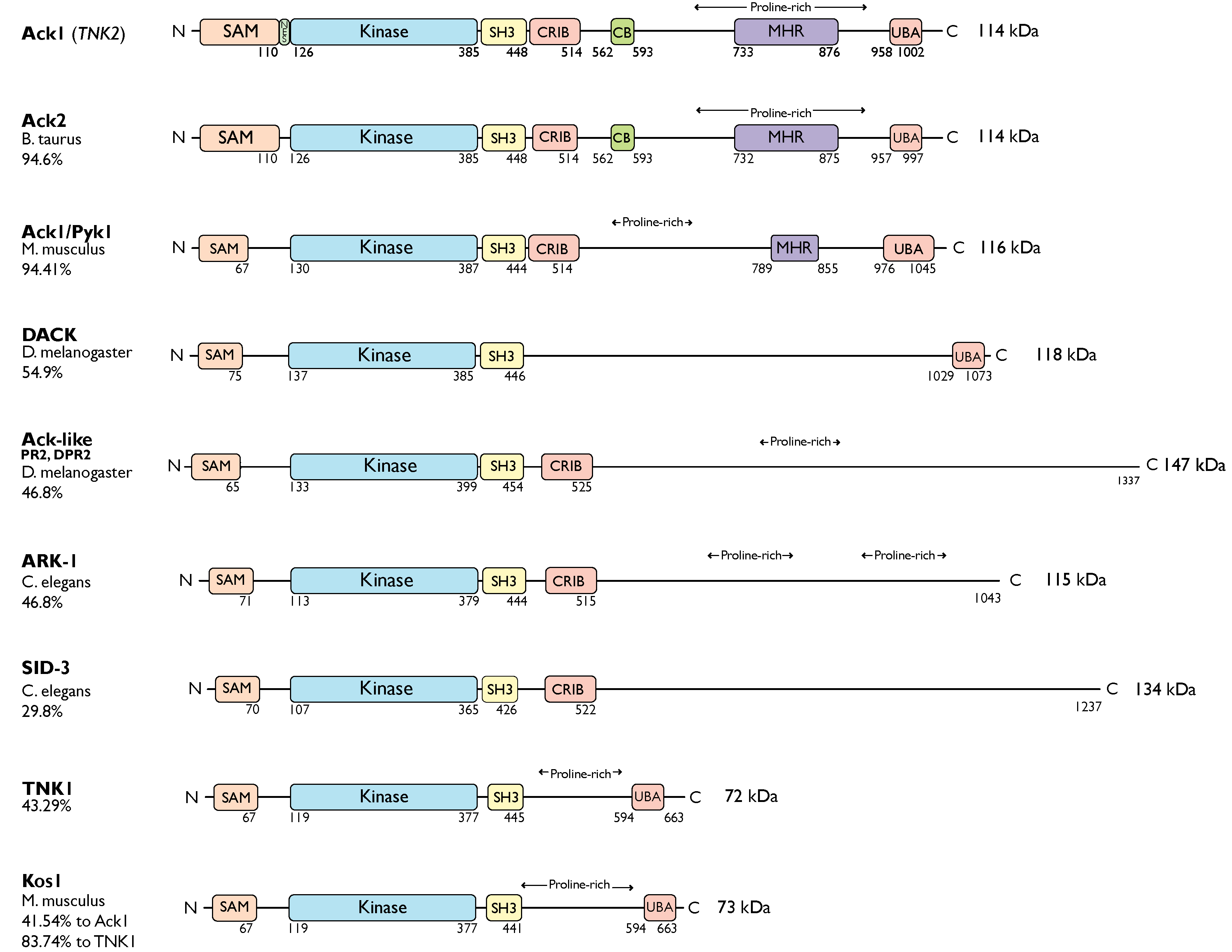
The Ack kinase family consists of Ack1 and TNK1 in humans, their homologs Ark-1 and sid-3 in Caenorhabditis elegans, Ack-like and DACK in Drosophila, bovine Ack2 and Ack1/Pyk1 and Kos1 in mice (Figure 1). All members of the Ack kinase family possess a SAM domain, which is uncharacteristic of nonreceptor tyrosine kinases, and an SH3 domain C-terminal to the catalytic domain. Notably, Acks are the only NRTKs known to have an SH3 domain at the C-terminus of their kinase domain.
Figure 1. Ack family kinases. Linear representation of Ack family members. The residue numbers below each domain depict the boundaries of the domains retrieved from the UniProtKB [
10]. SAM, sterile alpha motif; NES, nuclear export signal; CRIB, Cdc42, and Rac-interactive binding (CRIB) domain; CB, clathrin-binding motif; proline-rich region; MHR, Mig6 homology region; UBA, ubiquitin-associated domain. The sequence identity between each member and human Ack1 (or TNK1) is indicated to the left of the linear diagram as percentage identity. UniProtKB IDs used to depict the domain arrangements and to determine percent sequence identity are human Ack1, Q07912; bovine Ack2, Q17R13; mouse Ack1/Pyk1, O54967; fruit fly DACK, Q9VZI2; fruit fly Ack-like/DPR2/PR2, Q9I7F7; worm Ark-1, G5EBZ8; worm sid-3, Q10925; human TNK1, Q13470; and mouse TNK1/Kos1, Q99ML2.
The TNK1 is encoded by the human chromosome 17p13.1 [
11]. Similar to Ack1, TNK1 also contains a UBA domain with a high affinity for polyubiquitin [
12]. However, it lacks the CB, CRIB, and MHR domains. The murine homolog of TNK1, Kos1 [
13], is located in the middle region of chromosome 11 in mice, which corresponds to chromosome 17p13.1 in humans [
12,
14], and it is thought to be a splice variant of TNK1 [
13]. The Kos1 knockout mice develop spontaneous tumors, suggesting a role in cancer development [
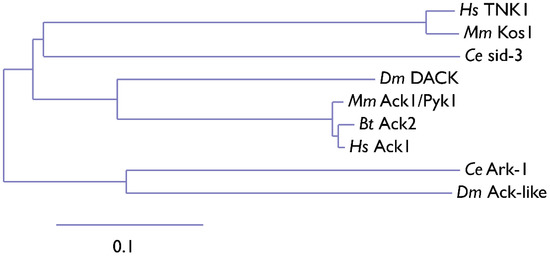
15]. Phylogenetic analysis of Ack kinase domains shows that the closest relative of TNK1 and Kos1 is
C. elegans sid-3. The Sid-3 kinase is more closely related to human Ack1 than to worm ARK-1, an Ack-related kinase (
Figure 2). Similar to its human homolog, Ark-1 downregulates let-23, the
C. elegans homolog of EGFR [
16]. Signaling events involving the downregulation of let-23 have been proposed to be mediated by sem-5, the
C. elegans homolog of Grb2.
Figure 2. Phylogenetic tree of the Ack1 family. Ce,
Caenorhabditis elegans (nematode); Dm,
Drosophila melanogaster (fruit fly); Bt,
Bos taurus (cow); Hs,
Homo sapiens (human); Mm,
Mus musculus (mouse). The scale indicates the rate of amino acid substitution per residue. The amino acid sequences of the kinase domains of Ack family proteins from
C. elegans (sid-3, Ark-1),
D. melanogaster (DACK, Ack-like),
B. taurus (Ack2),
M. musculus (Ack1, Kos1), and
H. sapiens (Ack1, TNK1) were used for phylogenetic analysis. The phylogenetic tree was manually compiled using MAFFT, Gblocks, PhyML, and TreeDyn at Phylogeny.fr [
17].
The murine homolog of Ack1 was identified as a Grb2-binding protein and was initially named proline-rich tyrosine kinase Pyk1 [
18]. Similar to its human counterpart, mouse Ack1 is highly expressed in the developing and adult brain [
18]. The expression of Ack1 is upregulated with increased neural activity, suggesting a role for Ack1 in mouse synaptic function, plasticity, and brain development.
Similarly, as a splice variant of human Ack1, the bovine homolog of Ack1, Ack2 [
19], retains the CRIB domain and CB motif following its SH3 domain. The Ack2 shares ~95% sequence identity with Ack1. Overexpression of Ack2 in NIH3T3 cells severely impairs cell growth [
20]. In fruit flies (
D. melanogaster), DACK and Ack-like (DPR2, PR2) are two Ack family kinases that are also effectors of the small GTPase Cdc42 [
21,
22,
23]. Compared to Ack-like, DACK is more closely related to Ack1.
The worm ARK-1 and SID-3, and fly DACK and Ack-like, only have the CRIB domain following the SH3 domain and lack the other domains present in Ack1. The ARK-1 acts as a negative regulator of EGFR signaling in
C. elegans [
24] paralleling its human counterpart. While ARK-1 suppresses cell division in embryos [
16,
25], SID-3 modulates the efficient import of dsRNA in worms [
26]. Human Ack1 is known to associate with endocytic vesicles, which are also involved in dsRNA transport.
Overall, the Ack family kinases regulate many important biological activities, including cell adhesion, apoptosis, tumor cell survival, proliferation, T-cell activation, DNA repair, synapse formation, and membrane trafficking.
3. Mechanism of Action
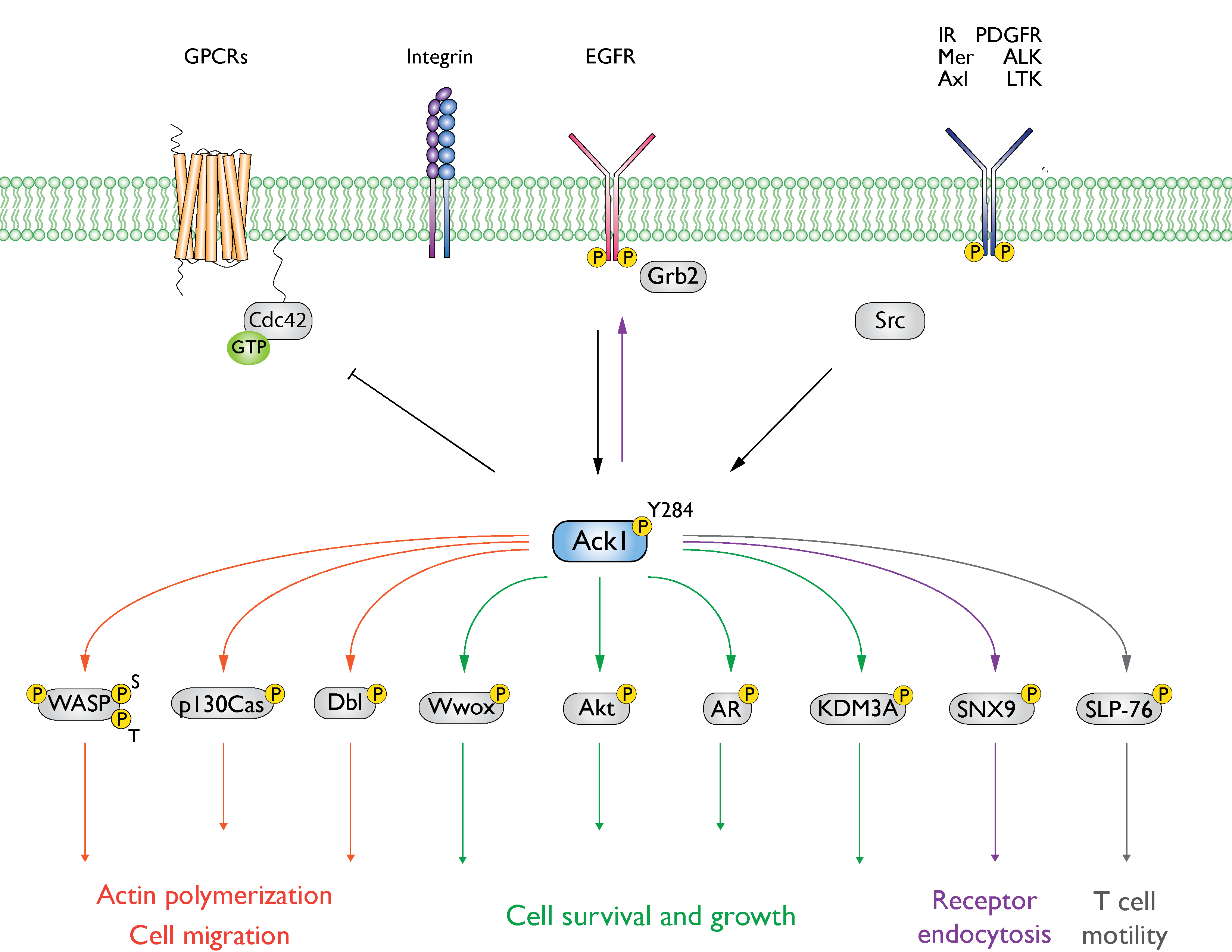
The Ack1 is activated downstream of a broad range of receptors such as integrins, muscarinic M3 receptors, PDGFR, Axl, IR, Mer, Trk, and EGFR, as summarized in
Figure 3 [
2,
27,
28,
29]. Similar to the Src family kinases, the activation of Ack1 involves autophosphorylation (at Y284). In vitro studies using purified Ack1 have demonstrated that autophosphorylation moderately enhances its activity and that phosphorylation is regulated by dimerization via the SAM domain. Phosphorylation of the activation loop does not have a significant effect on the substrate or ATP binding [
30].
Figure 3. Summary of Ack1-mediated signaling events. Ack1 is activated in response to growth factors, G-protein coupled receptors (GPCRs), and integrin-mediated cell adhesion and is recruited to activated RTKs such as platelet-derived growth factor receptor (PDGFR), insulin receptor (IR), Mer, and epidermal growth factor receptor (EGFR) [
2,
3,
25,
28,
31]. In cancer cells, Ack1 phosphorylates the tumor suppressor Wwox [
3] and facilitates uncontrolled activation of pro-proliferative signals (Akt and AR). A detailed discussion of the signaling pathways can be found in the study by Fox et al. [
7].
The Ack1 adopts a Src-like active conformation in its phosphorylated (Protein Data Bank [
32], PDB: 1U4D) and unphosphorylated states (PDB: 1U46). The activation loop of both structures is well-ordered, with R247 forming hydrogen bonds with the backbone carbonyl of P278 and N281, suggesting that both structures are in the active conformation. Typically, the activation loop in NRTKs adopts an autoinhibitory conformation in the unphosphorylated state. However, in Ack1, M274 of the activation loop extends outside the loop to position the hydrogen interactions between the loop and C-lobe, facilitating the stabilization of the unphosphorylated activation loop. Most TKs have a small residue at this position; a rather large hydrophobic M274, which is conserved throughout the Ack family, stabilizes the unphosphorylated state of the activation loop in Ack1.
The catalytic activity of Ack1 increases 20- to 30-fold by a head-to-head symmetric dimerization. This dimerization-mediated activation model is further supported by evidence that phosphorylation has a minimal effect on activity [
33]. Notably, Ack1 does not undergo significant structural changes upon binding to nucleotides. The structure of Ack1 bound to the nucleotide analog, AMP-PCP (PDB: 1U54), is comparable to nucleotide-free Ack1 in both phosphorylated (PDB: 1U4D) and unphosphorylated form (PDB: 1U46) with an RMSD of 0.5 Å overall Cα pairs [
30].
4. Emerging Roles in Signaling Pathways
Other than its established proto-oncogenic roles, recent work has provided evidence for the involvement of Ack1 in (1) neural signaling and (2) immune signaling pathways.
4.1. Role of Ack1 in Neural Signaling Pathways
The Ack1 is expressed ubiquitously in all tissues in humans, with especially high expression in neurons of the developing and adult brain, at both the mRNA and protein levels. In neurons, Ack1 plays a role in neurotrophin signaling cascades [
29], which are mainly mediated by nerve growth factors, neurotrophins, and Trk receptors that regulate neuronal development. The Ack1 interacts with Trk receptors and is tyrosine phosphorylated in response to neurotrophins. The Ack1 overexpression in primary neuronal cells induces neurite outgrowth and promotes branching in neurotrophin-treated neuronal cells. In addition, Ack1 is involved in the Ras-GRF1 signaling cascade in neuronal cells and mediates calcium influx in the brain [
34].
Another involvement of Ack1 in neural signaling pathways is through dopamine transporters. Dopamine is a vital regulator of physiological and behavioral pathways such as voluntary motor movement and reward [
35]. The dopamine transporter protein DAT controls dopamine neurotransmission and facilitates its clearance from synapses, resulting in the termination of dopamine signaling. The DAT is regulated and internalized via clathrin-mediated endocytosis. A key modulator of DAT endocytosis is the activation of protein kinase C (PKC), which promotes the internalization of DAT. The Ack1 works antagonistically to PKC and prevents the endocytosis of DAT, sustaining DAT in the plasma membrane. This effect is overcome by PKC activation, which inactivates Ack1 [
36]. A reduction in DAT density in the plasma membrane is implicated in Parkinson’s disease, which emphasizes the importance of endocytic regulation of DAT. Further research is needed to address Ack1’s connection to dopamine signaling and to identify the upstream and downstream modulators involved.
4.2. Role of Ack1 in Immune Signaling Pathways
The second emerging area of research involving Ack1 is in immune cell signaling cascades. The Ack1 expression is associated with immune cell infiltration and immunomodulators, which are pronounced in cancer immunity. In lung cancer, Ack1 mRNA levels are inversely correlated with the infiltration levels of B cells, CD8+ T-cells, CD4+ T-cells, macrophages, neutrophils, and dendritic cells [
37]. In addition to immune cell infiltration, dysregulation of Ack1 is linked to abnormal apoptotic activity [
38], which is crucial for the elimination of cytotoxins and self-antigens.
Silencing of the TNK2 gene, which encodes Ack1, leads to the activation of several immune-related signaling pathways, including T-cell receptor (TCR), chemokine, JAK-STAT, and Toll-like receptor (TLR) signaling pathways. Specifically, Ack1 regulates the activation of TLR signaling pathways such as TLR4, TLR7, and TLR9, which mediate inflammation and autoimmunity by controlling macrophages and dendritic cells [
39]. In macrophages, Ack1 overexpression promotes the activation of the TLR4, TLR7, and TLR9 pathways, while Ack1 knockdown inhibits their activation. Pharmacological inhibition of Ack1 activity reduces TLR-mediated activation of macrophages, relieving endotoxic shock and lupus symptoms in mouse models [
39]. These mouse models are the first to document the role of Ack1 in inflammation and autoimmunity, suggesting a new avenue where Ack1 inhibition could serve as a therapeutic.
Furthermore, Ack1 mediates early T-cell activation events in a process involving the SAM domain of Ack1. The Ack1 interacts with SLP-76, a protein known to play a crucial role in signal transmission to the transcriptional machinery [
40]. Both SLP-76 and Ack1 interact through a SAM–SAM interaction where the SAM domain of Ack1 binds to the SAM domain of SLP-76. The Ack1 phosphorylates SLP-76 at Y113, Y128, and Y145, and phosphorylation of these residues is required for the TCR activation [
41,
42].
5. Ack1 Substrates
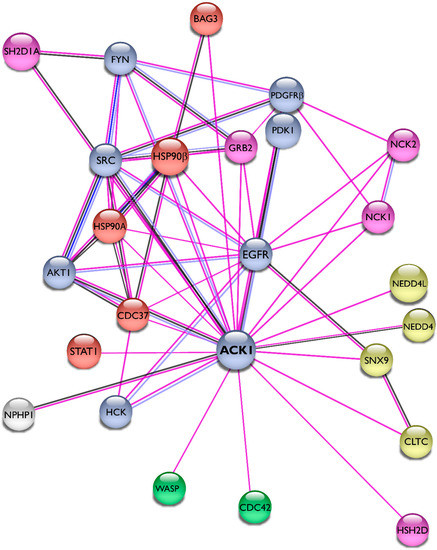
The researchers summarized the protein–protein interactions of Ack1 as a STRING network [
43] and included only experimentally validated interactions representing both physical and functional associations in
Figure 4. The interaction network of Ack1 includes protein kinases (EGFR, PDGFR, Akt, Src, Fyn, Hck, PDK1), adaptor molecules (Grb2, HSH2, NCKs), Heat shock protein 90 and its co-chaperones (HSP90, Cdc37, Bag3), proteins regulating actin dynamics (Cdc42, WASP), and proteins involved in vesicle dynamics (CLTC, SNX9, NEDD4). More details on interacting proteins and substrates of Ack1 are described in the review by Mahajan and Mahajan [
44].
Figure 4. Interaction network of Ack1. Using the STRING database [
43], the researchers mapped the Ack1-interacting proteins from Mahajan [
44]. Lines are color-coded according to the nature of the association, which is based on experimental evidence (pink), co-expression (dark blue), or protein homology (gray). Nodes are color-coded by function (protein kinases, light blue; adaptor proteins, pink; heat shock protein complex, orange) or signaling networks (actin polymerization, green; receptor endocytosis, yellow).
6. Ack1 Involvement in Disease
The role of Ack1 in different diseases has been extensively studied and reviewed by Mahajan [
8] and Owen [
7]. Generally, Ack1 involvement in cancer and other diseases occurs at one of three different levels: genomic (amplification and mutations), transcriptional (overexpression), and post-transcriptional (downregulation of tumor suppressors).
6.1. Genomic Level
The gene encoding Ack1, TNK2, is amplified in human cancers, including prostate, breast, esophageal, lung, ovarian, and pancreatic cancers, with the highest gene amplification being 9% in ovarian and 14% in lung primary tumors [
45]. The Ack1 activity is elevated in multiple cancer cell lines, including breast [
46], colon [
47,
48], prostate [
3], gastric [
49,
50], ovarian [
51], and liver cancers [
52]. In gastric cancer, the DNA copy numbers of TNK2 were significantly higher compared to those of normal gastric tissues [
49,
50]. Existing data shows that Ack1 is amplified or mutated in breast, ovarian [
53], colorectal cancers [
54], esophageal squamous cell carcinoma [
55], non-small-cell lung cancer [
56], osteosarcoma [
57], and chronic myelomonocytic leukemia [
58].
6.2. Transcriptional Level
Overexpression of TNK2 has been identified in 42% of aggressive lung tumors, with elevated RNA levels ranging from 6- to 35-fold [
38]. In metastatic hormone-refractory prostate tumors, overexpression is more prevalent, with 77% of the tissues exhibiting elevated RNA levels (5- to > 100-fold). In triple-negative breast cancer cell lines, Ack1 expression correlates with high proliferation, invasion, and colony-forming abilities [
59]. Overexpression of Ack1 also promotes hepatocellular carcinoma progression [
52], which is associated with tumor recurrence and poor survival [
60].
In addition to cancer, the transcriptional dysregulation of Ack1 has been implicated in several neural disorders. For example, overexpression of Ack1/EGFR has been linked to epilepsy [
61]. A homozygous missense variant of Ack1, V638M, has been documented in a family with infantile-onset autosomal recessive epilepsy and intellectual disability. The variant resulted in overexpression of Ack1 owing to its improper degradation. Further research is needed to fully understand gain-of-function mutations in Ack1 and their implications for perturbed signaling in neural diseases.
6.3. Post-Transcriptional Level
Another mechanism by which Ack1 is involved in cancer progression is by negatively regulating tumor suppressors such as Wwox and positively regulating pro-survival signaling pathways (reviewed in [
31]). Through Akt phosphorylation and activation of the PI3K pathway, Ack1 regulates the expression of 147 different proteins associated with metastasis and epithelial-mesenchymal transition (EMT) in gastric cancer [
50]. The activation of Akt is also implicated in glioblastoma multiforme through the elevation of Ack1 phosphorylation and increased PDGFR signaling [
62].
A novel role of Ack1 as an epigenetic regulator has been reported in castration-resistant prostate cancer (CRPC). The Ack1 phosphorylates histones (H4) upstream of the androgen receptor (AR) transcription start site, promoting its expression in the absence of androgen. Consequently, through epigenetic regulation and tyrosine phosphorylation, Ack1 is a key player in dysregulated signaling events in androgen-independent prostate cancer [
63,
64,
65,
66,
67].
7. Ack1-Mediated Drug Resistance
Several lines of evidence have demonstrated Ack1-mediated drug resistance in hormone-dependent cancers. In breast cancer, Ack1 drives the expression of the HOXA oncogene, making cells resistant to tamoxifen therapy [
4]. In HER2 overexpressing tumors, Ack1 is activated downstream of HER2 and acts as an indirect epigenetic regulator. The Ack1 phosphorylates the histone demethylase KDM3A, which in turn promotes estrogen receptor (ER)-driven transcription of HOXA in the absence of estrogen. In this manner, Ack1-driven transcription of HOXA, a critical mediator of breast cancer progression, overrides treatment with the ER antagonist tamoxifen.
The Ack1 serves as a prognostic marker in numerous types of cancer, and its overexpression or hyperphosphorylation tends to correlate with poor prognosis. As reviewed by Mahajan and Mahajan [
31], Ack1 Y284 is a biomarker for prostate cancer disease progression and negatively correlates with survival [
3,
63,
65,
68,
69,
70].
In contrast to its hyperactivation or overexpression, Ack1 is downregulated in vemurafenib-resistant melanoma with activated EGFR [
71]. Downregulation of Ack1 in these cells causes a decrease in EGFR degradation, as evidenced by in vitro and in vivo melanoma models. This presents another post-transcriptional mechanism through which Ack1 contributes to drug resistance.
8. Domain Localization of Cancer-Associated Mutations
To date, 863 different somatic mutations have been documented in the Catalogue of Somatic Mutations in Cancer (COSMIC) [
72,
73].
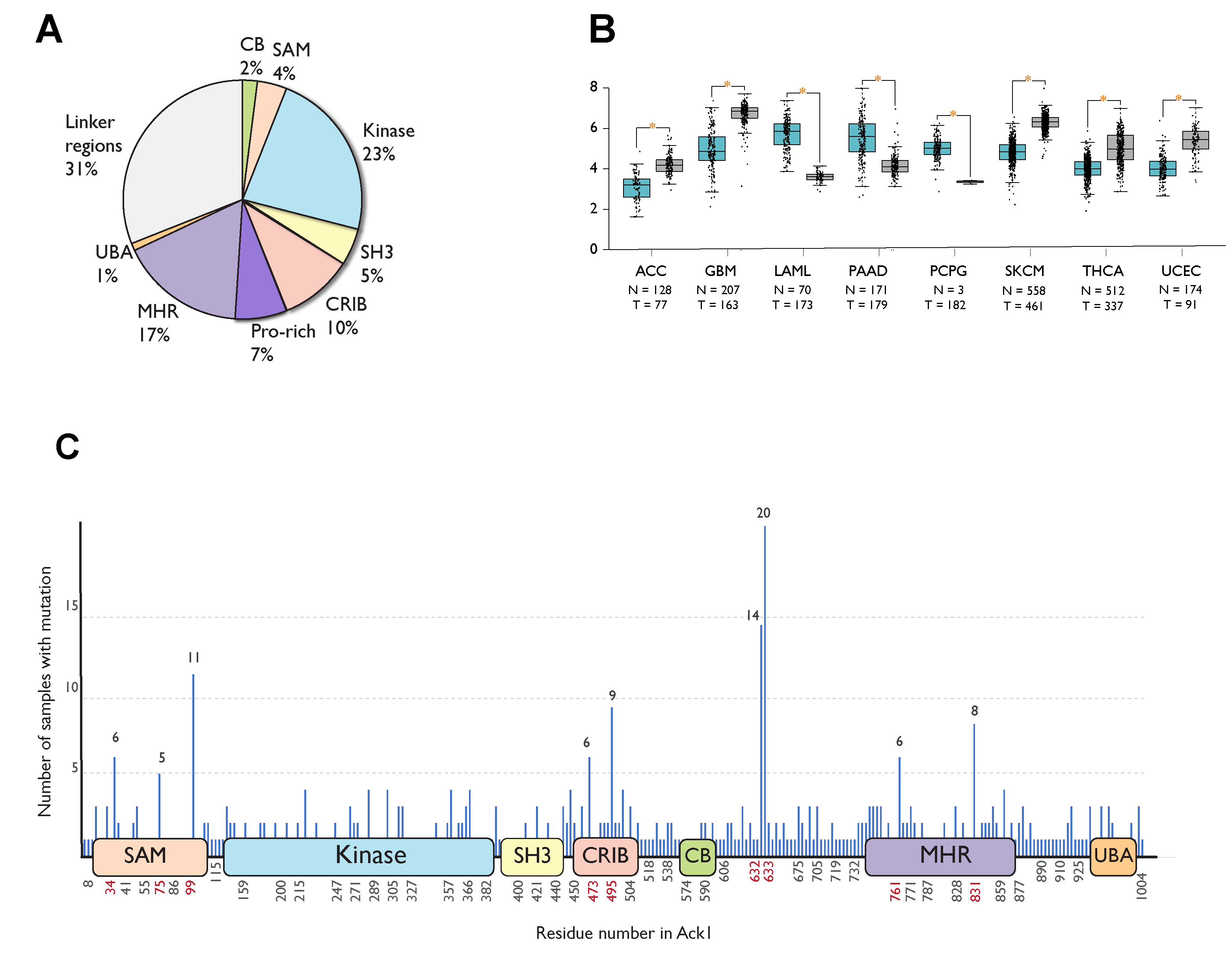
Figure 5A summarizes the distribution of these mutations across the domains of Ack1. Most mutations occur in the linker regions (31%), the kinase domain (23%), followed by the MHR (17%).
Figure 5. Cancer-associated mutations in Ack1 in the COSMIC database. (A) Distribution of nonsynonymous mutations. SAM, sterile alpha motif; CRIB, Cdc42, and Rac-interactive binding (CRIB) domain; CB, clathrin-binding motif; proline-rich region; MHR, Mig6 homology region; UBA, ubiquitin-associated domain (B) Ack1 expression in normal (blue) vs. tumor (gray) tissues. ACC: adrenocortical carcinoma, GBM: glioblastoma multiforme, LAML: acute myeloid leukemia, PAAD: pancreatic adenocarcinoma, PCPG: pheochromocytoma, SKCM: skin cutaneous melanoma, THCA: thyroid carcinoma, UCEC: uterine corpus endometrial carcinoma. (C) Locations of cancer-associated mutations outlined in the linear structure of Ack1. SAM, sterile alpha motif; NES, nuclear export signal; CRIB, Cdc42, and Rac-interactive binding (CRIB) domain; clathrin-binding motif; proline-rich region; MHR, Mig6 homology region; UBA, ubiquitin-associated domain. Each horizontal line represents a mutation, with the number of mutations positively correlated with the length depicted on the y-axis. The x-axis shows the location of the mutations. Highly mutated residues are shown in red. Note that the numbering convention used in COSMIC is based on an isoform different from the canonical one (UniProtKB: A0A5F9ZHL4) and that the figure uses the isoform 1 (UniProtKB: Q07912) convention.
Similar to COSMIC, the Gene Expression Database of Normal and Tumor Tissues 2 (GENT2) compiles gene expression patterns across different normal and tumor tissues from public databases [
74]. As opposed to statistically significant upregulation in adrenocortical carcinoma, glioblastoma, skin cutaneous melanoma, thyroid, and endometrial cancer, TNK2 expression is downregulated in acute myeloid leukemia (AML), pancreatic cancer, and pheochromocytoma compared to the corresponding healthy tissues (
Figure 5B).
A significant portion (67 of 508) of cancer-associated substitution or deletion mutations in Ack1 is localized to six different residues: R34, R99, D495, P633, P632, and Q831. The mutations in residues P632 and P633 are truncating frameshift mutations that remove the C-terminal domains MHR and UBA, while R34 and R99 are located in the SAM domain (Figure 5C).
9. Ack1 Drug Development Efforts
The association of Ack1 with the human disease has driven significant efforts toward the development of Ack1 inhibitors. The Ack1 was found to modulate sensitivity to tyrosine kinase inhibitors downstream of mutant CSF3R in patient-derived cell models of chronic neutrophilic leukemia and atypical chronic myeloid leukemia, making it a possible candidate for therapeutic intervention [
75]. Indeed, Ack1 is an important therapeutic target for acute myeloid leukemia (AML) with constitutively active NRAS mutations [
76,
77] and in chronic myelomonocytic leukemia since these mutations are sensitive to inhibitors of Ack1 [
58,
78]. In juvenile myelomonocytic leukemia (JMML) and AML cells, Ack1 activates PTPN11, and PTPN11-mutant JMML and AML cells are sensitive to Ack1 inhibition [
78].
An enantiomer of piperazine-substituted chloropyrimidine, (R)-9b, has been identified as a potent inhibitor of Ack1 in vitro (IC
50 = 56 nM,
33P HotSpot assay) and in vivo (IC
50 < 2 μM, human cancer cell lines) and is stable in human plasma (half-life > 6h) [
79]. In addition to (R)-9b, other potential inhibitors have been identified from existing clinical drugs [
9,
79,
80]. Dasatinib is also a potent inhibitor of Ack1, which inhibits Ack1 with an IC
50 of 1nM [
81].
This entry is adapted from the peer-reviewed paper 10.3390/cells12060900