
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yunyin Niu | -- | 7993 | 2023-04-12 09:56:29 | | | |
| 2 | Lindsay Dong | -1266 word(s) | 6727 | 2023-04-13 07:35:41 | | | | |
| 3 | Chenfei Ren | -1937 word(s) | 3096 | 2023-04-14 15:17:19 | | | | |
| 4 | Chenfei Ren | + 1610 word(s) | 4706 | 2023-04-17 12:19:34 | | | | |
| 5 | Lindsay Dong | -113 word(s) | 4593 | 2023-04-18 10:24:22 | | | | |
| 6 | Chenfei Ren | -35 word(s) | 4558 | 2023-04-18 13:11:51 | | |
Video Upload Options
In the process of using lubricating oil, it is inevitable that bubbles will be produced, which can not only accelerate the oil’s oxidation and shorten the oil change cycle but also reduce its fluidity and lubricity, aggravate the wear of mechanical parts and produce an air lock that interrupts the oil pump supply and causes an oil shortage accident.
1. Introduction
Lubricating oil is commonly used to reduce friction and wear as well as extend the service life of equipment. Lubricating oil is composed of 90% base oil and a variety of functional additives [1]. Lubricating oil is mainly divided into industrial oil and automobile lubricating oil; automobile lubricating oil is mainly divided into internal combustion engine oil [2], automobile gear oil [3], automobile brake oil, engine oil, water tank and cooling system oil, automatic wave tank oil, grease and so on [4]. When used, due to rapid agitation, impact and injection, lubricating oil will inevitably contact air and produce foam. If the foam cannot be eliminated in time, it will bring a lot of harm to the oil-using equipment and the lubricating oil itself [5]. In addition, in the face of an increasingly harsh working environment, various additives need to be added to the lubricating oil to enhance its performance. Graphene-family materials are used as lubricating additives for various liquids. Graphene-family additives can be dispersed well and stabilized in water-based lubricants due to hydrogen bond interactions [6]. However, for lubricating oils, graphene family additives are generally difficult to disperse and stabilize in oils [7]. Graphene oxide quantum dots (GOQDs) can be used as nano-additives to achieve macro super lubricity. This discovery is conducive to the development of new functional additives for industrial applications [8]. However, the interaction between additives, while improving one property of the oil, may have adverse effects on other properties. For example, cleansing dispersants, antioxidants and anticorrosive agents, and other additives are mostly surfactants that increase the foam formation trend and foam stability of oil products [9]. In running mechanical equipment, foam not only reduces the lubrication effect and aggravates the wear of mechanical parts, but also can produce an air lock to interrupt the oil pump supply and cause an oil shortage accident. As for the lubricating oil itself, the contact area between the foam and the air increases, and operation under high temperature conditions accelerates the oxidation and deterioration of the lubricating oil and shortens the oil change cycle. Therefore, the elimination of harmful foam is of great safety and economic significance for reducing non-essential losses and extending mechanical life. At present, adding antioxidant or defoaming agents (also known as antifoaming agents) into lubricating oil is a relatively simple and effective method [10][11].
2. Formation and Harm of Foam
2.1. Formation of Foam
Foam is a kind of gas–liquid interfacial phenomenon formed by air and oil, which is a dispersion system with lubricating oil as the dispersion medium and air as the dispersion phase. When bubbles in lubricating oil rise, they are surrounded by a certain thickness of oil film and then form bubble aggregates. In addition, lubricating oil produces bubbles in contact with air due to rapid stirring in use [12].
However, foams are not prone to forming in pure substances, and even frothing will break up and disappear immediately [11]. Therefore, foam that can exist stably in lubricating oil must be caused by the large amount of surfactants in the oil. Foam formation is influenced by the chemical and physical properties of the lubricant as well as by the operating conditions (temperature, pressure, circulating rate of oil in the system, etc.). In some cases, foaming may be caused by additives in the oil formulation [13]. For example, most of cleansing dispersants, antioxidants, anticorrosive agents, coagulants [14] and other additives used in general oils are surfactants. The polar groups of surfactants enriched at the gas–liquid interface point to the liquid, while the non-polar groups point inside the bubbles, forming a single-molecule layer film to reduce interfacial tension, and leaving the bubbles in a more stable thermodynamic state; when the bubbles float up to the liquid surface and escape, the bubble film generates a bimolecular layer film.
2.2. Harm of Foam
During the actual use of lubricating oil, due to shock, stirring and other effects, air is mixed into the oil, resulting in the formation of bubbles, which makes the fluidity of lubricating oil worse, the lubricating performance worse, and even produces an air lock that affects the oil supply, so some parts are not lubricated and are worn out or sintered. The harmfulness of foam is as follows:
- (1)
-
Degradation of lubrication and wear reduction performance:Foam destroys the continuity of the oil film at the friction pair where relative sliding occurs, reduces lubrication performance and causes the parts to lose sufficient lubrication protection, resulting in serious wear and even sintering [9].
- (2)
-
Degradation of cooling and heat dissipation performance:Partial heat of mechanical equipment can be carried away and dissipated by the lubricating oil when it circulates. However, a large amount of air contained in lubricating oil affects the cooling effect and the heat dissipation effect of the lubricating oil on the machine [15].
- (3)
-
Degradation of the cleaning and dispersing effect:The contact area between oil and air increases due to foam, and the oxidative metamorphism of lubricating oil at high temperatures intensifies, generating more carbides and sludge; at the same time, lubricating oil with insufficient fluidity cannot adequately flush away the dirty stuffs on the working surface of the parts [9].
- (4)
-
Degradation of the anticorrosion and antirust effect:Lubricating oil is absorbed on the surface of the parts to form a layer of oil film to isolate oxygen, water, acidic substances and harmful gases in the air to prevent corrosion. Foam not only destroys the oil film but also releases bubbles at high temperatures, creating cavitation [9].
- (5)
-
Phenomenon of air lock and flow interruption:Because of gas in the oil, on the one hand, the oil produces certain compressibility, which affects pressure transmission; on the other hand, steam resistance is generated, which blocks the oil circuit and affects the oil supply, thus affecting power transmission, making the system unable to work normally, or even interrupting flow and making the lubrication system unable to work normally [16].
- (6)
-
Aggravating oxidation and deterioration of lubricating oil:When bubbles are generated on the surface or inside the tank, the contact area between the lubricating oil and air increases and, coupled with an increase in oil temperature, aggravates the oxidation and deterioration of the base oil, resulting in a large accumulation of sludge at the bottom of the tank [17].
- (7)
-
Potential safety hazard:Foam in the lubricating oil increases the volume of the lubricating oil, and lubricating oil may overflow from the oil tank, resulting in oil loss, fire and other unsafe factors [18].
3. Defoaming Mechanism
4. Defoaming Methods
There are many defoaming methods. Generally, they could be divided into physical defoaming methods and chemical defoaming methods, as shown in Figure 1. However, there are two more implications, i.e., bubble suppression and bubble breaking [19], as shown in Figure 2. To suppress bubbles is to prevent the generation of bubbles, that is, nip in the bud; to destroy bubbles is to eliminate the bubbles that have been created, that is, suit the remedy to the case. Among them, the method of adding defoaming agents belongs to chemical methods.
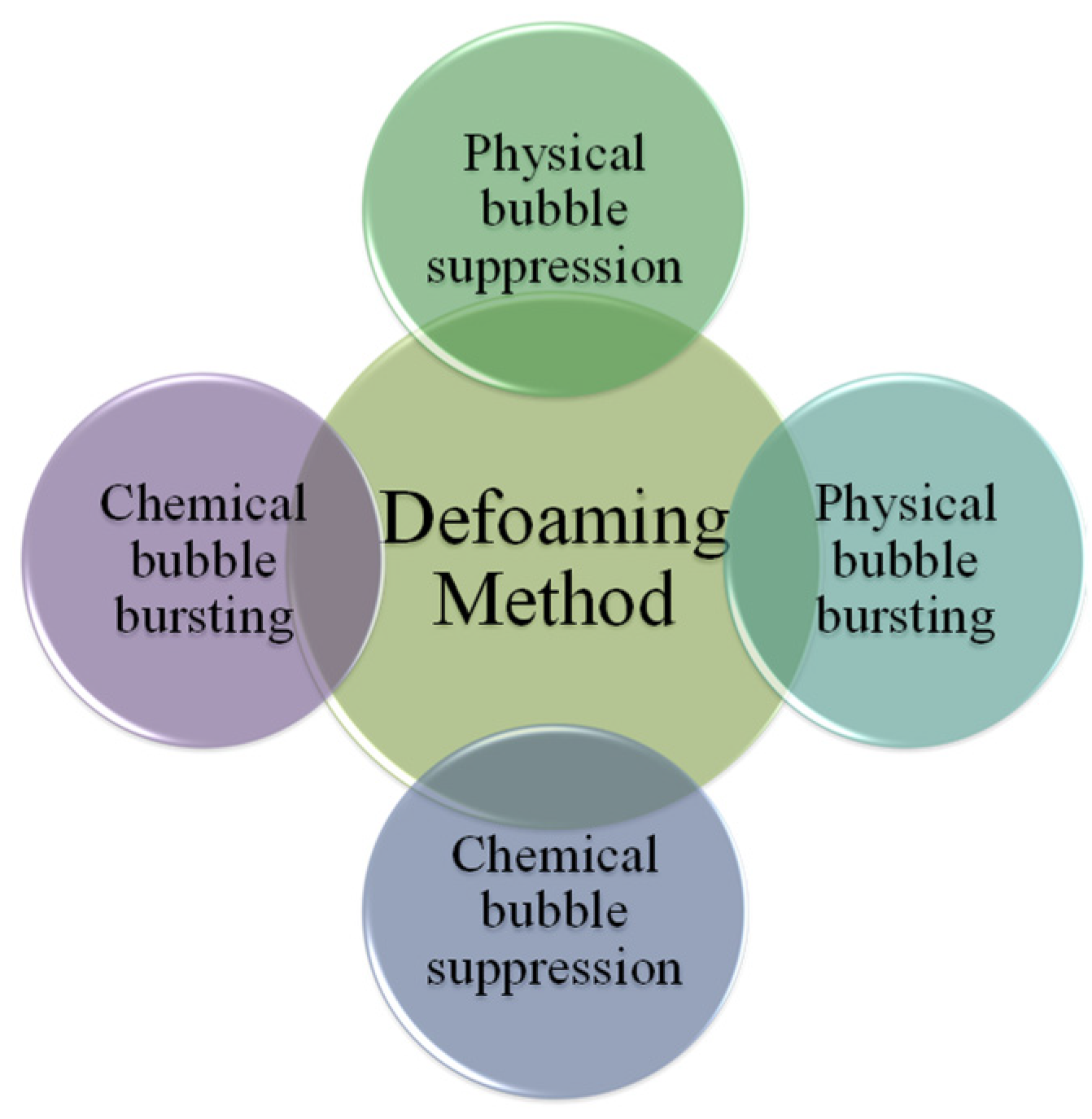
Figure 1. Defoaming methods.
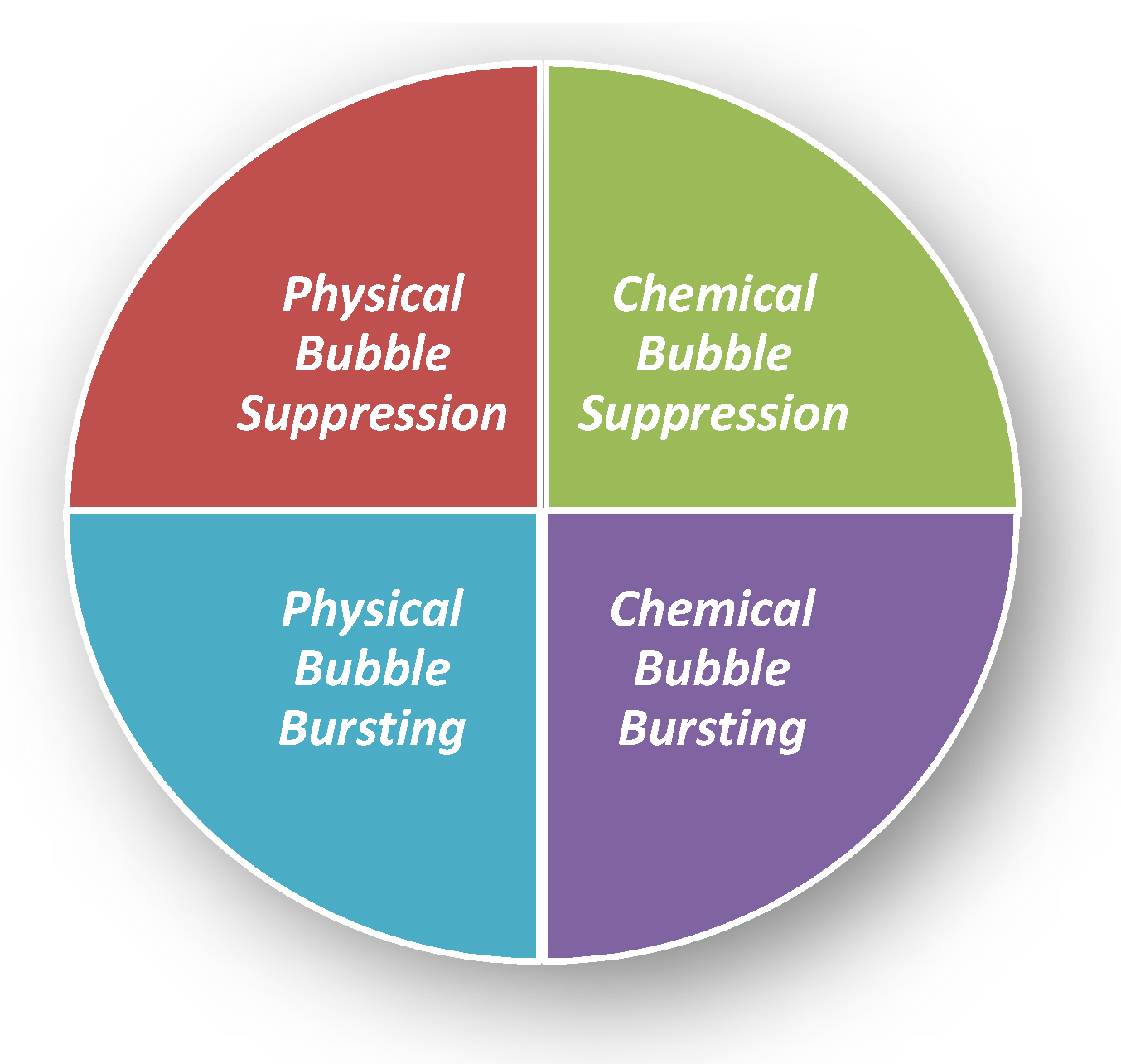
Figure 2. Four kinds of defoaming methods. Physical Bubble Suppression: Temperature change [20]; filtering to remove floating materials [19]; making the vessel open, to remove the mechanical foaming factors (to avoid violent boiling, oscillation, decompression, splashing). Physical Bubble Bursting: Temperature change [20] (freezing, heating [21], evaporation, drying); pressure change [22] (ultrasonic wave [23] and air injection); liquid injection; stirring and tapping with a hydrophobic metal mesh. Chemical Bubble Suppression: Adding defoaming agents; adding defoaming gases; using low foaming surfactants; adding electrolytes; adding substances that eliminate foam stability [19]. Chemical Bubble Bursting: Addition of electrolytes or by electrolysis to weaken the repulsion of the double electrical layers, adding substances discharging liquid ([19]; salting out [24]).
4.1. Physical Defoaming
4.1.1. Physical Bubble Suppression
4.1.2. Physical Bubble Bursting
These physical methods all promote the rate of gas transmission at both ends of the liquid film and the discharge of the bubble film to varying degrees, making the stabilization factor of the foam lower than the decay factor, thus gradually reducing the amount of foam. However, the common disadvantage of these methods is that their use is strongly constrained by environmental factors and the defoaming rate is not high; the advantages are environmental protection and high reusability [25].
4.2. Chemical Defoaming
4.2.1. Chemical Bubble Suppression
Adding defoaming agents; adding defoaming gases; using low-foaming surfactants; removing foaming substances by using adsorption, precipitation and chemical reactions; adjusting pH [26] and HLB; coating the entire vessel wall with adsorbent agents (to prevent violent boiling); adding substances that increase the solubility of foaming substances; adding electrolytes; adding substances that eliminate foam stability [19] are chemical bubble suppression methods.
4.2.2. Chemical Bubble Bursting
Adding defoaming agents [27]; using adsorption, dissolution, dilution and chemical reaction to remove foaming substances; contacting with volatile gases; adjustment of pH [26] and HLB by the addition of acid and base; removing dispersive bubbles by defoaming agents [21]; addition of electrolytes or by electrolysis to weaken the repulsion of the double electrical layers, adding substances discharging liquid [19]; salting out [24] are chemical bubble bursting methods.
These chemical methods have some shortcomings, such as the uncertainty of foaming substance’s composition, insolubility and harm to system equipment [25]. Nowadays, the most widely used defoaming method is adding a defoaming agent. The biggest advantage of this method lies in high defoaming efficiency and convenient use, but finding a suitable and efficient defoaming agent is the key.
4.3. Defoaming Agent
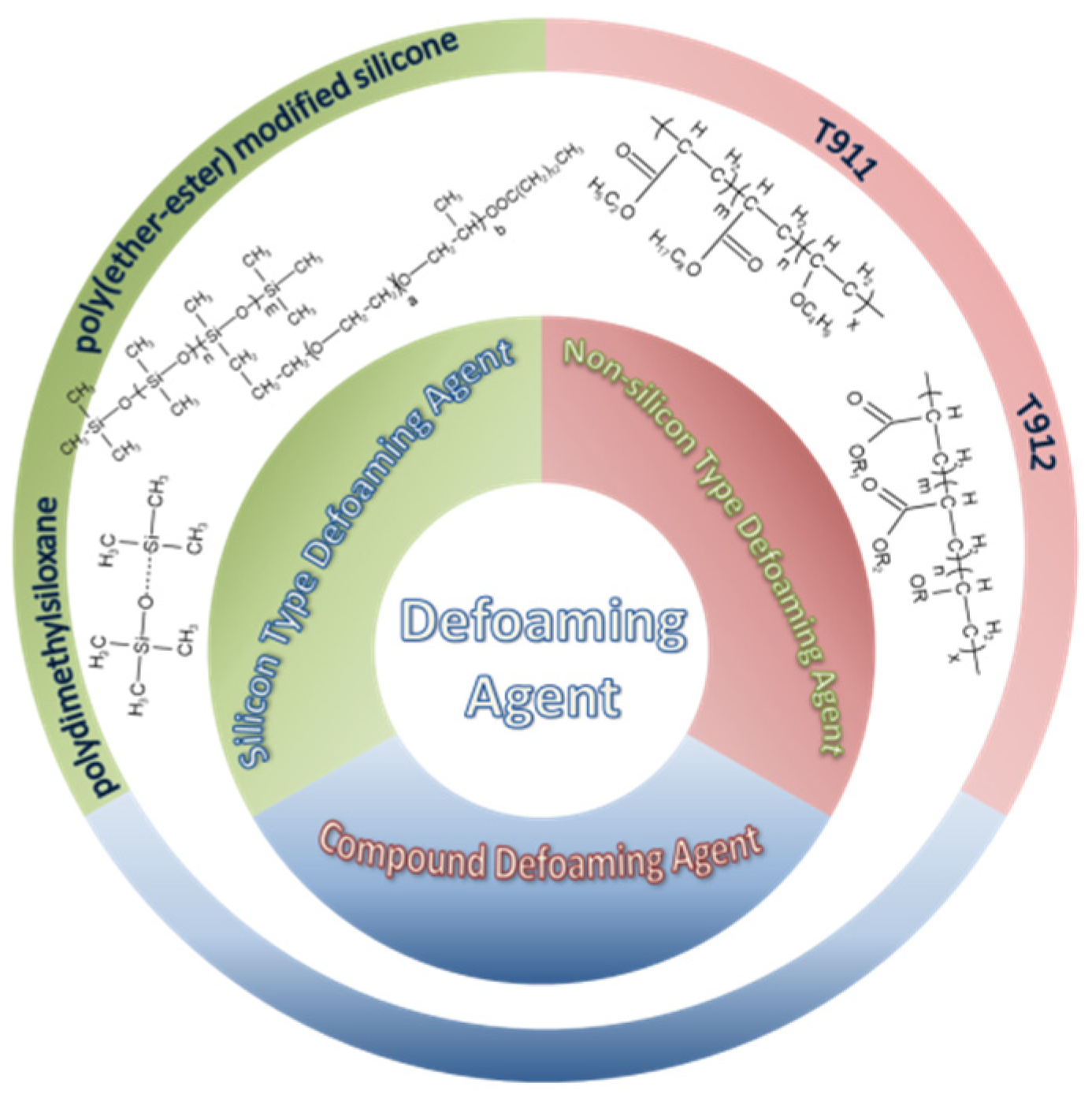
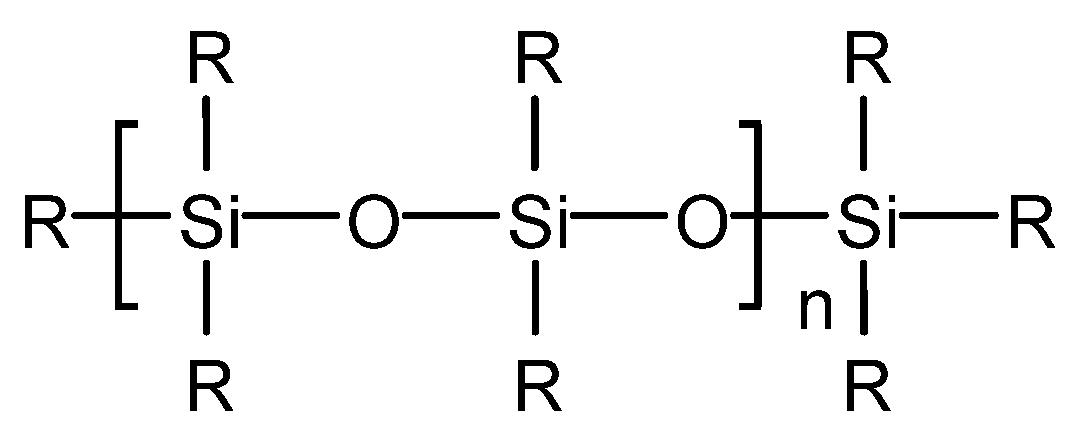
4.3.1. Silicone-Type Defoaming Agent
In addition, T901 is sensitive to blending technology, is prone to settling and accumulating in lubricating oil and has poor defoaming performance after storage. Zhang Liang et al. [28] have proved through experiments that with the increase of T901 addition, fine particles of the defoaming agent gather into droplets, which damages the surface tension system of the lubricating oil itself, resulting in undesirable phenomena such as the decrease of defoaming performance and the increase of turbidity of the lubricating oil.
Polyether-modified polysiloxane defoaming agent [29] is also a hot research topic in recent years. The main focus is on introducing polyether segments into the polysiloxane chain through block copolymerization or graft copolymerization. The hydrophilic polyether chain segments endow it with water solubility, and the hydrophobic polysiloxane chain segments endow it with low surface tension [30]. This kind of defoaming agent has the advantages of both polyether and silicone defoaming agent. Therefore, it has the characteristics of low surface tension, rapid defoaming, long effective foam suppression time, no toxicity and harm, good stability, low cost, lower dosage, wide application and so on. It is also the most ideal new variety in silicone and has good development prospects.
Yinchen Dou et al. synthesized a poly (ether-ester)-modified silicone defoaming agent [31]. The defoaming performance of the product was tested, and it was concluded that the surface tension is 28.6 mN/m when the mass concentration of poly (ether-ester)-modified silicone solution is 0.3 g/L. The defoaming time of poly (ether-ester)-modified silicone defoaming agent is 5 s, which is superior to that of a polyether defoaming agent GPE and a silicone defoaming agent X-100F and inferior to that of a silicone defoaming agent SAG. Its foam inhibition height is 300 mL, which is superior to that of a polyether defoaming agent GPE and a silicone defoaming agent X-100F and is equal to that of a silicone defoaming agent SAG. Poly (ether-ester)-modified silicone can rapidly reduce the surface tension at a low mass concentration and has excellent surface performance. Its structure diagram is shown in Figure 5. For the determination of surface tension, the poly (ether-ester)-modified silicone oil was prepared into different concentrations of an aqueous solution at room temperature and measured by a HARKE-A surface tensiometer. For the determination of defoaming performance, the foaming solution was prepared according to GB/T26527-2011 “silicone defoaming agent”, the defoaming performance was determined, and the foaming force and defoaming performance were measured by a cyclic bubbling meter.
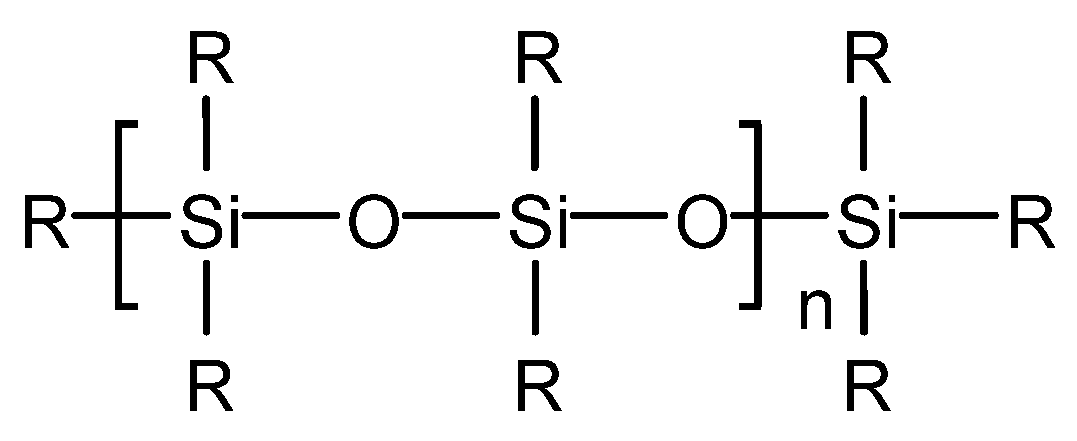
Figure 5. Structure of poly (ether-ester)-modified silicone.
Qiufeng An et al. [32] prepared hydroxyl-capped polyoxypropylene polyoxyethylene oxypropyl-b-polydimethylsiloxane (polyether-b-polysiloxane for short), which is denoted as PESO, and its structure is shown in Figure 6. PESO, dimethyl silicone oil, hydroxyl silicone oil and silicone rubber complex and hydrophobic silica white were added into the three-necked flask equipped with a stirrer, a reflux condenser and a thermometer according to the metering ratio, stirred, heated and warmed up to the set reaction temperature for 30 min. Then, an emulsifier was added and mixed evenly. Finally, deionized water was added while stirring until the solid mass fraction was 45% and a milky white homogeneous liquid could be obtained, namely a nano-effective polyether silicone defoaming agent. The nano-effective polyether silicone defoaming agent has fast foam-bursting speed, relatively long-lasting foam inhibition time, and its performance is close to the level of similar samples.
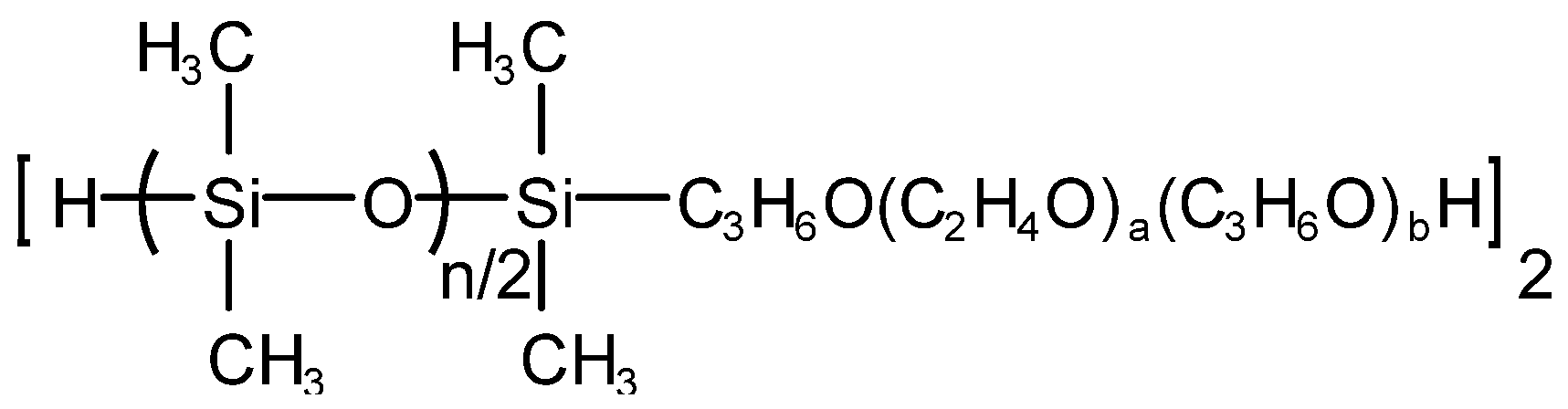
Figure 6. Structure of PESO.
Yan Hu et al. [33] synthesized a low-silicone defoaming agent with a variety of polyether-modified polysiloxanes as the main body (named polyether-modified polysiloxane defoaming agent) and used a refinery residual oil from CNOOC (China National Offshore Oil Corporation) as the foaming fluid to simulate the delayed coking foaming process in the laboratory. The performance was evaluated by comparing it with that of many different types of delayed coking defoaming agents on the market.
4.3.2. Non-Silicone-Type Defoaming Agent
Figure 8. Structure of T912.
Polyacrylate has good solubility in mineral oil and has the advantages of low dosage (0.005% to 0.1%), large diameter of generated bubbles, easy release, low impact on air release, insensitivity to various blending techniques, high defoaming efficiency and good defoaming durability in acidic media [34]. Since the bubble suppression effect of a non-silicone defoaming agent is greater than its foam elimination, it is inferred that the action mechanism of a non-silicone defoaming agent is to increase the surface tension between lubricating oil and air, which changes the original system’s tendency of low surface tension and easy-to-form foam. Because of surface activity, it is decided that a non-silicone-type defoaming agent can only properly increase the surface tension of the liquid interface in the system containing surfactants. Therefore, the defoaming property of a non-silicone-type defoaming agent is influenced by the existing surfactant in the system. Wenxuan Huang [35] conducted a test of non-silicone defoaming agent T912 and silicone oil in 250SN base oil with commonly used additives and found that the defoaming performance of T912 deteriorates when used in combination with three additives, T601 (polyvinyl n-butyl ether), T109 (calcium alkyl salicylate) and T705 (barium dinonyl naphthalene sulfonate), and foaming ability was even enhanced. These results indicate that the poor compatibility between a non-silicone defoaming agent and some additives will cause a decline in the defoaming effect and even promote foam generation. Therefore, more attention should be paid to the use of non-silicone defoaming agents.
Hong Zhou et al. [36] developed a new non-silicone defoaming agent AR-1101 that has an effect similar to that of an NF defoaming agent. The formula was: polyether surfactant 9.7%, saturated alkane 44%, triethanolamine 4.4%, solid Q 9.7% and water 32.2%, as shown in Figure 9. The above materials were heated and stirred, and when the temperature reached about 90 °C, the oil/water phase emulsion was obtained by holding for several hours. AR-1101 has the appearance of a creamy white thick liquid, non-ionic, with a solid content of more than 40%, pH 6–8, and is non-corrosive. AR-1101, a new multi-component non-silicone defoaming agent, has good stability and a long-lasting defoaming effect. At 50 °C, its defoaming height up to 25 mm, and it is widely used. Although it was popularized in 1987, the critical defoaming temperature needs to be further increased. The full-type measuring cylinder method (AR-1101 and NF comparison test) was used.
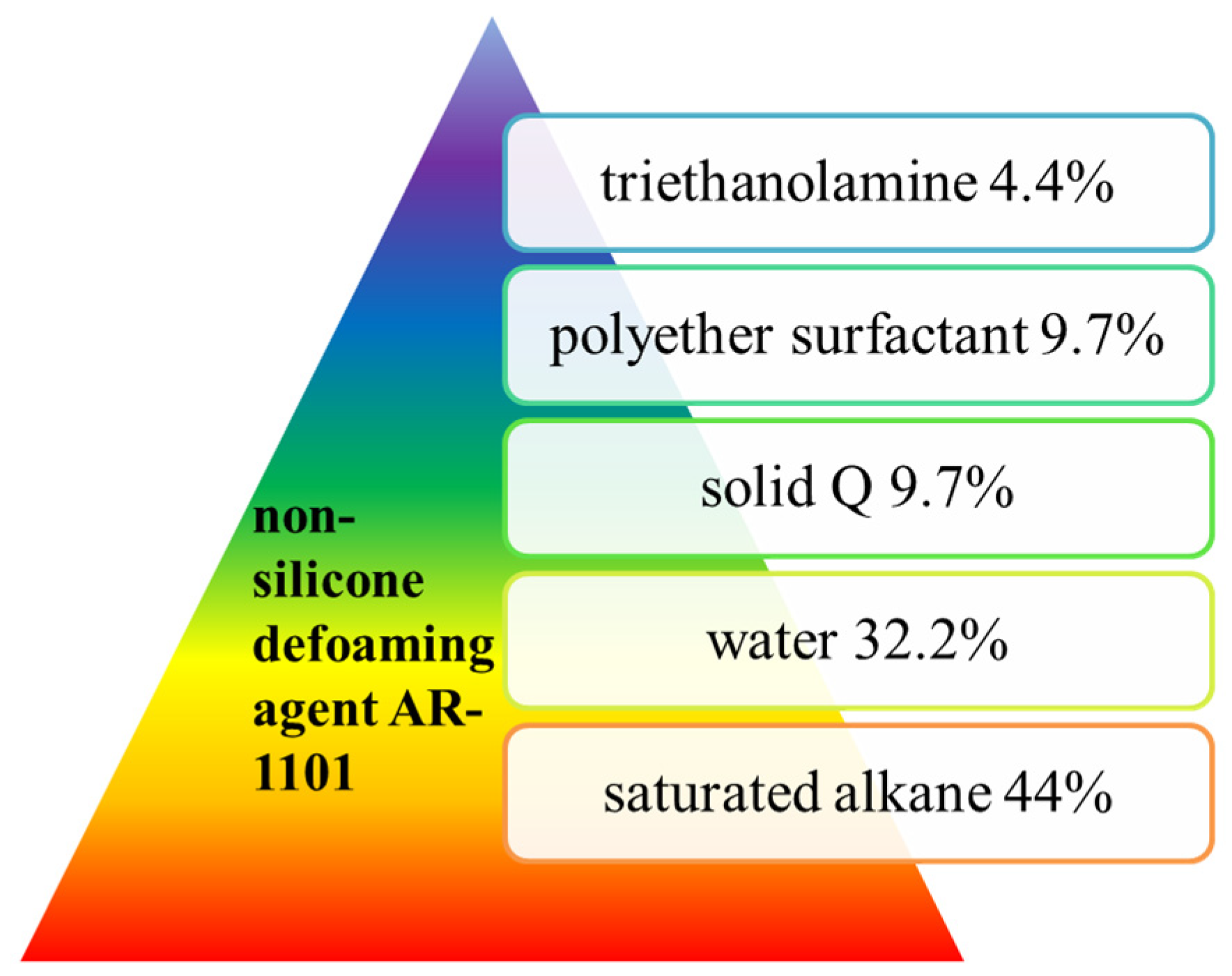
Figure 9. Raw materials for the preparation of a non-silicone defoaming agent AR-1101.
Jing Xiong et al. [37] invented a terpolymer-type non-silicone defoaming agent whose formula was nitrogen, toluene, n-butanol, potassium hydroxide, propylene oxide, epoxy butane, dibenzoyl peroxide and ethylene acetate decyl acrylate, as shown in Figure 10. The patent is characterized by the copolymerization of three monomers, including acrylate, ethylene acetate and an epoxy compound. The three monomers have mass percentages of: acrylate 40–60%, ethylene acetate 10–30% and epoxy compound 20–40%; the epoxy compound is a mixture of propylene oxide and epoxy butane. The terpolymer-type non-silicone defoaming agent prepared using this method can be uniformly dispersed in lubricating oil and effectively inhibit the tendency of foam generation. The copolymer is applicable to the field of lubricating oil, and the blended product has efficient defoaming performance and long-term stable performance, which can meet the actual use requirements of the oil. The defoaming performance was carried out by GB/T12579-2002 “determination of foaming characteristics of lubricating oils”.
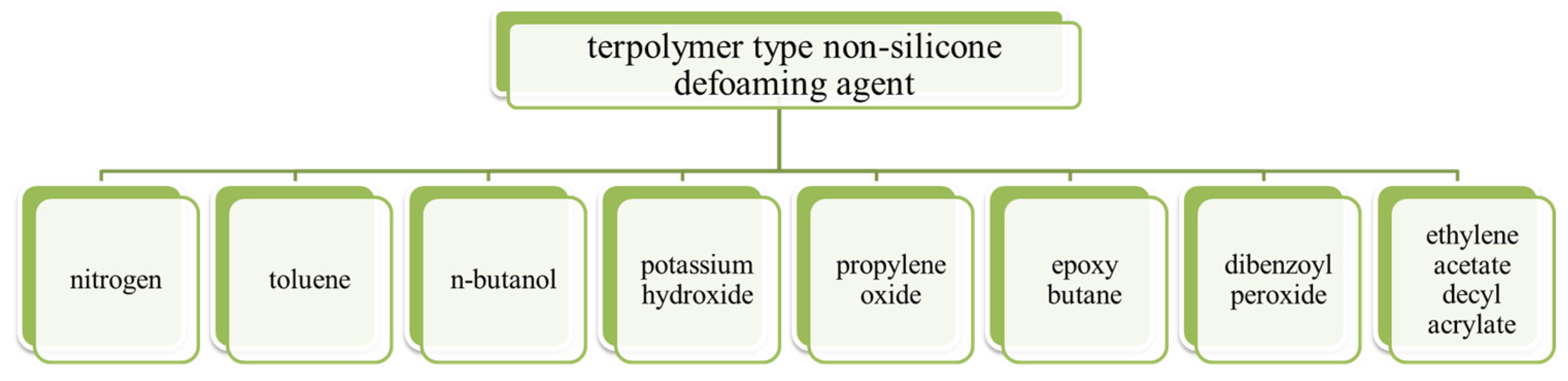
Figure 10. Raw materials for the preparation of a terpolymer-type non-silicone defoaming agent.
Yujuan Chen et al. [38] invented a non-silicone defoaming agent and its preparation method. Vegetable oil and its derivatives were used as the supporter, and silica and fatty acid metal soap were used as the main defoaming substances. Because the fatty acid metal soap cannot be swollen in vegetable oil and its derivatives, it exists in the form of particles; although it has good defoaming performance, it can cause the delamination of the defoaming agent. In order to solve this problem, the invention introduces a hydrogenated castor oil substance. On the one hand, through the special process, defoaming performance is ensured, and at the same time, the stability of the product is guaranteed. Through the special process and the secondary introduction of hydrogenated castor oil, the defoaming performance of the product is further improved. Moreover, the introduction of castor oil polyoxyethylene polyoxylactone oleate with a special structure (shown in Figure 11) ensures good compatibility of the product.

Figure 11. Structure of castor oil polyoxyethylene polyoxylactone oleate (R is selected from H, –OC(CH2)7CH=CH(CH2)7CH3 at least one R group is –OC(CH2)7CH=CH(CH2)7CH3, a + b + c = 0–40 and d + e + f = 0–20.).
Yu Wu et al. synthesized a high-efficiency 2-EHA/VAC copolymer defoaming agent for lubricating oil [39].
4.3.3. Compound Defoaming Agent
Yunfang Cao [42] produced a novel 410 compound defoaming agent that was mixed with silicone defoaming agent methylsilicone oil (T901) and non-silicone defoaming agent acrylate ether copolymer (T912). 410 is an effective compound defoaming agent that is suitable for internal combustion engine oil. The recommended dosage is 10–1200 μg/g. When using, the oil is heated to 60 ± 5 °C under mechanical stirring conditions, the 410 compound defoaming agent is added directly and slowly to the oil according to the required amount and stirred evenly. With adding 0.02% of the 410 compound defoaming agent, the foaming characteristic was 10/0 mL/mL, which proved its good defoaming property (Table 1).
Wei Xu et al. invented a variety of compound defoaming agents for lubricating oil [43], including compound defoaming agents 1, 2, 3, 4 and 5 (Table 1).
Table 1. Defoaming performance parameters of some typical defoaming agents.
|
Defoaming Agents |
Dosage |
Foaming Characteristics (Foam Tendency/Foam Stability) (24 °C, mL/mL) |
Oil for Test |
Data Source |
|
|
silicone-type defoaming agent |
Polydimethylsiloxane |
0 |
650/600 |
TBN25 marine medium-speed oil |
[12] [44] [45] [46] |
|
(T901) |
0.03% |
570/470 |
|||
|
Non-silicone-type defoaming agent |
Acrylate ether copolymer |
0 |
435/20 |
Medium extreme-pressure gear oil |
[47] |
|
T911 |
0.03%~0.1% |
0/0 |
[48] |
||
|
Acrylate ether copolymer |
0 |
650/600 |
TBN25 marine medium-speed oil |
[47] |
|
|
T912 |
0.14% |
570/280 |
[46] |
||
|
2-EHA/VAC copolymer high-efficiency defoaming agent |
0 |
600/520 |
Cold heading gear oil |
[39] |
|
|
0.05% |
0/0 |
||||
|
T921 |
0 |
/ |
Advanced anti-wear hydraulic oil |
[48] |
|
|
0.005%~0.1% |
5/0 |
||||
|
Compound defoaming agent |
T922 |
0 |
620/560 |
Shanghai 4040 medium-speed engine oil |
[41] |
|
0.1% |
355/0 |
||||
|
T923 |
0 |
620/560 |
Shanghai 4040 medium-speed engine oil |
[41] |
|
|
0.05% |
10/0 |
||||
|
410 |
0 |
570/530 |
Diesel engine three-generation oil |
[42] |
|
|
0.02% |
10/0 |
||||
|
412 |
0 |
650/600 |
TBN25 marine medium-speed oil |
[46] |
|
|
0.1% |
10/0 |
||||
|
Compound defoaming agent 1 |
0 |
240/30 (150 °C) |
Internal combustion engine oil |
[43] |
|
|
0.005% |
70/0 (150 °C) |
SL5W-30 |
|||
|
Compound defoaming agent 2 |
0 |
210/0 (150 °C) |
Internal combustion engine oil |
[43] |
|
|
0.01% |
70/0 (150 °C) |
SM5W-30 |
|||
|
Compound defoaming agent 3 |
0 |
210/0 (150 °C) |
Internal combustion engine oil |
[43] | |
|
0.005% |
40/0 (150 °C) |
SM5W-30 |
|||
|
Compound defoaming agent 4 |
0 |
210/0 (150 °C) |
Internal combustion engine oil |
[43] |
|
|
0.005% |
30/0 (150 °C) |
SM5W-30 |
|||
|
Compound defoaming agent 5 |
0 |
190/10 (150 °C) |
Continuously variable transmission oil |
[43] |
|
|
0.005% |
50/0 (150 °C) |
CVTF |
|||
5. Foam Resistance Parameters of Defoaming Agent
The defoaming parameters of three kinds of defoaming agents are listed in Table 1.
6. Conclusions and Outlook
The application of silicone-based compounds in the separation tank can inhibit the formation of foam but can then cause serious catalyst deactivation in the later stage of the refining process [49]. Therefore, silicone defoaming agents have a good ability to eliminate foam, but the effect of long-term foam inhibition is poor. The initial foam elimination effect of non-silicone defoaming agents is not as good as that of silicone defoaming agents, but their defoaming ability is stable and does not decrease significantly after long-term storage. Compound defoaming agents with good bubble suppression and bubble bursting effects, high dispersibility and good durability will occupy the dominant position in the market to replace single defoaming agents with poor performance and unstable chemical properties [50]. However, the application of compound defoaming agents is a new, and their application scope and methods need to be further studied [51]. The synergistic defoaming effect of the mixture of insoluble hydrophobic particles and hydrophobic oil (filled defoaming agent) dispersed in aqueous media has been well confirmed in the patent literature in the early 1950s. These mixed defoaming agents are very effective at low concentrations (10–1000 ppm) and are widely used [52].
However, there is little information in the public literature on the inactivation of other types of defoaming agents [53]. In the future, research on polyether-modified polysiloxane defoaming agents can be carried out from the following aspects [54]: (1) Optimizing the structure of polyether-modified polysiloxane from the perspective of molecular design, and preparing polyether-modified polysiloxane with high yield, good performance, strong stability, and environmental protection by adjusting the amount and arrangement formula of ethylene oxide and propylene oxide in the polyether chain segment, the type of polyether end group and the structure of hydrogen-containing silicone oil. (2) Introducing some functional groups to impart other properties to polyether-modified polysiloxanes, suitable for some special foaming systems. (3) For -Si-C- polyether-modified polysiloxanes, seeking low-cost catalysts to reduce production costs; For -Si-O-C- polyether-modified polysiloxanes, seeking suitable additives to reduce the hydrolysis rate of the product and extend the product’s shelf life. (4) Continued exploring of the defoaming mechanism of this type of defoaming agent and optimizing the molecular structure of polyether-modified polysiloxane and composite additives based on the mechanism.
References
- Xia, L.; Long, J.; Zhao, Y.; Wu, Z.; Dai, Z.; Wang, L. Molecular Dynamics Simulation on the Aggregation of Lubricant Oxidation Products. Tribol. Lett. 2018, 66, 104.
- Dyson, C.J.; Priest, M.; Lee, P.M. Simulating the Misting of Lubricant in the Piston Assembly of an Automotive Gasoline Engine: The Effect of Viscosity Modifiers and Other Key Lubricant Components. Tribol. Lett. 2022, 70, 49. [Google Scholar] [CrossRef]
- Tuszynski, W.; Michalczewski, R.; Piekoszewski, W.; Szczerek, M. Effect of ageing automotive gear oils on scuffing and pitting. Tribol. Int. 2008, 41, 875–888. [Google Scholar] [CrossRef]
- Mohamed, A.; Ali, S.; Osman, T.A.; Kamel, B.M. Development and manufacturing an automated lubrication machine test for nano grease. J. Mater. Res. Technol. 2020, 9, 2054–2062. [Google Scholar] [CrossRef]
- Li, Z.T. Discussion on the mechanism and characteristics of antifoaming agent for lubricating oils. Synth. Lubr. 2020, 47, 38–41. [Google Scholar]
- Liu, Y.F.; Ge, X.Y.; Li, J.J. Graphene lubrication. Appl. Mater. Today 2020, 20, 100662. [Google Scholar] [CrossRef]
- Ge, X.Y.; Chai, Z.Y.; Shi, Q.Y.; Liu, Y.F.; Wang, W.Z. Graphene superlubricity: A review. Friction 2023. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, S.; Li, J.; Ge, X.; Zhao, Z.; Wang, W. Quantum dots of graphene oxide as nano-additives trigger macroscale superlubricity with an extremely short running-in period. Mater. Today Nano 2022, 18, 100219. [Google Scholar] [CrossRef]
- Cen, Z.Y.; Chen, B.Y.; Yang, S.J. Talking about antifoaming agents in lubricating oils. J. Shandong Ind. Technol. 2019, 6, 38. [Google Scholar] [CrossRef]
- Mao, J.X.; Hu, J.Q.; Xu, X.; Guo, L. Application and development of defoaming method in lubricating oil. Chem. Ind. Times 2019, 33, 34–36. [Google Scholar]
- Zhan, C.; Saint-Jalmes, A.; Receveur, M.; El Bahi, H.; Rondelez, F.; Leroy, V. Detailed characterization of aeration in lubricating oils by an ultrasonic approach. Tribol. Int. 2022, 175, 107782.
- Binks, B.P.; Davies, C.A.; Fletcher, P.D.I.; Sharp, E.L. Non-aqueous foams in lubricating oil systems. Colloids Surf. A 2010, 360, 198–204.
- Kichkin, G.I. Foam formation in lubricating oils. Chem. Technol. Fuels Oils 1966, 2, 272–275.
- Faujdar, E.; Negi, H.; Singh, R.K.; Varshney, V.K. Study on Biodegradable Poly(α-Olefins–co–α-Pinene) Architectures as Pour Point Depressant and Viscosity Index Improver Additive for Lubricating Oils. J. Polym. Environ. 2020, 28, 3019–3027.
- Zhao, R.P. Causes, hazards and treatment measures of lubricating oil foam in gear box of wind turbine. Constr. Mach. Equip. 2020, 51, 108–112.
- Zou, X.R. The cause, harm and treatment of foaming of lubricating oil. Mech. Eng. Autom. 2001, S1, 164–165.
- Wu, Y. Study on the Foaming Problems of the Cold Heading Process Lubricating Oil; East China University of Science and Technology: Shanghai, China, 2015.
- Chang, J.H. Study on the Problems of Gear Oil Bubble Properties; East China University of Science and Technology: Shanghai, China, 2015.
- Jiao, X.S. Preparation and Application of Antifoaming Agent; China Light Industry Press: Beijing, China, 2004; pp. 2–5.
- Wang, C.W.; Ni, H.J.; Wang, R.H.; Du, Y.K. Defoaming technology for foam drilling fluid. Drill. Prod. Technol. 2011, 34, 106–108.
- Blázquez, C.; Dalmazzone, C.; Emond, E.; Schneider, S. Crude Oil Foams: Testing and Ranking of Antifoams with the Depressurization Test. Energy Fuels 2017, 31, 1285–1294.
- Kang, S.; Li, R.; Wu, Z.; Guo, S.; Gao, Y. Effective improvement of defoaming efficiency using foam breaker with synthetic sponge cylinders in foam fractionation. Chem. Eng. Process. 2016, 106, 26–32.
- Garrett, P.R. Defoaming: Antifoams and mechanical methods. Curr. Opin. Colloid Interface Sci. 2015, 20, 81–91.
- Wei, Y.; Deng, C.L.; Xiao, Y.; Li, J.J.; Tang, X.D. Research on defoaming method of LuKeQin foam heavy oil. Yunnan Chem. Technol. 2019, 46, 16–18.
- Chang, Q. Chapter 11—Emulsion, Foam, and Gel. In Colloid and Interface Chemistry for Water Quality Control; Chang, Q., Ed.; Academic Press: Beijing, China, 2016; pp. 227–245.
- Ge, C.C.; Wang, Y.S.; Yu, H.W.; Wei, Z. Study on foam and antifoaming agent. Dev. Appl. Mater. 2010, 25, 81–85.
- Zhu, M.Y. Study on Preparation and Performance of Multifunctional Composite Antifoam Agent; Zhengzhou University: Zhengzhou, China, 2020.
- Zhang, L.; Wang, Y. Effect of antifoaming agent on lubricating oil. Lubr. Oil 2019, 34, 40–43. [Google Scholar]
- Zhang, F.J.; Wang, M. Research progress of silicone defoamer. J. Xuchang Univ. 2012, 31, 76–79. [Google Scholar]
- Cevada, E.; Hernández, E.; Flores, C.; Zavala, G.; Álvarez, F.; Vázquez, F. Novel silicon free defoaming agents, based on alkylacrylates, for petroleum: Effect of the molecular weight on their efficiency. Fuel 2020, 278, 118401. [Google Scholar] [CrossRef]
- Dou, Y.C.; Guo, R.; Qiao, Y.; Shi, D.N. Preparation of poly(ether-ester) modified silicone defoaming agent. Fine Chem. 2014, 31, 36–39.
- An, Q.F.; Guo, K.; Li, M.T.; Huang, L.X. Synthesis and characterization of polyether-b-polysiloxane and its applications in antifoaming agent. Silicone Mater. 2008, 22, 344–348.
- Hu, T.; Zhang, G.X.; Lu, Y.; Zhang, Y.; Cheng, Y.; Wei, Q. Synthesis of silicone defoamer and evaluation of its residue defoaming performance. Spec. Petrochem. 2022, 39, 28–30.
- Feng, H.C.; Ge, Q.W.; Wang, X.L.; Wang, Y.H.; Ji, C.Y. Effect of the defoaming agent on the properties of lubricating oil. Lubr. Oil 2010, 25, 24–27.
- Huang, W.X. Lecture 17: The action mechanism, main varieties and applications of antifoaming agents. Pet Prod. Appl. Res. 2018, 36, 83–94.
- Zhou, H.; Wang, Z.X.; Li, J.M.; Lin, C.M. Preparation and application of AR-1101 non-silicon defoaming agent. Text Aux 1987, 4, 25–29.
- Li, L.; Xiong, J. A Terpolymer Type Non-Silicone Anti-Foaming Agent and Its Preparation Method. China Patent CN108359513B, 11 May 2021.
- Chen, Y.J.; Zhang, Z.; Liu, Y.; Cao, T.; Huang, W.; D’Arcy, A.K.; Chen, J. A Non-Silicone Defoamer and Its Preparation Method. China Patent CN108786189B, 2 July 2019.
- Wu, Y.; Li, S.P.; Chang, J.H. Synthesis and performance of 2-EHA/VAC copolymer super-antifoaming agents for lubricating oils. Mod. Chem. Ind. 2014, 34, 92–95.
- Chen, P.H.; Jiang, H.L.; Shu, H.Y.; Zhao, T.T. Study and progress trend of organicsilicon antifoam. Jiangxi Chem. Ind. 2007, 3, 5–7.
- Wang, K.Y.; Xu, W. Preparation and application of No.3 compound anti-foaming agent. Lubr. Oil 2002, 17, 48–52.
- Cao, Y.F. Development and application of 410 antifoam package. Lubr. Oil 1997, 12, 43–45.
- Xu, W.; Shui, L.; Zhang, J.; Zhang, G.R.; Zhou, Y.; Song, Z.X.; Xue, Y.L.; An, A.F.; Chen, L.; Zhao, H.P. The Utility Model Relates to a Compound Antifoaming Agent for Lubricating Oil and Its Application. China Patent CN104232246B, 1 February 2017.
- Chen, J.; Huang, X.; He, L.; Luo, X. Foaming of Oils: Effect of Poly(dimethylsiloxanes) and Silica Nanoparticles. ACS Omega 2019, 4, 6502–6510. [Google Scholar] [CrossRef][Green Version]
- Politova-Brinkova, N.; Hristova, M.; Georgiev, V.; Tcholakova, S.; Denkov, N.; Grandl, M.; Achenbach, F. Role of surfactant adsorption and surface properties for the efficiency of PDMS-silica antifoams. Colloids Surf. A 2021, 610, 125747. [Google Scholar] [CrossRef]
- Liu, H.C. Application of 412 compound anti-foam agent. Lubr. Oil 1998, 13, 45–46. [Google Scholar]
- Hu, N.; Hu, M.M.; Li, X.; Li, Z.X.; Pan, M.H.; Gao, L.F.; Yin, J.H. Study on Preparation and Performance of a Highly Efficient GPES Defoamer. J. Salt. Sci. Chem. Ind. 2021, 50, 9–13. [Google Scholar]
- Wang, K.Y. The properties and application of series products of non-silicon antifoamers. Lubr. Oil 1993, 36–38.
- Cevada, E.; Fuentes, J.V.; Zamora, E.B.; Hernandez, E.I.; Flores, C.A.; Zavala, G.; Alvarez-Ramirez, F.; Vazquez, F. Effect of the Chemical Structure of Alkyl Acrylates on Their Defoaming Activity in Crude Oil: Experimental and Theoretical Studies. Energy Fuels 2021, 35, 9047–9058.
- Zhu, T.I.; Li, M.; Cheng, L.; Liu, X.L.; Zhu, J.; Ma, X.Y.; Sun, X.T. Types and characteristics introduction of antifoaming agent. Lubr. Oil 2017, 32, 23–25. [Google Scholar]
- Wang, K.Y. Study on application of complex antifoamers. Pet. Process. Petrochem. 1994, 25, 15–19. [Google Scholar]
- Pugh, R.J. Foaming, foam films, antifoaming and defoaming. Adv. Colloid Interface Sci. 1996, 64, 67–142. [Google Scholar] [CrossRef]
- McClure, D.D.; Lamy, M.; Black, L.; Kavanagh, J.M.; Barton, G.W. An experimental investigation into the behaviour of antifoaming agents. Chem. Eng. Sci. 2017, 160, 269–274. [Google Scholar] [CrossRef]
- Hu, N.; Hu, M.M.; Li, Z.X.; Li, X.; Gao, L.F.; Yin, J.H. Research Progress and Prospect of Defoamer. J. Salt. Sci. Chem. Ind. 2021, 50, 10–16.




