Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Michail Karypidis | -- | 2285 | 2023-04-15 10:14:42 | | | |
| 2 | Catherine Yang | Meta information modification | 2285 | 2023-04-17 02:48:21 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Karypidis, M.; Karanikas, E.; Papadaki, A.; Andriotis, E.G. Synthetic Organic Antimicrobial Agents. Encyclopedia. Available online: https://encyclopedia.pub/entry/43082 (accessed on 01 March 2026).
Karypidis M, Karanikas E, Papadaki A, Andriotis EG. Synthetic Organic Antimicrobial Agents. Encyclopedia. Available at: https://encyclopedia.pub/entry/43082. Accessed March 01, 2026.
Karypidis, Michail, Evangelos Karanikas, Aikaterini Papadaki, Eleftherios G. Andriotis. "Synthetic Organic Antimicrobial Agents" Encyclopedia, https://encyclopedia.pub/entry/43082 (accessed March 01, 2026).
Karypidis, M., Karanikas, E., Papadaki, A., & Andriotis, E.G. (2023, April 15). Synthetic Organic Antimicrobial Agents. In Encyclopedia. https://encyclopedia.pub/entry/43082
Karypidis, Michail, et al. "Synthetic Organic Antimicrobial Agents." Encyclopedia. Web. 15 April, 2023.
Copy Citation
Synthetic organic antimicrobial agents are organic compounds and polymers that exhibit antimicrobial activity, which is self-activated through the aforementioned mode of action. Their chemical structure is critical for their categorisation. Recently, a huge number of antimicrobial polymers were synthesized. These species could be quaternary ammonium compounds (QACs), halogen-containing compounds (molecules containing fluorine or chlorine, N-halamines or triclosan), guanidine-containing polymers (polyhexamethylene vinyguanide), polymers containing phospho- and sulpho-derivatives, polymers of phenol and benzoic acid derivatives, nitro compounds, urea, amines, formaldehyde, organometallic polymers and others.
N-halamine
silver nanoparticles AgNPs
copper nanoparticles CNPs
1. Quaternary Ammonium Compounds (QACs)
Quaternary ammonium compounds (QACs) are cationic surface active agents known as cationic surfactants, which bear a positive charge on the nitrogen (N) atom and typically adsorb to the surface of an anionic fibre through ionic interaction [1][2][3]. They consist of a nitrogen (N) atom attached to four different moieties through a covalent bond. QACs refer to a subgroup of linear alkylammonium compounds, which consist of a hydrophobic alkyl chain and a hydrophilic part. In textiles, compounds containing long alkyl chains (12–18 carbon atoms) are mainly used for cellulosic substrates, polyester, nylon and wool [1][2][4][5]. The general formula of QAC is N+R1R2R3R4X−, where R can be a hydrogen atom, a plain alkyl group or an alkyl group substituted with other functional groups, and X represents an anion; see Figure 1. They are commonly used in textiles, and they had an essential role as biocides for many years, being characterised as effective antiseptic and disinfectant agents [6]. Generally, long-chain QACs with 8–18 carbon atoms possess good germicidal activity. Important representatives of this class are benzalkonium chloride, stearalkonium chloride and cetrimonium chloride [7]. These compounds can react with both Gram-positive and Gram-negative bacteria, fungi and certain types of viruses [1]. The antimicrobial activity of QACs depends on several factors such as the length of the alkyl chain, the presence of the halogenated group and the number of cationic ammonium groups in the molecule. The antimicrobial action starts from electrostatic interactions between the positive charge of the N+ atom and the microbe’s negatively charged cell membrane, creating surfactant–microbe complexes interrupting its essential functions and protein activity, as described in the antimicrobial action section [6].
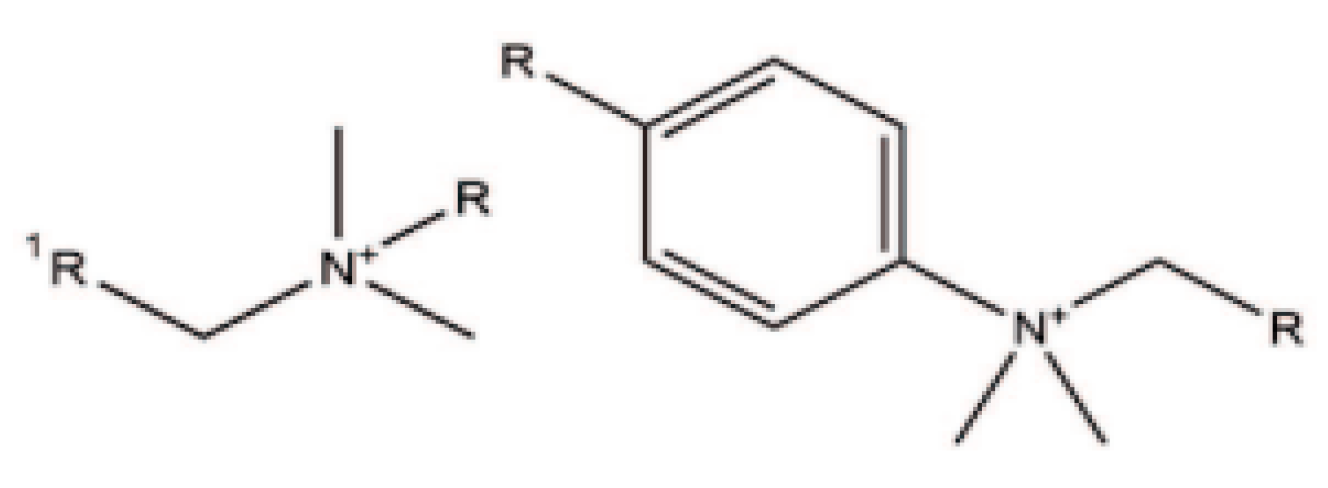
Figure 1. General chemical structure of quaternary ammonium compounds (QACs).
Carbon atoms with alkyl chains between 11 and 16 have been widely used as disinfectants; these compounds exert a positive charge on the N atom, which in reaction with the bacteria causes various adverse effects, resulting in microbial death [8][9]. When the length of the alkyl chain contains between 12–14 carbon atoms, optimal antibacterial activity against Gram-positive bacteria is achieved, while the alkyl group with 14–16 carbon atoms is superior in the attack against Gram-negative bacteria [7]. A type of QAC frequently applied to apparel textiles is 3-(trihydroxysilyl) propyldimethyl-octadecyl ammonium chloride, where a silane group is bonded to the long non-polar chain and the positively charged ammonium group and is often referred to as Si-QAC [4].
QACs have many favourable antimicrobial properties; however, they have no reactive functional groups in their structure to create a chemical bond with the fibres. This causes a gradual detachment from the textile due to the lack of physical bonding, called leaching. As a consequence, a fast concentration decrease in the QACs in the textile is observed. New studies report the synthesis of polymerisable QACs [1][6]. This is achieved by the incorporation of acrylate or methacrylate groups in their structure which is capable of forming permanent bonds, which are known as non-leaching QACs’ biocidal. The QACs monomers are named surfactant monomers or “surfmers”. The “surfmers” can polymerise into a bulk polymer network. They exhibit a polycationic chemical structure under the appropriate conditions, which include groups chemically side-bonded to the main polyacrylate chain. The outcome is that the QAC groups can function as a biological barrier and kill microorganisms when they come into contact. The formation of the polymer network on the surface of the fibres improves the coating’s wash fastness and durability of antimicrobial agents in general [6].
Sol–gel technology has also been used in antimicrobial textiles, where QACs regulate textile fibre behaviour. A nanocomposite polymer network with an organic–inorganic hybrid structure is formatted by this method. Colloidal solutions (sols) have been formulated for this purpose by incorporating mixtures of tetraalkoxysilane (Si(OR)4) with different structures of QACs or organic–inorganic hybrids, including alkyl- trialkoxysilanes (Rx-Si(OR)3). The increased durability and wash fastness on the finished fibres are provided by the formation of covalent bonds between sol–gel –SiOH groups and –OH groups of the fibres [6].
Novel cationic antimicrobial dyes are obtained by attaching QACs to the chromophore structure. In this manner, one or two QAC groups of different structures are in the molecule of mono-, diazo and anthraquinone dyes. Antimicrobial activity increases with the number of chemical substitutions and QAC groups. Moreover, the chain length of the hydrocarbons of the QACs’ component in the dye structure also promotes antimicrobial activity [6]. Consequently, when used on acrylic fibres, cationic anthraquinone dyes bear a double role; firstly, they confer colouration and simultaneously exert an antimicrobial function [10]. Similarly, n anthraquinone cationic reactive dye has been synthesised to exhibit antimicrobial characteristics to improve the wash fastness of the antimicrobial when applying dye on the cellulosic substrate [11] where high exhaustion and fixation values were recorded, even if applied without the electrolyte addition in the dyebath. However, repeated laundering seems to decrease the antimicrobial activity of both these dyes.
QAC antimicrobial agents are used on both natural fibres, such as cotton and wool, as well as man-made fibres, such as polyester and polyamide [1][12]. The QAC antimicrobial activity has been tested in the above substrates, where the concentration of 10–100 mg/L presented good reproducibility, as well as adequate wash fastness [13]. Due to its high solubility in water, its usage as an antimicrobial agent in textile finishes is, however, limited [14].
2. The N-Halamines
N-halamines are heterocyclic organic compounds bearing at least one or more nitrogen–halogen (N–X) covalent bonds, as shown in Figure 2. They are typically formed by the halogenation of amine, amide or imide groups, which is responsible for the stability of the structure and shows a controlled release of free active halogen species into the environment [6][15]. Chlorine is the most frequently used halogen in this category, but the activity of other halogens, such as bromine and iodine, is not unusual. N-halamines are environmentally friendlier and healthier, with enhanced antimicrobial efficacy against a broad spectrum of microorganisms [7]. While the bonded N–H, formed by a substitution reaction, exerts no antimicrobial properties, the further exposure of the reagent to dilute sodium hypochlorite develops its antimicrobial activity [6]. The last takes place with the release of chlorine by its electrophilic substitution with H in the N–Cl bond and is effective against a vast range of bacteria, fungi and viruses. The reaction occurs once water is present, allowing chlorine free-cations to be released, thus allowing them to bind to the acceptor regions of the bacteria. As a consequence, the enzymes and metabolism of the microorganism are hindered, leading to its gradual deterioration [13]. The application procedure composes the pad-dry method followed by exposure to chlorine bleach for the formation of antimicrobial cotton fabric. The chlorinated substrate exhibits strong antimicrobial properties against Gram-positive and Gram-negative pathogens. The chlorinated fabric can be recharged to the extent of 85% after being stored for fifteen days. This proves the strong efficacy of N-halamine compounds as antibacterial agents for medical textile finishes [16][17].
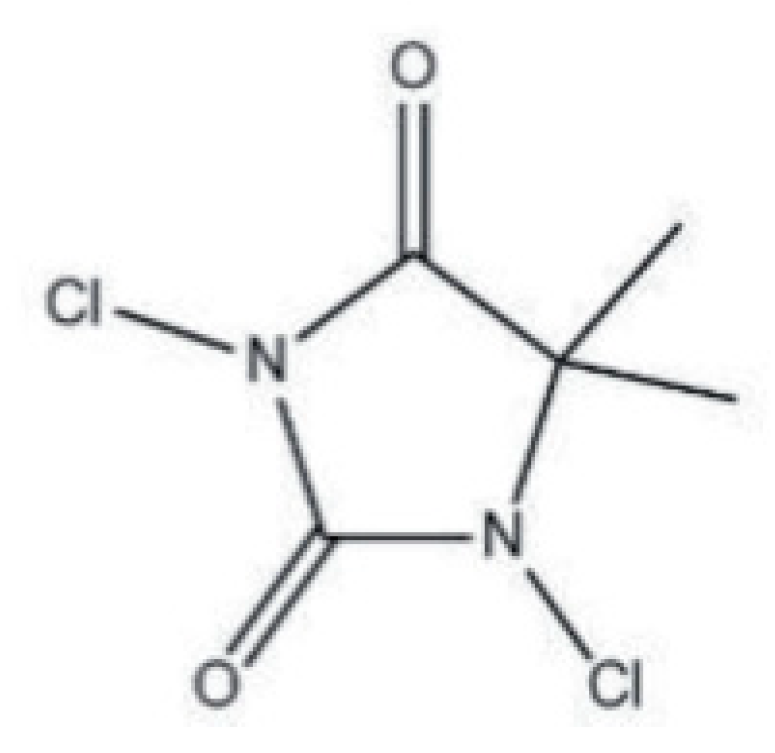
Figure 2. Molecular structure of N-halamines.
A wide range of textile surfaces, such as cellulose, wool, cotton, polyamide and polyester fibres, can be treated by the above process with N-amines [1][6][12]. The effectiveness and durability of the antimicrobial finishing can be further enhanced by the synthesis of N-halamide monomers with the incorporation of a reactive vinyl group.
A variation to the above reagent is the N-halamide monomer, which possesses enhanced durability and antimicrobial action. The molecule is obtained by the addition of the vinyl reactive group that, under the proper conditions, can polymerise cellulose fibres to form a coating with excellent fastness to washing. In addition, the preparation of the N-halamine precursors with two hydroxyl groups promotes covalent bonding to the cellulose surface. The species can be chemically bonded to hydroxyl groups in cellulose fibres in the presence of 1,2,3,4-butantetracarboxylic acid as a crosslinking agent. Alternatively, bonding can also be achieved by synthesising an N-halamine siloxane monomer precursor, which allows the silanol groups to react with hydroxyl groups of cellulose, forming a nanocomposite coating. Consequently, combining N-halamines with N-halamine siloxane and QACs siloxane confers a synergistic effect of antimicrobial action [6].
3. Triclosan
Triclosan 5-chloro-2-(2.4-dichlorophenoxy) phenol (C12H7Cl3O2) is an odourless synthetic chlorinated bisphenol, as shown in Figure 3. Triclosan differs from most cationic biocides, and it is not ionised in solutions, which improves its wash fastness. It has a reliable antimicrobial activity against Gram-negative and Gram-positive bacteria, but it also has antifungal and antiviral characteristics. The mechanism of action of this biocide agent works by blocking lipid biosynthesis, such as phospholipids, lipopolysaccharides and lipoproteins, affecting the integrity of cell membranes, as explained in the respective section. Triclosan, throughout the last 30 years, has become the most efficient and widely used biocide. It is included in many consumer and professional healthcare products, such as soaps, lotions and creams, toothpastes, mouthwashes and underarm deodorants, and it is also incorporated into textile fabrics and plastics. It is also mainly used in synthetic fibres such as polyester, nylon, polypropylene, cellulose acetate and acrylic fibres. Several products are available on the market, either as isolated antimicrobial agents for a finishing option or to incorporate into fibres, such as Microban® Cannock, United Kingdom and Irgaguard® (Ludwigshafen, Germany) 1000. Some are already incorporated into fibre or fabric, such as BiofresH™ (Salem, MA, USA) and Silfresh® (Magenta, Italy) [1].
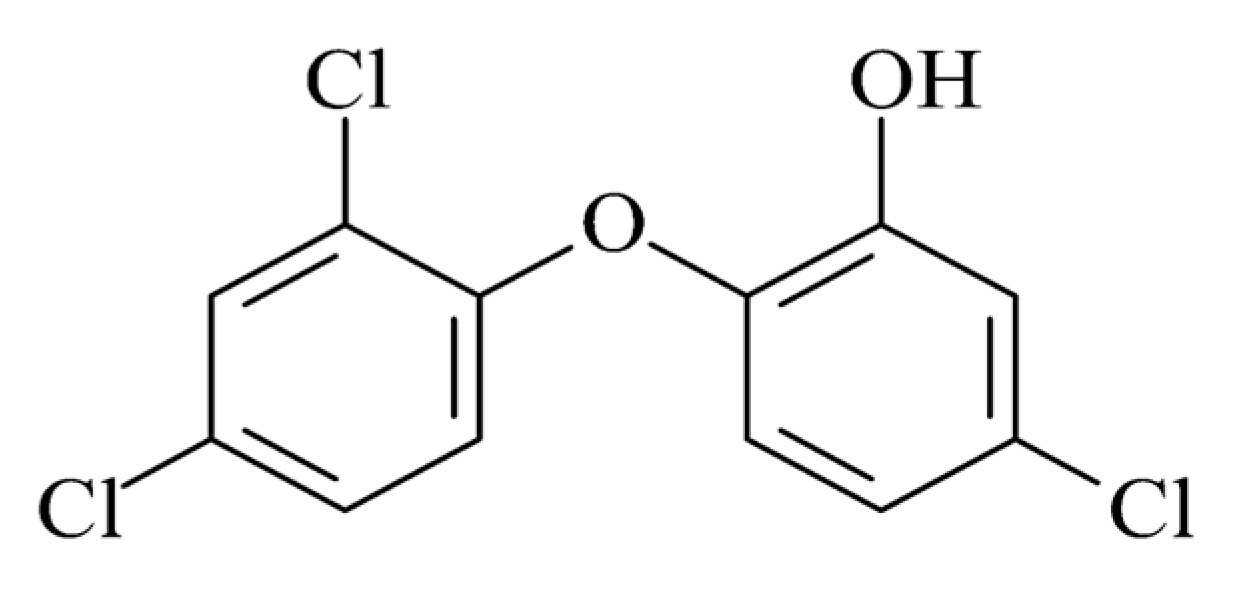
Figure 3. Molecular structure of triclosan.
Triclosan has been applied to cellulose fibres in combination with polycarboxylic acids as crosslinking agents. The pretreatment application of polycarboxylic acid to fibres followed by after-treatment with triclosan enhances the washing durability of the coating. Furthermore, another mode of the application consists of adding triclosan molecules to novel host–guest complexes with pretreatment with cationic β-cyclodextrins. β-cyclodextrins are cyclic oligosaccharides which contain six to eight glucose units linked by β-1,4 bonds. Antimicrobial activity, water solubility and stability have been examined for the host–guest complexes. The complexes are nearly all adsorbed to the surface of cellulose fibres because of the strong electrostatic attraction. Finally, triclosan can be used for finishing non-woven textiles when encapsulated in biodegradable polylactide as a carrier [6].
However, in recent times, the phenomenon of the extensive use of triclosan in non-healthcare settings has been observed. This is a major concern because bacterial microorganisms develop a resistance to triclosan [1]. Another significant concern is that the photochemical exposure of triclosan leads to the formation of 2,8-dichlorodibenzo-p-dioxin in aqueous solutions, which is highly toxic [18][19]. Triclosan is used as an antimicrobial agent on cellulose acetate, polyester, nylon and polypropylene fibres [1][12].
4. Polybiguanidines
Polybiguanides are polymeric polycationic amines that contain cationic biguanide repeat units separated by aliphatic chain linkers. This can be of identical or dissimilar lengths; see Figure 4. The main representative antimicrobial is poly(hexamethylenebiguanide) (PHMB) ((C8H17N5)n), with an average of 11 biguanide units [6]. It consists of a hydrophobic backbone, in which the cationic biguanide groups are interspersed between hydrophobic hexamethylene groups [20][21]. The cationic and hydrophobic character of PHMB reassures the interactivity with microbial cell membranes through electrostatic and hydrophobic interactions [22]. This mechanism causes a cell membrane disruption and a lethal leakage of cytoplasmic materials, and its activity increases the levels of polymerisation [1][16][20][21].
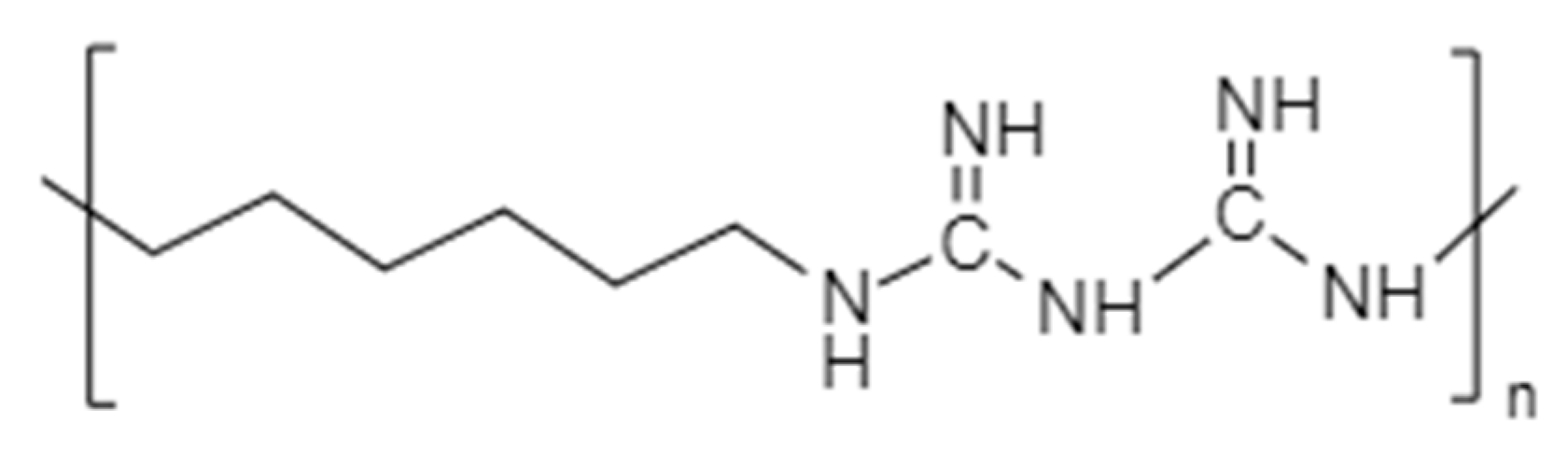
Figure 4. Molecular Structure of poly(hexamethylenebiguanide) (PHMB).
Guanidine-based polymers can be obtained from guanidine salt, biguanidine salt, cyanamide, dicyandiamide and other similar small molecules. These act as monomers to confer the antimicrobial guanidio group to the final guanidine polymer. The guanidio group possesses a strong antimicrobial activity caused by its cationic charge. The negatively charged membrane of bacteria attracts and binds with the positive polymer, which causes the displacement of Mg2+ and Ca2+ ions. The lipopolysaccharide and peptidoglycan components of the cell wall are also bound, causing a major change to the phospholipid environment of the membrane, thus destroying the cytoplasmic membrane and resulting in the bacteria’s death [23].
Both monomeric and dimeric biguanides exert antimicrobial activity, while the latter is more effective against all types of bacteria. The properties of PHMB can be summarised as highly water soluble, chemically stable, less toxic, cost-effective with high antimicrobial activity and good wash fastness, provoking less skin irritation [8]. PHMB is found in health products (mouthwash and wound dressings), clothing, pharmacy and food industries, household and water treatment textiles [1][21]. It is applied during the finishing process of the products, such as underwear and towels, to stop microbial growth. In addition, it is commonly used in medicine as an antiseptic agent for the prevention of wound infection by antibiotic-resistant bacteria. For the aforementioned reasons of biocidal activity and low toxicity, it is used to protect sensitive textile fibres such as cotton [6], but it is also used as an antimicrobial agent to polyester and nylon fibres [1][12]. The highest antibacterial inhibition effect of PHMB is observed at a slightly acidic area of pH 5–6 [8][13][16].
Antimicrobial tests on polyester samples with 2% and 4% of the weight of the fibre (o.w.f.) of PHMB demonstrated that the bacterial-free area was >99.9% in both cases of the treated area for K. pneumonaie. The same test was repeated again after 20 washings. The results remained excellent, with a 99.9% reduction of K. pneumonaie, for polyester treated with 2% and then >99.9% for polyester treated with 4% o.w.f. PHMB. Consequently, this indicates that PHMB remained in the substrate after repeated wet treatments, making it a valuable antibacterial additive [24]. A similar test, performed on cotton fabric with 2% o.w.f. PHMB, demonstrated excellent results (>99.9% and 99.9% reduction after 20 washings) [25].
References
- Morais, D.S.; Guedes, R.M.; Lopes, M.A. Antimicrobial Approaches for Textiles: From Research to Market. Materials 2016, 9, 498.
- Gao, Y.; Cranston, R. Recent Advances in Antimicrobial Treatments of Textiles. Text. Res. J. 2008, 78, 60–72.
- McDonnell, G.; Russell, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179.
- Windler, L.; Height, M.; Nowack, B. Comparative Evaluation of Antimicrobials for Textile Applications. Environ. Int. 2013, 53, 62–73.
- Kegley, S.E.; Hill, B.R.; Orme, S.; Choi, A.H. Pan Pesticide Database, Pesticide Action Network, North America; Pesticide Action Network: Oakland, CA, USA; San Francisco, CA, USA, 2010.
- Simoncic, B.; Tomsic, B. Structures of Novel Antimicrobial Agents for Textiles—A Review. Text. Res. J. 2010, 80, 1721–1737.
- Jain, A.; Duvvuri, L.S.; Farah, S.; Beyth, N.; Domb, A.J.; Khan, W. Antimicrobial Polymers. Adv. Healthcare Mater. 2014, 3, 1969–1985.
- Kamel, M.Y.; Hassabo, A.G. Anti-Microbial Finishing for Natural Textile Fabrics. J. Text. Color. Polym. Sci. 2021, 18, 83–95.
- El-Ola, S.M.A. Recent Developments in Finishing of Synthetic Fibres for Medical Applications. Des Monomers Polym. 2008, 11, 483–533.
- Ma, M.; Sun, G. Antimicrobial Cationic Dyes. Part 3: Simultaneous Dyeing and Antimicrobial Finishing of Acrylic Fabrics. Dye Pigments 2005, 66, 33–41.
- Zhao, T.; Sun, G.; Song, X. An Antimicrobial Cationic Reactive Dye: Synthesis and Applications on Cellulosic Fibres. J. Appl. Polym. Sci. 2008, 108, 1917–1923.
- Nortjie, E.; Basitere, M.; Moyo, D.; Nyamukamba, P. Extraction Methods, Quantitative and Qualitative Phytochemical Screening of Medicinal Plants for Antimicrobial Textiles: A Review. Plants 2022, 11, 2011.
- Zanoaga, M.; Tanasa, F. Antimicrobial Reagents as Functional Finishing for Textiles Intended for Biomedical Applications. I. Synthetic Organic Compounds. Chem. J. Mold. 2014, 9, 14–32.
- Choudhury, A.K.R. Finishes for Protection against Microbial, Insect and Uv Radiation. In Principles of Textile Finishing; Woodhead Publishing: Sawston, UK, 2017; Chapter 11.
- Hui, F.; Debiemme-Chouvy, C. Antimicrobial N-Halamine Polymers and Coatings: A Review of Their Synthesis, Characterization, and Applications. Biomacromolecules 2013, 14, 585–601.
- Afraz, N.; Uddin, F.; Syed, U.; Mahmood, A. Antimicrobial Finishes for Textiles. Curr. Trends Fash. Technol. Text. Eng. 2019, 4, 87–94.
- Ramadan, A.M.; Gawish, S.M. Review on Recent Applications of Antimicrobial Agents for Polyamide and Polypropylene. Al-Azhar J. Dent. Sci. 2012, 23, 1–28.
- Latch, D.E.; Packer, J.L.; Arnold, W.A.; McNeill, K. Photochemical Conversion of Triclosan to 2,8-Dichlorodibenzo-p-Dioxin in Aqueous Solution. J. Photochem. Photobiol. A Chem. 2003, 158, 63–66.
- Buth, J.M.; Steen, P.O.; Sueper, C.; Blumentritt, D.; Vikesland, P.J.; Arnold, W.A.; McNeill, K. Dioxin Photoproducts of Triclosan and Its Chlorinated Derivatives in Sediment Cores. Environ. Sci. Technol. 2010, 44, 4545–4551.
- Wang, W.Y.; Chiou, J.C.; Yip, J.; Yung, K.F.; Kan, C.W. Development of Durable Antibacterial Textile Fabrics for Potential Application in Healthcare Environment. Coatings 2020, 10, 520.
- Chadeau, E.; Brunon, C.; Degraeve, P.; Léonard, D.; Grossiord, C.; Bessueille, F.; Oulahal, N. Evaluation of Antimicrobial Activity of a Polyhexamethylene Biguanide-Coated Textile by Monitoring Both Bacterial Growth (Iso 20743/2005 Standard) and Viability (Live/Dead Baclight Kit). J. Food Saf. 2012, 32, 141–151.
- Gulati, R.; Sharma, S.; Sharma, R.K. Antimicrobial Textile: Recent Developments and Functional Perspective. Polym. Bull. 2021, 79, 5747–5771.
- Liu, R.; Chen, X.; Hayouka, Z.; Chakraborty, S.; Falk, S.P.; Weisblum, B.; Gellman, S.H. Nylon-3 Polymers with Selective Antifungal Activity. J. Am. Chem. Soc. 2013, 135, 5270–5273.
- Karanikas, E.; Nikolaidis, N.; Tsatsaroni, E. Disperse Inkjet Inks with a New Antibacterial Agent as Additive: Properties and Application to Polyester and Polyamide Fibres. Fibers Polym. 2016, 17, 248–256.
- Tsatsaroni, E.; Karanikas, E.; Nikolaidis, N. Polyhexamethylene Bisguanidine: A New Antimicrobial Agent for Textile Applications. Int. J. Curr. Res. 2016, 8, 41705–41710.
More
Information
Subjects:
Materials Science, Textiles
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
977
Entry Collection:
Organic Synthesis
Revisions:
2 times
(View History)
Update Date:
17 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





