
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Md. Wasim Khan | -- | 2686 | 2023-04-13 19:38:46 | | | |
| 2 | Md. Wasim Khan | Meta information modification | 2686 | 2023-04-13 19:42:15 | | | | |
| 3 | Dean Liu | -1 word(s) | 2685 | 2023-04-14 04:32:37 | | |
Video Upload Options
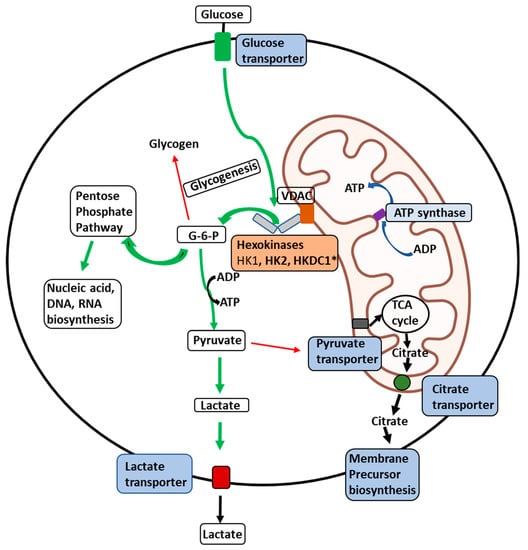
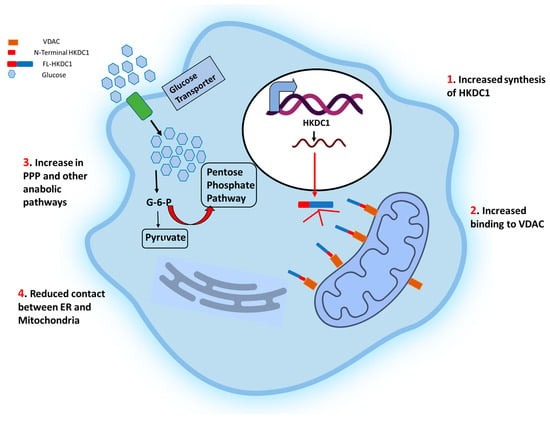
The role of hexokinases in metabolic reprogramming in cancer is multifaceted and pivotal for the altered metabolic phenotype observed in cancer cells. Hexokinases, a group of enzymes responsible for catalyzing the first step of glycolysis, play a critical role in regulating glucose metabolism in cancer cells. In cancer, hexokinases are upregulated and exhibit distinct isoform preferences. Hexokinases facilitate the Warburg effect, a hallmark metabolic alteration in cancer cells characterized by increased glycolysis and decreased oxidative phosphorylation by promoting high glucose consumption and ATP production. Furthermore, hexokinases also participate in other metabolic pathways, such as the pentose phosphate pathway and mitochondrial metabolism, contributing to the rewiring of cancer cell metabolism. The overexpression of hexokinases in cancer cells supports the high bioenergetic and biosynthetic demands of rapidly proliferating cells and confers survival advantages by modulating cellular redox status and apoptosis. The dysregulation of hexokinases in cancer cells presents a promising target for cancer therapy. Understanding their role in metabolic reprogramming provides crucial insights into cancer metabolism and potential therapeutic strategies.
1. Regulation of Hexokinase Activity
2. Roles of Hexokinases in Cancer-Mediated Metabolic Reprogramming
References
- Wilson, J.E. Isozymes of mammalian hexokinase: Structure, subcellular localization and metabolic function. J. Exp. Biol. 2003, 206 Pt 13, 2049–2057.
- Katzen, H.M.; Schimke, R.T. Multiple forms of hexokinase in the rat: Tissue distribution, age dependency, and properties. Proc. Natl. Acad. Sci. USA 1965, 54, 1218–1225.
- Sebastian, S.; Hoebee, B.; Hande, M.; Kenkare, U.; Natarajan, A. Assignment of hexokinase types 1, 2, 3 (Hk1, 2, 3) and glucokinase (Gck) to rat chromosome band 20q11, 4q34, 17q12 and 14q21 respectively, by in situ hybridization. Cytogenet. Cell Genet. 1997, 77, 266–267.
- Wilson, J.E. Hexokinases. Rev. Physiol. Biochem. Pharmacol. 1995, 126, 65–198.
- Cárdenas, M.L.; Cornish-Bowden, A.; Ureta, T. Evolution and regulatory role of the hexokinases. Biochim. Biophys. Acta Mol. Cell Res. 1998, 1401, 242–264.
- Sebastian, S.; Horton, J.D.; Wilson, J.E. Anabolic function of the Type II isozyme of hexokinase in hepatic lipid synthesis. Biochem. Biophys. Res. Commun. 2000, 270, 886–891.
- Kaselonis, G.L.; McCabe, E.R.; Gray, S.M. Expression of hexokinase 1 and hexokinase 2 in mammary tissue of nonlactating and lactating rats: Evaluation by RT-PCR. Mol. Genet. Metab. 1999, 68, 371–374.
- Kawai, S. Hypothesis: Structures, evolution, and ancestor of glucose kinases in the hexokinase family. J. Biosci. Bioeng. 2005, 99, 320–330.
- Velho, G.; Froguel, P.; Clement, K.; Pueyo, M.E.; Rakotoambinina, B.; Zouali, H.; Passa, P.; Cohen, D.; Robert, J.J. Primary pancreatic beta-cell secretory defect caused by mutations in glucokinase gene in kindreds of maturity onset diabetes of the young. Lancet 1992, 340, 444–448.
- Velho, G.; Petersen, K.F.; Perseghin, G.; Hwang, J.H.; Rothman, D.L.; Pueyo, M.E.; Cline, G.W.; Froguel, P.; Shulman, G.I. Impaired hepatic glycogen synthesis in glucokinase-deficient (MODY-2) subjects. J. Clin. Investig. 1996, 98, 1755–1761.
- Osbak, K.K.; Colclough, K.; Saint-Martin, C.; Beer, N.L.; Bellanné-Chantelot, C.; Ellard, S.; Gloyn, A.L. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum. Mutat. 2009, 30, 1512–1526.
- Byrne, M.M.; Sturis, J.; Clement, K.; Vionnet, N.; Pueyo, M.E.; Stoffel, M.; Takeda, J.; Passa, P.; Cohen, D.; Bell, G.I.; et al. Insulin secretory abnormalities in subjects with hyperglycemia due to glucokinase mutations. J. Clin. Investig. 1994, 93, 1120–1130.
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314.
- Liberti, M.V.; Locasale, J.W. The Warburg effect: How does it benefit cancer cells? Trends Biochem. Sci. 2016, 41, 211–218.
- Matschinsky, F.M.; Wilson, D.F. The central role of glucokinase in glucose homeostasis: A perspective 50 years after demonstrating the presence of the enzyme in islets of Langerhans. Front. Physiol. 2019, 10, 148.
- Martínez-Reyes, I.; Chandel, N.S. Cancer metabolism: Looking forward. Nat. Rev. Cancer 2021, 21, 669–680.
- Postic, C.; Shiota, M.; Magnuson, M.A. Cell-specific roles of glucokinase in glucose homeostasis. Recent Prog. Hormone Res. 2001, 56, 195–217.
- Franz, M.M. Glucokinase, glucose homeostasis, and diabetes mellitus. Curr. Diab. Rep. 2005, 5, 171–176.
- Iynedjian, P.B. Molecular physiology of mammalian glucokinase. Cell Mol. Life Sci. 2009, 66, 27–42.
- Moates, J.M.; Nanda, S.; Cissell, M.A.; Tsai, M.-J.; Stein, R. BETA2 activates transcription from the upstream glucokinase gene promoter in islet β-cells and gut endocrine cells. Diabetes 2003, 52, 403–408.
- Jetton, T.L.; Liang, Y.; Pettepher, C.C.; Zimmerman, E.C.; Cox, F.G.; Horvath, K.; Matschinsky, F.M.; Magnuson, M.A. Analysis of up-stream glucokinase promoter activity in transgenic mice and identification of glucokinase in rare neuroendocrine cells in the brain and gut. J. Biol. Chem. 1994, 269, 3641–3654.
- Moates, J.M.; Magnuson, M.A. The Pal elements in the upstream glucokinase promoter exhibit dyad symmetry and display cell-specific enhancer activity when multimerized. Diabetologia 2004, 47, 1632–1640.
- Sternisha, S.M.; Miller, B.G. Molecular and Cellular Regulation of Human Glucokinase. Arch. Biochem. Biophys.
- Peter, A.; Stefan, N.; Cegan, A.; Walenta, M.; Wagner, S.; Königsrainer, A.; Königsrain-er, A.; Machicao, F.; Schick, F.; Häring, H.-U.; et al. Hepatic Glucokinase Expression Is Associated with Lipogenesis and Fatty Liver in Humans. J. Clin. Endocrinol. Metab. 2011, 96, E1126–E1130.
- Iynedjian, P.B. Mammalian glucokinase and its gene. Biochem. J. 1993, 293 Pt 1, 1–13.
- Iynedjian, P.B.; Gjinovci, A.; Renold, A.E. Stimulation by insulin of glucokinase gene transcription in liver of diabetic rats. J. Biol. Chem. 1988, 263, 740–744.
- Wu, C.; Okar, D.A.; Stoeckman, A.K.; Peng, L.J.; Herrera, A.H.; Herrera, J.E.; Towle, H.C.; Lange, A.J. A potential role for fructose-2,6-bisphosphate in the stimulation of hepatic glucokinase gene expression. Endocrinology 2004, 145, 650–658.
- Roth, U.; Curth, K.; Unterman, T.G.; Kietzmann, T. The transcription factors HIF-1 and HNF-4 and the coactivator p300 are involved in insulin-regulated glucokinase gene expression via the phosphatidylinositol 3-kinase/protein kinase B pathway. J. Biol. Chem. 2004, 279, 2623–2631.
- Iynedjian, P.B.; Marie, S.; Gjinovci, A.; Genin, B.; Deng, S.P.; Buhler, L.; Morel, P.; Mentha, G. Glucokinase and cytosolic phosphoenolpyruvate carboxykinase (GTP) in the human liver. Regulation of gene expression in cultured hepatocytes. J. Clin. Investig. 1995, 95, 1966–1973.
- Agius, L. Hormonal and Metabolite Regulation of Hepatic Glucokinase. Annu. Rev. Nutr. 2016, 36, 389–415.
- Zapater, J.L.; Lednovich, K.R.; Khan, M.W.; Pusec, C.M.; Layden, B.T. Hexokinase domain-containing protein-1 in metabolic diseases and beyond. Trends Endocrinol. Metab. 2022, 33, 72–84.
- Guo, C.; Ludvik, A.E.; Arlotto, M.E.; Hayes, M.G.; Armstrong, L.L.; Scholtens, D.M. Coordinated regulatory variation associated with gestational hyperglycemia regulates expression of the novel hexokinase HKDC1. Nat. Commun. 2015, 6, 6069.
- Khan, M.W.; Terry, A.R.; Priyadarshini, M.; Ilievski, V.; Farooq, Z.; Guzman, G.; Cordoba-Chacon, J.; Ben-Sahra, I.; Wicksteed, B.; Layden, B.T. The hexokinase “HKDC1” interaction with the mitochondria is essential for liver cancer progression. Cell Death Dis. 2022, 28, 660.
- Labrecque, M.P.; Brown, L.G.; Coleman, I.M.; Nguyen, H.M.; Lin, D.W.; Corey, E.; Nelson, P.S.; Morrissey, C. Cabozantinib can block growth of neuroendocrine prostate cancer patient-derived xenografts by disrupting tumor vasculature. PLoS ONE 2021, 16, e0245602.
- Lee, H.G.; Kim, H.; Son, T.; Jeong, Y.; Kim, S.U.; Dong, S.M.; Park, Y.N.; Lee, J.D.; Lee, J.M.; Park, J.H. Regulation of HK2 expression through alterations in CpG methylation of the HK2 promoter during progression of hepatocellular carcinoma. Oncotarget 2016, 7, 41798–41810.
- Sun, L.; Shukair, S.; Naik, T.J.; Moazed, F.; Ardehali, H. Glucose phosphorylation and mitochondrial binding are required for the protective effects of hexokinases I and, II. Mol. Cell Biol. 2008, 28, 1007–1017.
- Wilson, J.E. Regulation of mammalian hexokinase activity. In Regulation of Carbohydrate Metabolism; Beitner, R., Ed.; CRC: Boca Raton, FL, USA, 1985; pp. 45–85.
- Li, Y.; Tian, H.; Luo, H.; Fu, J.; Jiao, Y.; Li, Y. Prognostic significance and related mechanisms of hexokinase 1 in ovarian cancer. Onco Targets Ther. 2020, 13, 11583–11594.
- Xu, S.; Catapang, A.; Doh, H.M.; Bayley, N.A.; Lee, J.T.; Braas, D.; Graeber, T.G.; Herschman, H.R. Hexokinase 2 is targetable for HK1 negative, HK2 positive tumors from a wide variety of tissues of origin. J. Nucl. Med. 2019, 60, 212–217.
- Xu, S.; Catapang, A.; Braas, D.; Stiles, L.; Doh, H.M.; Lee, J.T.; Graeber, T.G.; Damoiseaux, R.; Shirihai, O.; Herschman, H.R. A precision therapeutic strategy for hexokinase 1-null, hexokinase 2-positive cancers. Cancer Metab. 2018, 6, 7.
- Šimčíková, D.; Gardáš, D.; Hložková, K.; Hruda, M.; Žáček, P.; Rob, L.; Heneberg, P. Loss of hexokinase 1 sensitizes ovarian cancer to high-dose metformin. Cancer Metab. 2021, 9, 41.
- Amendola, C.R.; Mahaffey, J.P.; Parker, S.J.; Ahearn, I.M.; Chen, W.C.; Zhou, M.; Court, H.; Shi, J.; Mendoza, S.L.; Morten, M.J.; et al. KRAS4 directly regulates HK1. Nature 2019, 576, 482–486.
- Bacci, M.; Giannoni, E.; Fearns, A.; Ribas, R.; Gao, Q.; Taddei, M.L.; Pintus, G.; Dowsett, M.; Isacke, C.M.; Martin, L.A. miR-155 drives metabolic reprogramming of ER+ breast cancer cells following long-term estrogen deprivation and predicts clinical response to aromatase inhibitors. Cancer Res. 2016, 76, 1615–1626.
- van ‘t Veer, L.J.; Dai, H.; van de Vijver, M.J.; He, Y.D.; Hart, A.A.M.; Mao, M.; Peterse, H.L.; van der Kooy, K.; Marton, M.J.; Witteveen, A.T.; et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002, 415, 530–536.
- Liu, X.; Miao, W.; Huang, M.; Li, L.; Dai, X.; Wang, Y. Elevated hexokinase II expression confers acquired resistance to 4-hydroxytamoxifen in breast cancer cells. Mol. Cell Proteomics 2019, 18, 2273–2284.
- Palmieri, D.; Fitzgerald, D.; Shreeve, S.M.; Hua, E.; Bronder, J.L.; Weil, R.J.; Davis, S.; Stark, A.M.; Merino, M.J.; Kurek, R.; et al. Analyses of resected human brain metastases of breast cancer reveal the association between up-regulation of hexokinase 2 and poor prognosis. Mol. Cancer Res. 2009, 7, 1438–1445.
- Yang, X.; Cheng, Y.; Li, P.; Tao, J.; Deng, X.; Zhang, X.; Gu, M.; Lu, Q.; Yin, C. A lentiviral sponge for miRNA-21 diminishes aerobic glycolysis in bladder cancer T24 cells via the PTEN/PI3K/AKT/mTOR axis. Tumour Biol. 2015, 36, 383–391.
- Huang, X.; Liu, M.; Sun, H.; Wang, F.; Xie, X.; Chen, X.; Su, J.; He, Y.; Dai, Y.; Wu, H.; et al. HK2 is a radiation resistant and independent negative prognostic factor for patients with locally advanced cervical squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 4054–4063.
- Iwamoto, M.; Kawada, K.; Nakamoto, Y.; Itatani, Y.; Inamoto, S.; Toda, K.; Kimura, H.; Sasazuki, T.; Shirasawa, S.; Okuyama, H.; et al. Regulation of ^18F-FDG accumulation in colorectal cancer cells with mutated KRAS. J. Nucl. Med. 2014, 55, 2038–2044.
- Demetrius, L.; Tuszynski, J.A. Quantum metabolism explains the allometric scaling of metabolic rates. J. R. Soc. Interface 2010, 7, 507–514.
- Kwee, S.A.; Hernandez, B.; Chan, O.; Wong, L. Choline kinase alpha and hexokinase-2 protein expression in hepatocellular carcinoma: Association with survival. PLoS ONE 2012, 7, e46591.
- Chen, J.; Zhang, S.; Li, Y.; Tang, Z.; Kong, W. Hexokinase 2 overexpression promotes the proliferation and survival of laryngeal squamous cell carcinoma. Tumor Biol. 2014, 35, 3743–3753.
- Wang, H.; Wang, L.; Zhang, Y.; Wang, J.; Deng, Y.; Lin, D. Inhibition of glycolytic enzyme hexokinase II (HK2) suppresses lung tumor growth. Cancer Cell Int. 2016, 16, 38.
- Botzer, L.E.; Maman, S.; Sagi-Assif, O.; Meshel, T.; Nevo, I.; Yron, I.; Witz, I.P. Hexokinase 2 is a determinant of neuroblastoma metastasis. Br. J. Cancer 2016, 114, 759–766.
- Ogawa, H.; Nagano, H.; Konno, M.; Eguchi, H.; Koseki, J.; Kawamoto, K.; Nishida, N.; Colvin, H.; Tomokuni, A.; Tomimaru, Y.; et al. The combination of the expression of hexokinase 2 and pyruvate kinase M2 is a prognostic marker in patients with pancreatic cancer. Mol. Clin. Oncol. 2015, 3, 563–571.
- He, H.C.; Bi, X.C.; Zheng, Z.W.; Dai, Q.S.; Han, Z.D.; Liang, Y.X.; Ye, Y.K.; Zeng, G.H.; Zhu, G.; Zhong, W.D. Real-time quantitative RT-PCR assessment of PIM-1 and hK2 mRNA expression in benign prostate hyperplasia and prostate cancer. Med. Oncol. 2009, 26, 303–308.
- Patra, K.C.; Wang, Q.; Bhaskar, P.T.; Miller, L.; Wang, Z.; Wheaton, W.; Chandel, N.; Laakso, M.; Muller, W.J.; Allen, E.L. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell 2013, 24, 213–228.
- Christofk, H.R.; Vander Heiden, M.G.; Harris, M.H.; Ramanathan, A.; Gerszten, R.E.; Wei, R.; Fleming, M.D.; Schreiber, S.L.; Cantley, L.C. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 2008, 452, 230–233.
- Marybeth, A.; Raoud, M.; Richard, M.; Jen, J.Y. Hexokinase 2 promotes tumor growth and metastasis by regulating lactate production in pancreatic cancer. Oncotarget 2016, 8, 56081–56094.
- Wang, L.; Xiong, H.; Wu, F.; Zhang, Y.; Wang, J.; Zhao, L.; Guo, X.; Chang, L.J.; Zhang, Y.; You, M.J.; et al. Hexokinase 2-mediated Warburg effect is required for PTEN- and p53-deficiency-driven prostate cancer growth. Cell Rep. 2014, 8, 1461–1474.
- Wolf, A.; Agnihotri, S.; Micallef, J.; Mukherjee, J.; Sabha, N.; Cairns, R.; Hawkins, C.; Guha, A. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J. Exp. Med. 2011, 208, 313–326.
- Semenza, G.L. HIF-1: Upstream and downstream of cancer metabolism. Curr. Opin. Genet. Dev. 2010, 20, 51–56.
- Cheung, E.C.; Ludwig, R.L.; Vousden, K.H. Mitochondrial localization of TIGAR under hypoxia stimulates HK2 and lowers ROS and cell death. Proc. Natl. Acad. Sci. USA 2012, 109, 20491–20496.
- Neary, C.L.; Pastorino, J.G. Akt inhibition promotes hexokinase 2 redistribution and glucose uptake in cancer cells. J. Cell Physiol. 2013, 228, 1943–1948.
- Gao, F.; Li, M.; Liu, W.B.; Zhou, Z.S.; Zhang, R.; Li, J.L.; Zhou, K.C. Epigallocatechin gallate inhibits human tongue carcinoma cells via HK2-mediated glycolysis. Oncol. Rep. 2015, 33, 1533–1539.
- Hou, X.; Liu, Y.; Liu, H.; Chen, X.; Liu, M.; Che, H.; Guo, F.; Wang, C.; Zhang, D.; Wu, J.; et al. PERK silence inhibits glioma cell growth under low glucose stress by blockage of p-AKT and subsequent HK2’s mitochondria translocation. Sci. Rep. 2015, 5, 9065.
- Yoshino, H.; Enokida, H.; Itesako, T.; Kojima, S.; Kinoshita, T.; Tatarano, S.; Chiyomaru, T.; Nakagawa, M.; Seki, N. Tumor-suppressive microRNA-143/145 cluster targets hexokinase-2 in renal cell carcinoma. Cancer Sci. 2013, 104, 1567–1574.
- Guo, W.; Qiu, Z.; Wang, Z.; Wang, Q.; Tan, N.; Chen, T.; Chen, Z.; Huang, S.; Gu, J.; Li, J.; et al. MiR-199a-5p is negatively associated with malignancies and regulates glycolysis and lactate production by targeting hexokinase 2 in liver cancer. Hepatology 2015, 62, 1132–1144.
- Qin, Y.; Cheng, C.; Lu, H.; Wang, Y. miR-4458 suppresses glycolysis and lactate production by directly targeting hexokinase2 in colon cancer cells. Biochem. Biophys. Res. Commun. 2016, 469, 37–43.
- Jiang, S.; Zhang, L.F.; Zhang, H.W.; Hu, S.; Lu, M.H.; Liang, S.; Li, B.; Li, Y.; Li, D.; Wang, E.D.; et al. A novel miR-155/miR-143 cascade controls glycolysis by regulating hexokinase 2 in breast cancer cells. EMBO J. 2012, 31, 1985–1998.
- Gregersen, L.H.; Jacobsen, A.; Frankel, L.B.; Wen, J.; Krogh, A.; Lund, A.H. MicroRNA-143 down-regulates Hexokinase 2 in colon cancer cells. BMC Cancer 2012, 12, 232.
- Dai, W.; Wang, F.; Lu, J.; Xia, Y.; He, L.; Chen, K.; Li, J.; Li, S.; Liu, T.; Zheng, Y.; et al. By reducing hexokinase 2, resveratrol induces apoptosis in HCC cells addicted to aerobic glycolysis and inhibits tumor growth in mice. Oncotarget 2015, 6, 13703–13717.
- Federzoni, E.A.; Valk, P.J.; Torbett, B.E.; Haferlach, T.; Löwenberg, B.; Fey, M.F.; Tschan, M.P. PU.1 is linking the glycolytic enzyme HK3 in neutrophil differentiation and survival of APL cells. Blood 2012, 119, 4963–4970.
- Hai-Yan, G.; Xin-Guo, L.; Xi, C.; Jing-Hua, W. Identification of key genes affecting disease free survival time of pediatric acute lymphoblastic leukemia based on bioinformatic analysis. Blood Cells Mol. Dis. 2015, 54, 38–43.
- Lu, J. The Warburg metabolism fuels tumor metastasis. Cancer Metastasis Rev. 2019, 38, 157–164.
- Jose, C.; Bellance, N.; Rossignol, R. Choosing between glycolysis and oxidative phosphorylation: A tumor’s dilemma? Biochim. Biophys. Acta 2011, 1807, 552–561.
- Seiler, K.; Humbert, M.; Minder, P.; Mashimo, I.; Schläfli, A.M.; Krauer, D.; Federzoni, E.A.; Vu, B.; Moresco, J.J.; Yates, J.R., 3rd; et al. Hexokinase 3 enhances myeloid cell survival via non-glycolytic functions. Cell Death Dis. 2022, 13, 448.
- Xu, W.; Liu, W.R.; Xu, Y.; Tian, X.; Anwaier, A.; Su, J.Q.; Zhu, W.K.; Shi, G.H.; Wei, G.M. Hexokinase 3 dysfunction promotes tumorigenesis and immune escape by upregulating monocyte/macrophage infiltration into the clear cell renal cell carcinoma microenvironment. Int. J. Biol. Sci. 2021, 17, 2205–2222.
- Board, M.; Colquhoun, A.; Newsholme, E.A. High Km glucose-phosphorylating (glucokinase) activities in a range of tumor cell lines and inhibition of rates of tumor growth by the specific enzyme inhibitor mannoheptulose. Cancer Res. 1995, 55, 3278–3285.
- Danial, N.N.; Gramm, C.F.; Scorrano, L.; Zhang, C.Y.; Krauss, S.; Ranger, A.M.; Datta, S.R.; Greenberg, M.E.; Licklider, L.J.; Lowell, B.B.; et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature 2003, 424, 952–956.
- Bui, N.L.C.; Pandey, V.; Zhu, T.; Ma, L.; Basappa, L.P.E. Bad phosphorylation as a target of inhibition in oncology. Cancer Lett. 2018, 415, 17–26.
- Danial, N.N. BAD: Undertaker by night, candyman by day. Oncogene 2008, 27 (Suppl. 1), S53–S70.
- Jiang, P.; Du, W.; Heese, K.; Wu, M. The Bad guy cooperates with good cop p53: Bad is transcriptionally up-regulated by p53 and forms a Bad/p53 complex at the mitochondria to induce apoptosis. Mol. Cell Biol. 2006, 26, 9071–9082.
- Matschinsky, F.M.; Magnuson, M.A.; Zelent, D.; Jetton, T.L.; Doliba, N.; Han, Y.; Taub, R.; Grimsby, J. The network of glucokinase-expressing cells in glucose homeostasis and the potential of glucokinase activators for diabetes therapy. Diabetes 2006, 55, 1–12.
- Joseph, G.; Ramakanth, S.; Wendy, L.C.; Nancy-Ellen, H.; Fred, T.B.; John, W.C.; Kevin, R.G.; Darryl, H.; Robert, K. Allosteric Activators of Glucokinase: Potential Role in Diabetes Therapy. Science 2003, 301, 370–373.
- Matschinsky, F.M. Assessing the potential of glucokinase activators in diabetes therapy. Nat. Rev. Drug Discov. 2009, 8, 399–416.
- Akinobu N and Yasuo, T. Present status of clinical deployment of glucokinase activators. J. Diabetes Investig. 2015, 6, 124–132.
- Yoon, S.O.; Youn-Jung, L.; Kaapjoo, P.; Hyun Ho, C.; Sangjong, Y.; Hee-Sook, J. Treatment with glucokinase activator, YH-GKA, increases cell proliferation and decreases glucotoxic apoptosis in INS-1 cells. Eur. J. Pharm. Sci. 2014, 51, 137–145.
- Porat, S.; Weinberg-Corem, N.; Tornovsky-Babaey, S.; Schyr-Ben-Haroush, R.; Hija, A.; Stolovich-Rain, M.; Dadon, D.; Granot, Z.; Ben-Hur, V.; White, P.; et al. Control of pancreatic β cell regeneration by glucose metabolism. Cell Metab. 2011, 13, 440–449.
- Kassem, S.; Bhandari, S.; Rodríguez-Bada, P.; Motaghedi, R.; Heyman, M.; García-Gimeno, M.A.; Cobo-Vuilleumier, N.; Sanz, P.; Maclaren, N.K.; Rahier, J.; et al. Large islets, beta-cell proliferation, and a glucokinase mutation. N. Engl. J. Med. 2010, 362, 1348–1350.
- Shen, Q.; Cheng, F.; Song, H.; Lu, W.; Zhao, J.; An, X.; Liu, M.; Chen, G.; Zhao, Z.; Zhang, J. Proteome-Scale Investigation of Protein Allosteric Regulation Perturbed by Somatic Mutations in 7,000 Cancer Genomes. Am. J. Hum. Genet. 2017, 100, 5–20.
- Těšínský, M.; Šimčíková, D.; Heneberg, P. First evidence of changes in enzyme kinetics and stability of glucokinase affected by somatic cancer-associated variations. Biochim. Biophys. Acta Proteins Proteom. 2019, 1867, 213–218.
- Pusec, C.M.; De Jesus, A.; Khan, M.W.; Terry, A.R.; Ludvik, A.E.; Xu, K.; Giancola, N.; Pervaiz, H.; Smith, E.D.; Ding, X.; et al. Hepatic HKDC1 expression contributes to liver metabolism. Endocrinology 2019, 160, 313–330.
- Khan, M.W.; Ding, X.; Cotler, S.J.; Clarke, M.; Layden, B.T. Studies on the tissue localization of HKDC1, a putative novel fifth hexokinase, in humans. J. Histochem. Cytochem. 2018, 66, 385–392.
- Orci, L.A.; Sanduzzi-Zamparelli, M.; Caballol, B.; Sapena, V.; Colucci, N.; Torres, F.; Bruix, J.; Reig, M.; Toso, C. Incidence of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: A systematic review, meta-analysis, and meta-regression. Clin. Gastroenterol. Hepatol. 2021, 20, 283–292.e10.
- Nagaoki, Y.; Hyogo, H.; Ando, Y.; Kosaka, Y.; Uchikawa, S.; Nishida, Y.; Teraoka, Y.; Morio, K.; Fujino, H. Increasing incidence of non-HBV- and non-HCV-related hepatocellular carcinoma: Single-institution 20-year study. BMC Gastroenterol. 2021, 21, 306.
- Chen, X.; Lv, Y.; Sun, Y.; Zhang, H.; Xie, W.; Zhong, L.; Chen, Q.; Li, M.; Li, L.; Feng, J.; et al. PGC1β regulates breast tumor growth and metastasis by SREBP1-mediated HKDC1 expression. Front. Oncol. 2019, 9, 290.
- Li, J.; Wang, J.; Chen, Y.; Yang, L.; Chen, S. A prognostic 4-gene expression signature for squamous cell lung carcinoma. J. Cell. Physiol. 2017, 232, 3702–3713.
- Yixiang, Z.; Puyuan, X.; Junling, L. Treatment of advanced squamous cell lung cancer. Chin. J. Lung Cancer 2016, 19, 687–691.
- Jia, H.; Wang, A.; Lian, H.; Shen, Y.; Wang, Q.; Zhou, Z.; Zhang, R.; Li, K.; Liu, C. Identification of novel alternative splicing isoform biomarkers and their association with overall survival in colorectal cancer. BMC Gastroenterol. 2020, 20, 1–12.
- Majem, M.; Juan, O.; Insa, A.; Reguart, N.; Trigo, J.M.; Carcereny, E.; García-Campelo, R.; García, Y.; Guirado, M.; Provencio, M. SEOM clinical guidelines for the treatment of non-small cell lung cancer (2018). Clin Transl Oncol. 2019, 21, 3–17.
- Anders, S.; Reyes, A.; Huber, W. Detecting differential usage of exons from RNA-seq data. Genome Res. 2012, 22, 2008–2017.
- Fuhr, L.; El-Athman, R.; Scrima, R.; Cela, O.; Carbone, A.; Knoop, H.; Li, Y.; Hoffmann, K.; Laukkanen, M.O.; Corcione, F.; et al. The Circadian Clock Regulates Metabolic Phenotype Rewiring Via HKDC1 and Modulates Tumor Progression and Drug Response in Colorectal Cancer. EBioMedicine 2018, 33, 105–121.
- Fan, L.; Huang, C.; Li, J.; Gao, T.; Lin, Z.; Yao, T. Long non-coding RNA urothelial cancer associated 1 regulates radioresistance via the hexokinase 2/glycolytic pathway in cervical cancer. Int. J. Mol. Med. 2018, 42, 2247–2259.
- Ma, Y.; Hu, M.; Zhou, L.; Ling, S.; Li, Y.; Kong, B.; Huang, P. Long non-coding RNA HOTAIR promotes cancer cell energy metabolism in pancreatic adenocarcinoma by upregulating hexokinase-2. Oncol. Lett. 2019, 18, 2212–2219.
- Nabi, K.; Le, A. The Intratumoral Heterogeneity of Cancer Metabolism. Adv. Exp. Med. Biol. 2021, 1311, 149–160.
- Antonio, M.J.; Zhang, C.; Le, A. Different Tumor Microenvironments Lead to Different Metabolic Phenotypes. Adv. Exp. Med. Biol. 2021, 1311, 137–147.
- Evstafieva, A.G.; Kovaleva, I.E.; Shoshinova, M.S.; Budanov, A.V.; Chumakov, P.M. Implication of KRT16, FAM129A and HKDC1 genes as ATF4 regulated components of the integrated stress response. PLoS ONE 2018, 13, e0191107.





