Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Surekha K Satpute | -- | 1919 | 2023-04-12 10:41:42 | | | |
| 2 | Conner Chen | Meta information modification | 1919 | 2023-04-14 09:23:46 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Sharma, D.; Singh, D.; Sukhbir-Singh, G.M.; Karamchandani, B.M.; Aseri, G.K.; Banat, I.M.; Satpute, S.K. Biosurfactants as Food Additives. Encyclopedia. Available online: https://encyclopedia.pub/entry/42975 (accessed on 07 February 2026).
Sharma D, Singh D, Sukhbir-Singh GM, Karamchandani BM, Aseri GK, Banat IM, et al. Biosurfactants as Food Additives. Encyclopedia. Available at: https://encyclopedia.pub/entry/42975. Accessed February 07, 2026.
Sharma, Deepansh, Deepti Singh, Gadhwal Monika Sukhbir-Singh, Bhoomika M. Karamchandani, Gajender Kumar Aseri, Ibrahim M. Banat, Surekha K. Satpute. "Biosurfactants as Food Additives" Encyclopedia, https://encyclopedia.pub/entry/42975 (accessed February 07, 2026).
Sharma, D., Singh, D., Sukhbir-Singh, G.M., Karamchandani, B.M., Aseri, G.K., Banat, I.M., & Satpute, S.K. (2023, April 12). Biosurfactants as Food Additives. In Encyclopedia. https://encyclopedia.pub/entry/42975
Sharma, Deepansh, et al. "Biosurfactants as Food Additives." Encyclopedia. Web. 12 April, 2023.
Copy Citation
Microbial surfactants as food additives comprise molecules that may be introduced to food in order to confer emulsifying, foaming, thickening, texture-improving, and/or preserving properties, along with the encapsulation of fat-soluble substances such as vitamins (called “direct food additives”).
antimicrobial
acute toxicity
biosurfactants
1. Introduction
The term ‘food additives’ represents the substances that are added to food to retain or preserve and/or improve some physical properties, often taste, texture, freshness and appearance, along with its safety. However, it is additionally imperative to evaluate the food additives themselves for their potential harmful effects on human health before they are utilized for their desired applications. Among several substances available, surfactants are considered as the most multifarious agents explored for varied applications, such as detergents, pesticide application, cosmetics and microbial enhanced oil recovery processes, and the food-processing industry [1][2][3]. Surfactants are obtained from various sources, e.g., petrochemicals, fatty acids, microbial cells, etc. [4][5]. Some known natural surfactants, such as lecithin from egg yolk and milk proteins, are prominently used in salad dressings and for the enhancement of flavor, appearance, and texture of desserts [6][7]. The growing interest in surfactants and the identification of appropriate molecules with less toxicity and efficient surface characteristics have been of immense interest for both industrial and scientific communities.
Synthetic surfactants are linked with many health-related issues and drawbacks, among which intestinal dysfunction [8] is reported prominently. Surfactants are used in foods in relatively high concentrations, which might lead to severe intestinal permeability, which in turn may elicit various allergic and autoimmune diseases [9]. Surfactants increase intestinal permeability for a limited time in a precise and recurrent way in the presence of antigens and pathogens. It is crucial to note that there are no acceptable daily intake (ADI) guidelines for the use of surfactants in food production [10]. The ADI guidelines specify the highest amount or the limit of a particular chemical which can be consumed regularly over the period of a life span without any health-related issues or apparent side effects. Demands of green ingredients over synthetic additives (“green label”) have led to extensive research in pursuit of new microbial sources for the production of effective surface-active or emulsifying agents [11][12].
BSs are generally low-molecular-weight compounds with the ability to reduce surface tension noticeably, whereas BEs are high-molecular-weight molecules with efficient emulsifying abilities. BSs represent surface-active compounds of microbial origin. BEs are considered as BSs that are used as emulsifiers; therefore, the term BS is much wider and inclusive of BEs. Multifarious BS/BE molecules confer or provide various functional properties to food, such as emulsifying, additive, foam-forming, and wetting agents, in addition to pharmaceutical-related properties (antimicrobial, antiadhesive, antiviral, antibiofilm, etc.) [12][13]. Even though BSs and BEs have an unquestionable potential for replacing synthetic surfactants, with huge importance to food industries. Major blockages include high production cost and apprehension regarding their safety. The present research about BSs/BEs in food production is restricted to laboratory conditions, without detailing any assessment regarding their safety and hazard analysis, which has restricted their acceptance for several food-related applications [12][13][14]. It is important to note that committed guidelines for adopting BSs/BEs in food formulations do not exist; however, the recommendations to include them as all-purpose food additives might be accepted, and would grant their primary approval.
2. Biosurfactants as Food Additives
Food additives are compounds or substances that facilitate the enhancement of overall food properties. Since ancient times, some food additives such as salt, sugar, and SO2, have been utilized to preserve meats, fishes, beverages, etc. Currently, the journey of food additives has made huge advances, from kitchens and small factories to the advanced commercial scale. Food additives have been obtained from various natural sources, or synthesized chemically, and added to food items to achieve some positive technological benefits. Thus, food additives should solely serve the intended desired aims of preserving the nutritional quality of food, and not result in any negative effects. Based upon their functional properties, the World Health Organization (WHO) and the Food and Agriculture Organization (FAO) have broadly categorized additive compounds as (1) flavoring agents, (2) enzyme preparations, and (3) other additives.
Microbial surfactants as food additives comprise molecules that may be introduced to food in order to confer emulsifying, foaming, thickening, texture-improving, and/or preserving properties, along with the encapsulation of fat-soluble substances such as vitamins (called “direct food additives”). Attributes such as antiadhesive/antimicrobial or food surface cleaning are termed as “indirect food additives” that are fulfilled through packaging, coating, or transport and storage processes. Microbial BSs pose antiadhesive and antimicrobial activity against several pathogens and have been listed in Table 1. BSs may interact with porins (proteins of cross cellular membranes) and may lead to leakage of the cytoplasmic content of the cell, resulting in cell death [13]. However, prior to the inclusion of BSs/BEs in food processing, they must undergo critical toxicological assessment protocols which test synergistic compatibility with food molecules, dosage limit determinations for daily intake, and potential protective effects of their use. Rhamnolipids (RLs) have been reported to improve various properties, such as dough stability, batter texture, and the volume and shape of bakery products [14]. A patent on ‘RLs in bakery products’ emphasizes the improvement of dough characteristics and the volume of bakery products after mixing with RLs [15]. Basically, L-rhamnose is a methyl pentose natural sugar found in varied microbial RLs [16], which is useful as a food additive.
Kiran et al. [17] described the BSs of Nesterenkonia sp. for the enhancement of muffin texture. The Nesterenkonia sp. are obligate aerobes, grouped under the genus Micrococci, which grow optimally between 25 and 37 °C. Phylogenetic and chemotaxonomic analysis of isolates showed Kocuria, Kytococcus Dermacoccus, and Nesterenkonia under the genus Micrococcus [18]. Use of Nesterenkonia provides various additional benefits to the muffins, including a decrease in hardness, chewiness and gumminess compared to control treatments in the presence of 0.75% lipopeptide in the preparation mixture. The roles of microbial BSs/BEs (e.g., surfactin, RLs, lipopeptides, glycolipids, and emulsan) as emulsifiers, bakery additives, flavor enhancers, bread improvers, etc., are outlined in Figure 1 and listed in Table 2.
Table 1. Antiadhesive and antimicrobial roles of various microbial-originated biosurfactants against pathogens.
| ANTIADHESIVE | |||
| Microorganisms | Biosurfactant | Pathogens | Reference |
| Pseudomonas putida | Putisolvin I and II | Pseudomonas sp. | [19] |
| Psedofactin II | Enterobacter faecalis Proteus mirabilis, Candida sp. |
[20] | |
| Bacillus subtilis | Fengycin | Salmonella enterica | [21] |
| Bacillus tequilensis | Lipopeptide | Streptococcus mutans | [22] |
| Candida spaerica | Lunasan | Streptococcus agalactiae Pseudomonas aeruginosa |
[23] |
| Pseudomonas aeruginosa | Rhamnolipid | Yarrowia sp. | [24] |
| Candida lipolytica | Rufisan | Streptococcus sp. | [25] |
| Serretia marsecens | Glycolipid | Candida albicans Pseudomonas aeruginosa Bacillus pumilus |
[26] |
| ANTIMICROBIAL | |||
| Microorganisms | Biosurfactant (MIC µg/mL) |
Pathogens | Reference |
| Pseudomonas aeruginosa | Rhamnolipids (4–64) |
Alternaria alternata Aureobasidium pullulans Aspergillus niger Candida albicans Chaetonium globosum Gliocadium virens |
[27] |
| Pseudomonas aeruginosa | Rhamnolipids (20–50) |
Alternaria mali Brotrytis cinerea Fusarium sp. Rhizoctonia solani |
[28] |
| Pseudomonas aeruginosa | Rhamnolipids (0.5–1.70) |
Brotrytis cinerea Fusarium sp. Fusarium solani Gliocadium virens Penicillium funiculosum Rhizoctonia solani |
[29] |
| Pseudomonas aeruginosa | Rhamnolipids | Brotrytis cinereal | [30] |
| Pseudomonas aeruginosa | Rhamnolipids (64–256) |
Brotrytis cinereal Mucor miehei Staphylococcus aureus Bacillus cereus |
[31] |
| Pseudomonas sp. | Rhamnolipid | Pseudomonas aeruginosa | [32] |

Figure 1. Different roles of BSs/BEs in improving properties of food, including taste, texture, and flavor improvements.
Table 2. Potential applications of biosurfactant bio emulsifiers in food system.
| FOOD ADDITIVES | |||
| Microorganisms | Biosurfactant | Applications | Reference |
| B. subtilis | Surfactants | Emulsifier | [33] |
| C. utilis | - | Mayonnaise emulsifier | [34] |
| B. subtilis | - | Bakery additive | [35] |
| B. subtilis | Surfactin | Food preservative | [36] |
| Pseudomonas sp. | Rhamnolipid | Dough improvement | [15] |
| Pseudomonas aeruginosa | Rhamnolipid | [16] | |
| Nesterenkonia sp. | Lipopeptides | Texture improvement | [17] |
| Bacillus subtilis | - | Cookie dough | [37] |
| Bacillus subtilis | Lipopeptides | Bread improvement | [38] |
| Candida bombicola | Glycolipids | Cupcake additive | [39] |
| Starmerella bombicola | Sophorolipids | Sophorolipids + curcumin | [40] |
| Probiotic (GRAS) | - | Animal fodder | [41] |
| EMULSIFICATION | |||
| Microorganisms | Biosurfactant type | Emulsification material | Reference |
| Bacillus vallismortis | Exopolysaccharides | Essential oils | [42] |
| Pseudomonas fluorescens | Exopolysaccharides | Edible oils | [43] |
| Nesterenkonia sp. | Lipopeptide | Unsaturated hydrocarbons | [18] |
| Candida utilis | Glycolipids | Vegetable oil | [44] |
| Pseudomonas aeruginosa | Rhamnolipids | Saturated hydrocarbons | [45] |
| Kluyveromyces marxianus | Mannoprotien | Corn oil | [46] |
| Saccharomyces lipolytica | - | Cooking vegetable oil | [47] |
| Candida utilis | Glycolipids | Canola oil | [48] |
| Pseudomonas aeruginosa | Rhamnolipids | Nano-emulsion | [49] |
Campos et al. [34] established the varied formulations of mayonnaise with Candida utilis derived BE as a key ingredient to confer stability to the emulsion during storage process. Dough properties and volume were considerably improved with the use of the chemical emulsifier glycerol monostearate at the application of 0.1% BSs. In another example B. subtilis-derived surfactants were reported highlighting their ability to enhance dough structural properties and the textural quality of cookies [37]. Similarly, Mnif et al. [38] reported the enhancement of bread dough quality with a B. subtilis-derived BS at a concentration of 0.075% (w/w) in comparison to soya lecithin. Other additional improvements in the structural properties of bread, such as chewiness, cohesion, and reduction in firmness were also reported.
In a bakery-related application, Silva et al. [39] had incorporated a BS into cupcakes as a replacement of 50–100% of the plant fat contents. The replacement of plant fat by a BS resulted in some improvement in the nutritional value of the cupcake, through the reduction of trans-fatty acids (prevalent in plant fat). Microbial BSs have also been explored to improve animal feed by enriching rapeseed meal with GRAS microorganisms. Much longer ago (1951), it was already well known that surfactants encourage the growth of chickens [50]. It was also later suggested that non-ionic surfactants do have an additional impact on animals, in improving their weight, milk production capacity, and feed hydrolysis [51]. Enriching rapeseed meal with BSs produced by GRAS microorganisms successfully hydrolyzes the rapeseed meal and provides several benefits in terms of probiotic concepts. Therefore, BSs can be used as a magnificent substitute to antibiotics, some of which are restricted for use in animal feed [41].
The emulsification activity of BS/BE molecules is decisive for food industries and can be predicted by thorough understanding of their hydrophilic-lipophilic balance (HLB), which designates their usage in the preparations of water-in-oil (W/O) or oil-in water (O/W) emulsions. Based on the HLB scale (0–20), each BS/BE can be categorized, in order to explore them further for suitable applications. HLB values between 3 and 6 are desired for W/O microemulsions, while those between 8 and 18 buoy up O/W microemulsions. For instance, RLs, surfactin, and sophorolipids (SLs), according to their HLB values, favor the improvement of O/W emulsions. Some of the HLB values for representative BSs and Polysorbate 80 (as a reference) are listed in Table 3 [52].
The foremost role of surface-active agents is dropping the interfacial tension that permits the formation of small droplets in an insoluble liquid (oil and water). Surfactants reduce adverse interactions between a water–oil (W/O) interface and permit the dispersion of droplets of one phase into the other. The decrease in the droplet size of an emulsion improves the stability of suspension or liquid solutions [53]. Another potential application of BSs and BEs is their ability to form micro-emulsions, which can be utilized as carriers for fat-soluble vitamins and value-added molecules [54]. Research published by Farheen et al. [55] suggested P. aeruginosa-derived RLs, based in nano-BS preparation and its application in bakery industry facilitating enhanced emulsifying potential, as equated to synthetic surfactants.
Table 3. HLB value evaluation of different surfactants.
SLs are recognized for their substantial emulsification potential towards vegetable oil utilized in bakery preparations. Gaur et al. [62] reported the production of SLs by Candida spp. and further explored its applications as an emulsifier for the food industry. BSs exhibited substantial emulsification activity with olive (51%), soybean (39%), almond (50%), and mustard (50%) oils. It is a well-established fact that BSs can act as efficient emulsifying agents for several oils, and thus can probably be used in several food-related applications. Various other “indirect” applications, including biocidal, food preservation, and antibiofilm applications, need more substantiation and standardization protocols, environmental aspect assessments, along with synergy-supporting evidence [36][37]. Overall, applications of BSs/BEs in food, including their antimicrobial activities against pathogens, are certainly promising and are represented in Figure 2.
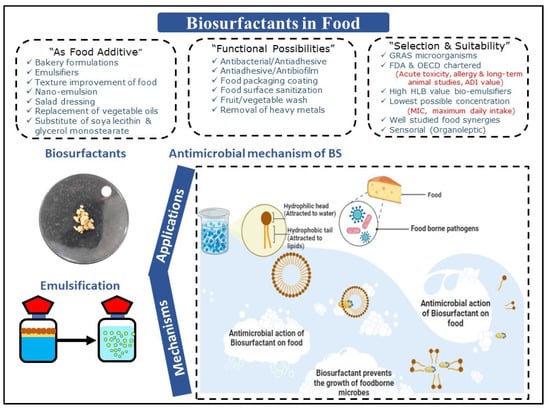
Figure 2. Applications of biosurfactants/bioemulsifiers in food, including their antimicrobial activities against pathogens.
References
- Sharma, D.; Saharan, B.S. Functional characterization of biomedical potential of biosurfactant produced by Lactobacillus helveticus. Biotechnol. Rep. 2016, 11, 27–35.
- Satpute, S.K.; Kulkarni, G.R.; Banpurkar, A.G.; Banat, I.M.; Mone, N.S.; Patil, R.H.; Cameotra, S.S. Biosurfactant/s from Lactobacilli species: Properties, challenges and potential biomedical applications. J. Basic Microbiol. 2016, 56, 1140–1158.
- Karamchandani, B.M.; Pawar, A.A.; Pawar, S.S.; Syed, S.; Mone, N.S.; Dalvi, S.G.; Rahman, P.K.; Banat, I.M.; Satpute, S.K. Biosurfactants’ multifarious functional potential for sustainable agricultural practices. Front. Bioeng. Biotechnol. 2022, 10, 1–20.
- Marchant, R.; Banat, I.M. Microbial biosurfactants: Challenges and opportunities for future exploitation. Trends Biotechnol. 2012, 30, 558–565.
- De Almeida, D.G.; Soares Da Silva, R.D.C.F.; Luna, J.M.; Rufino, R.D.; Santos, V.A.; Banat, I.M.; Sarubbo, L.A. Biosurfactants: Promising molecules for petroleum biotechnology advances. Front. Microbiol. 2016, 7, 1718.
- Campos, J.M.; Montenegro Stamford, T.L.; Sarubbo, L.A.; de Luna, J.M.; Rufino, R.D.; Banat, I.M. Microbial biosurfactants as additives for food industries. Biotechnol. Prog. 2013, 29, 1097–1108.
- Campos, J.M.; Banat, I.M.; Sarubbo, L.A. Natural and Microbial Biosurfactants’ Use in the Food Industry. In Microbial Biosurfactants and Their Environmental and Industrial Applications; CRC Press: Boca Raton, FL, USA, 2019; pp. 242–257.
- Glynn, A.; Igra, A.M.; Sand, S.; Ilbäck, N.G.; Hellenäs, K.E.; Rosén, J.; Aspenström-Fagerlund, B. Are additive effects of dietary surfactants on intestinal tight junction integrity an overlooked human health risk?—A mixture study on Caco-2 monolayers. Food Chem. Toxicol. 2017, 106, 314–323.
- Csáki, K.F. Synthetic surfactant food additives can cause intestinal barrier dysfunction. Med. Hypotheses 2011, 76, 676–681.
- Sharma, D. Biosurfactants: Greener Surface Active Agents for Sustainable Future; Springer: Singapore, 2021.
- da Silva, A.F.; Banat, I.M.; Giachini, A.J. Fungal biosurfactants, from nature to biotechnological product: Bioprospection, production and potential applications. Bioprocess Biosyst. Eng. 2021, 44, 2003–2034.
- Sarubbo, L.A.; Maria da Gloria, C.S.; Durval, I.J.B.; Bezerra, K.G.O.; Ribeiro, B.G.; Silva, I.A.; Twigg, M.S.; Banat, I.M. Biosurfactants: Production, properties, applications, trends, and general perspectives. Biochem. Eng. J. 2022, 181, 108377.
- Farias, C.B.B.; Almeida, F.C.; Silva, I.A.; Souza, T.C.; Meira, H.M.; Rita de Cássia, F.; Luna, J.M.; Santos, V.A.; Converti, A.; Banat, I.M.; et al. Production of green surfactants: Market prospects. Electron. J. Biotechnol. 2021, 51, 28–39.
- Sharma, D. Applications of biosurfactants in food. Biosurfactants Food 2016, 43–80.
- Van Haesendonck, I.; Vanzeveren, E. International patent PCT/BE/2003/000186. 2005 Patent Application No. 2006. 10/533,499, 4 November 2003.
- Trummler, K.; Effenberger, F.; Syldatk, C. An integrated microbial/enzymatic process for production of rhamnolipids and L-(+)-rhamnose from rapeseed oil with Pseudomonas sp. DSM 2874. Eur. J. Lipid Sci. Technol. 2003, 105, 563–571.
- Kiran, G.S.; Priyadharsini, S.; Sajayan, A.; Priyadharsini, G.B.; Poulose, N.; Selvin, J. Production of lipopeptide biosurfactant by a marine Nesterenkonia sp. and its application in food industry. Front. Microbiol. 2017, 8, 1138.
- Stackebrandt, E.; Koch, C.; Gvozdiak, O.; Schumann, P. Taxonomic Dissection of the Genus Micrococcus: Kocuria gen. nov., Nesterenkonia gen. nov., Kytococcus gen. nov., Dermacoccus gen. nov., and Micrococcus Cohn 1872 gen. emend. Int. J. Syst. Evolution. Microbiol. 1995, 45, 682–692.
- Kuiper, I.; Lagendijk, E.L.; Pickford, R.; Derrick, J.P.; Lamers, G.E.; Thomas-Oates, J.E.; Lugtenberg, B.J.J.; Bloemberg Bloemberg, G.V. Characterization of two Pseudomonas putida lipopeptide biosurfactants, putisolvin I and II, which inhibit biofilm formation and break down existing biofilms. Molec. Microbiol. 2004, 51, 97–113.
- Janek, T.; Łukaszewicz, M.; Rezanka, T.; Krasowska, A. Isolation and characterization of two new lipopeptide biosurfactants produced by Pseudomonas fluorescens BD5 isolated from water from the Arctic Archipelago of Svalbard. Bioresour. Technol. 2010, 101, 6118–6123.
- Rivardo, F.; Turner, R.J.; Allegrone, G.; Ceri, H.; Martinotti, M.G. Anti-adhesion activity of two biosurfactants produced by Bacillus spp. prevents biofilm formation of human bacterial pathogens. Appl. Microbiol. Biotechnol. 2009, 83, 541–553.
- Pradhan, A.K.; Pradhan, N.; Mall, G.; Panda, H.T.; Sukla, L.B.; Panda, P.K.; Mishra, B.K. Application of lipopeptide biosurfactant isolated from a halophile: Bacillus tequilensis CH for inhibition of biofilm. Appl. Biochem. Biotechnol. 2013, 171, 1362–1375.
- Luna, J.; Rufino, R.; Campos, G.; Sarubbo, L. Properties of the biosurfactant produced by Candida sphaerica cultivated in low-cost substrates. Chem. Eng. 2012, 27, 67–72.
- Dusane, D.H.; Dam, S.; Nancharaiah, Y.V.; Kumar, A.R.; Venugopalan, V.P.; Zinjarde, S.S. Disruption of Yarrowia lipolytica biofilms by rhamnolipid biosurfactant. Aqua. Biosy. 2012, 8, 17.
- Rufino, R.D.; Luna, J.M.; Sarubbo, L.A.; Rodrigues, L.R.M.; Teixeira, J.A.C.; Campos-Takaki, G.M. Antimicrobial and anti-adhesive potential of a biosurfactant Rufisan produced by Candida lipolytica UCP 0988. Coll. Surf. B Biointerfaces 2011, 84, 1–5.
- Dusane, D.H.; Pawar, V.S.; Nancharaiah, Y.V.; Venugopalan, V.P.; Kumar, A.R.; Zinjarde, S.S. Anti-biofilm potential of a glycolipid surfactant produced by a tropical marine strain of Serratia marcescens. Biofouling 2011, 27, 645–654.
- Benincasa, M.; Abalos, A.; Oliveira, I.; Manresa, A. Chemical structure, surface properties and biological activities of the biosurfactant produced by Pseudomonas aeruginosa LBI from soapstock. Antonie Van Leeuwenhoek 2004, 85, 1–8.
- Kim, B.S.; Lee, J.Y.; Hwang, B.K. In vivo control and in vitro antifungal activity of rhamnolipid B, a glycolipid antibiotic, against Phytophthora capsici and Colletotrichum orbiculare. Pest Manag. Sci. Former. Pestic. Sci. 2000, 56, 1029–1035.
- Haba, E.; Pinazo, A.; Jauregui, O.; Espuny, M.J.; Infante, M.R.; Manresa, A. Physicochemical characterization and antimicrobial properties of rhamnolipids produced by Pseudomonas aeruginosa 47T2 NCBIM 40044. Biotechnol. Bioeng. 2003, 81, 316–322.
- Varnier, A.L.; Sanchez, L.; Vatsa, P.; Boudesocque, L.; Garcia-Brugger, A.; Rabenoelina, F.; Sorokin, A.; Renault, J.H.; Kauffmann, S.; Pugin, A.; et al. Bacterial rhamnolipids are novel MAMPs conferring resistance to Botrytis cinerea in grapevine. Plant Cell Environ. 2009, 32, 178–193.
- Nitschke, M.; Costa, S.G.; Contiero, J. Structure and applications of a rhamnolipid surfactant produced in soybean oil waste. Appl. Biochem. Biotechnol. 2010, 160, 2066–2074.
- Sotirova, A.; Spasova, D.; Vasileva-Tonkova, E.; Galabova, D. Effects of rhamnolipid-biosurfactant on cell surface of Pseudomonas aeruginosa. Microbiol. Res. 2009, 164, 297–303.
- Chander, C.S.; Lohitnath, T.; Kumar, D.M.; Kalaichelvan, P.T. Production and characterization of biosurfactant from Bacillus subtilis MTCC441 and its evaluation to use as bioemulsifier for food bio-preservative. Adv. Appl. Sci. Res. 2012, 3, 1827–1831.
- Campos, J.M.; Stamford, T.L.; Rufino, R.D.; Luna, J.M.; Stamford, T.C.M.; Sarubbo, L.A. Formulation of mayonnaise with the addition of a bioemulsifier isolated from Candida utilis. Toxicol. Rep. 2015, 2, 1164–1170.
- Zouari, R.; Besbes, S.; Ellouze-Chaabouni, S.; Ghribi-Aydi, D. Cookies from composite wheat–sesame peels flours: Dough quality and effect of Bacillus subtilis SPB1 biosurfactant addition. Food Chem. 2016, 194, 758–769.
- Sharma, R.; Singh, J.; Verma, N. Production, characterization and environmental applications of biosurfactants from Bacillus amyloliquefaciens and Bacillus subtilis. Biocat. Agricul. Biotechnol. 2018, 16, 132–139.
- Zouari, R.; Ben Abdallah-Kolsi, R.; Hamden, K.; Feki, E.A.; Chaabouni, K.; Makni-Ayadi, F.; Sallemi, F.; Ellouze-Chaabouni, S.; Ghribi-Aydi, D. Assessment of the antidiabetic and antilipidemic properties of Bacillus subtilis SPB1 biosurfactant in alloxan-induced diabetic rats. Pept. Sci. 2015, 104, 764–774.
- Mnif, I.; Besbes, S.; Ellouze, R.; Ellouze-Chaabouni, S.; Ghribi, D. Improvement of bread quality and bread shelf-life by Bacillus subtilis biosurfactant addition. Food Sci. Biotechnol. 2012, 21, 1105–1112.
- Silva, I.A.; Veras, B.O.; Ribeiro, B.G.; Aguiar, J.S.; Guerra, J.M.C.; Luna, J.M.; Sarubbo, L.A. Production of cupcake-like dessert containing microbial biosurfactant as an emulsifier. Peer J. 2020, 8, e9064.
- Vasudevan, S.; Prabhune, A.A. Photophysical studies on curcumin-sophorolipid nanostructures: Applications in quorum quenching and imaging. R. Soc. Open Sci. 2008, 5, 170865.
- Konkol, D.; Szmigiel, I.; Domżał-Kędzia, M.; Kułażyński, M.; Krasowska, A.; Opaliński, S.; Korczyńskia, M.; Łukaszewicz, M. Biotransformation of rapeseed meal leading to production of polymers, biosurfactants, and fodder. Bioorganic Chem. 2019, 93, 102865.
- Song, B.; Zhu, W.; Song, R.; Yan, F.; Wang, Y. Exopolysaccharide from Bacillus vallismortis WF4 as an emulsifier for antifungal and antipruritic peppermint oil emulsion. Int. J. Biol. Macromol. 2019, 125, 436–444.
- Vidhyalakshmi, R.; Nachiyar, C.V.; Kumar, G.N.; Sunkar, S.; Badsha, I. Production, characterization and emulsifying property of exopolysaccharide produced by marine isolate of Pseudomonas fluorescens. Biocatal. Agri. Bbiotechnol. 2018, 16, 320–325.
- Campos, J.M.; Stamford, T.L.; Sarubbo, L.A. Production of a bioemulsifier with potential application in the food industry. Appl. Biochem. Biotechnol. 2014, 172, 3234–3252.
- Nitschke, M.; Costa, S.G.; Contiero, J. Rhamnolipid surfactants: An update on the general aspects of these remarkable biomolecules. Biotechnol. Prog. 2005, 21, 1593–1600.
- Nitschke, M.; Costa, S.G.V.A.O. Biosurfactants in food industry. Trends Food Sci. Technol. 2007, 18, 252–259.
- Lima, Á.S.; Alegre, R.M. Evaluation of emulsifier stability of biosurfactant produced by Saccharomyces lipolytica CCT-0913. Braz. Arch. Biol. Technol. 2009, 52, 285–290.
- Ribeiro, B.G.; Dos Santos, M.M.; Pinto, M.I.; Meira, H.M.; Durval, I.B.; Guerra, J.M. Production and optimization of the extraction conditions of a biosurfactant of Candida utilis UFPEDA1009 with potential of application in the food industry. Chem. Eng. Trans. 2019, 74, 1477–1482.
- Bai, L.; McClements, D.J. Formation and stabilization of nanoemulsions using biosurfactants: Rhamnolipids. J. Colloid Interface Sci. 2016, 479, 71–79.
- Ely, C.M. Chick-growth stimulation produced by surfactants. Science 1951, 114, 523–524.
- Kim, C.H.; Kim, J.N.; Ha, J.K.; Yun, S.G.; Lee, S.S. Effects of dietary addition of surfactant Tween 80 on ruminal fermentation and nutrient digestibility of Hanwoo steers. Asian-Australas. J. Anim. Sci. 2004, 17, 337–342.
- Gudiña, E.J.; Rangarajan, V.; Sen, R.; Rodrigues, L.R. Potential therapeutic applications of biosurfactants. Trends Pharmacol. Sci. 2013, 34, 667–675.
- Goodarzi, F.; Zendehboudi, S. A comprehensive review on emulsions and emulsion stability in chemical and energy industries. Can. J. Chem. Eng. 2019, 97, 281–309.
- Sagalowicz, L.; Leser, M.E. Delivery systems for liquid food products. Curr. Opin. Colloid Interface Sci. 2010, 15, 61–72.
- Farheen, V.; Saha, S.B.; Pyne, S.; Chowdhury, B.R. Production of nanobiosurfactant from Pseudomonas aeruginosa and it’s application in bakery industry. Int. J. Adv. Res. Biol. Eng. Sci. Technol. 2016, 2, 67.
- Khoshdast, H.; Abbasi, H.; Sam, A.; Noghabi, K.A. Frothability and surface behavior of a rhamnolipid biosurfactant produced by Pseudomonas aeruginosa MA01. Biochem. Eng. J. 2012, 60, 127–134.
- Vaughn, S.F.; Behle, R.W.; Skory, C.D.; Kurtzman, C.P.; Price, N.P.J. Utilization of sophorolipids as biosurfactants for postemergence herbicides. Crop. Prot. 2004, 59, 29–34.
- Randu, M.; Sylvie, H.E.R.Y.; Ravier, P.; Deprey, S. Concentrate comprising a MEL and a polyethylene glycol fatty acid ester having an HLB value greater than or equal to 12. U.S. Patent Application No. 16/085,348, 1 April 2021.
- Sekhar, K.P.; Adicherla, H.; Nayak, R.R. Impact of glycolipid hydrophobic chain length and headgroup size on self-assembly and hydrophobic guest release. Langmuir 2018, 34, 8875–8886.
- De Zoysa, G.H.; Glossop, H.D.; Sarojini, V. Unexplored antifungal activity of linear battacin lipopeptides against planktonic and mature biofilms of C. albicans. European J. Med. Chem. 2018, 146, 344–353.
- Braun, A.C.; Ilko, D.; Merget, B.; Gieseler, H.; Germershaus, O.; Holzgrabe, U.; Meinel, L. Predicting critical micelle concentration and micelle molecular weight of polysorbate 80 using compendial methods. Eur. J. Pharm. Biopharm. 2015, 94, 559–568.
- Gaur, V.K.; Regar, R.K.; Dhiman, N.; Gautam, K.; Srivastava, J.K.; Patnaik, S.; Manickam, N. Biosynthesis and characterization of sophorolipid biosurfactant by Candida spp.: Application as food emulsifier and antibacterial agent. Bioresour. Technol. 2019, 285, 121314.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
14 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




