在使用润滑油的过程中,不可避免地会产生气泡,这不仅会加速机油的氧化,缩短换油周期,还会降低其流动性和润滑性,加剧机械部件的磨损,产生气闸,中断油泵供应,造成缺油事故。本文主要全面论述了润滑油的发泡工艺及其危害、消泡机理和消泡方法,更具体地介绍了有机硅消泡剂、非有机硅消泡剂和复合消泡剂三种化学消泡剂的合成、应用、优缺点和现状。最后,本文展望了润滑油专用消泡剂的未来发展。
- lubricating oil
- silicone-type defoaming agent
- non-silicone-type defoaming agent
- compound defoaming agent
- copolymer
- review
1. 简介
2. 泡沫的形成和危害
2.1. 泡沫的形成
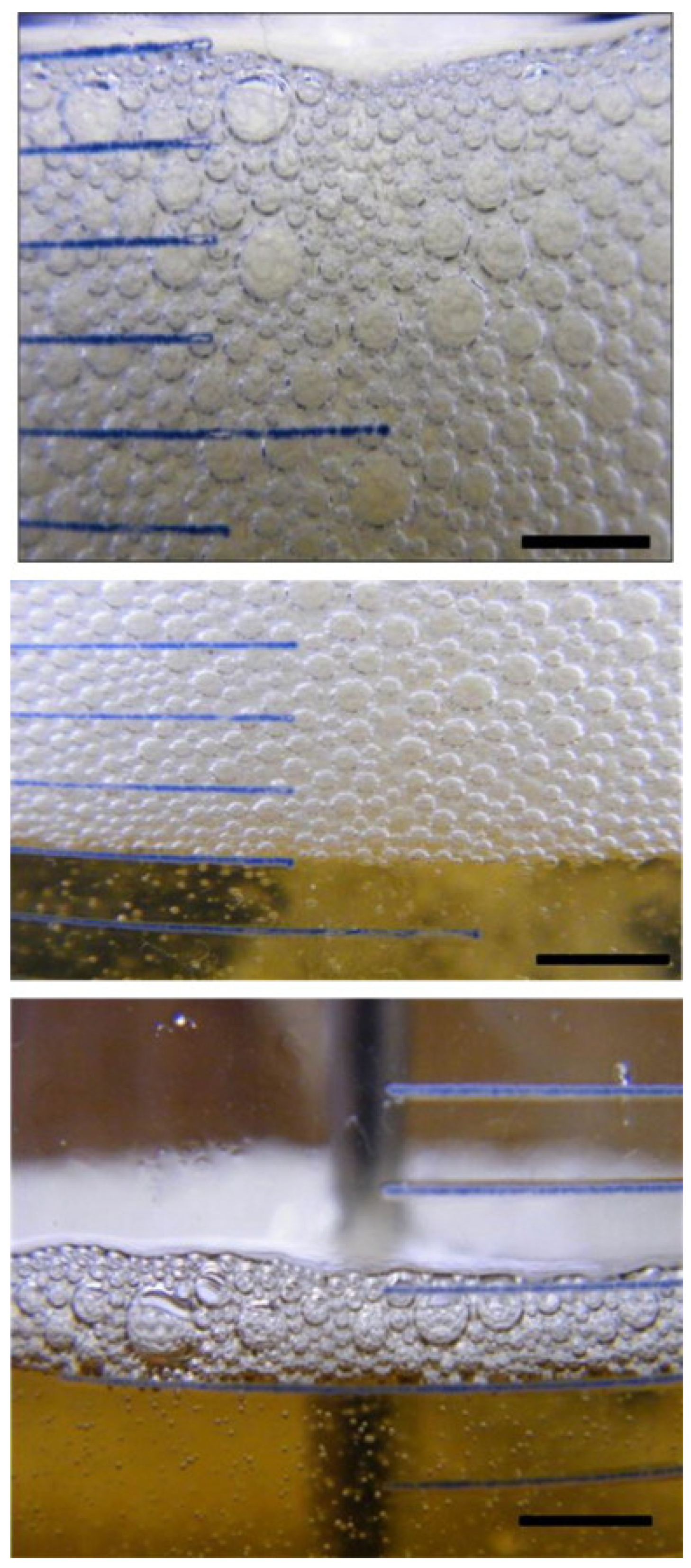
图1. 2°C下由20wt.%添加剂D在基础油中的溶液产生的泡沫的光学图像。 经许可转载[12]。版权所有 © 2010 爱思唯尔公司
2.2. 泡沫的危害
润滑油在实际使用过程中,由于冲击、搅拌等作用,空气混入油中,导致气泡的形成,使润滑油的流动性变差,润滑性能变差,甚至产生气闸,影响供油,因此有些零件不润滑,磨损或烧结。泡沫的危害性如下:
- (1)
-
Degradation of lubrication and wear reduction performance:Foam destroys the continuity of the oil film at the friction pair where relative sliding occurs, reduces lubrication performance and causes the parts to lose sufficient lubrication protection, resulting in serious wear and even sintering [9].
- (2)
-
Degradation of cooling and heat dissipation performance:Partial heat of mechanical equipment can be carried away and dissipated by the lubricating oil when it circulates. However, a large amount of air contained in lubricating oil affects the cooling effect and the heat dissipation effect of the lubricating oil on the machine [15].
- (3)
-
Degradation of the cleaning and dispersing effect:The contact area between oil and air increases due to foam, and the oxidative metamorphism of lubricating oil at high temperatures intensifies, generating more carbides and sludge; at the same time, lubricating oil with insufficient fluidity cannot adequately flush away the dirty stuffs on the working surface of the parts [9].
- (4)
-
Degradation of the anticorrosion and antirust effect:Lubricating oil is absorbed on the surface of the parts to form a layer of oil film to isolate oxygen, water, acidic substances and harmful gases in the air to prevent corrosion. Foam not only destroys the oil film but also releases bubbles at high temperatures, creating cavitation [9].
- (5)
-
Phenomenon of air lock and flow interruption:Because of gas in the oil, on the one hand, the oil produces certain compressibility, which affects pressure transmission; on the other hand, steam resistance is generated, which blocks the oil circuit and affects the oil supply, thus affecting power transmission, making the system unable to work normally, or even interrupting flow and making the lubrication system unable to work normally [16].
- (6)
-
Aggravating oxidation and deterioration of lubricating oil:When bubbles are generated on the surface or inside the tank, the contact area between the lubricating oil and air increases and, coupled with an increase in oil temperature, aggravates the oxidation and deterioration of the base oil, resulting in a large accumulation of sludge at the bottom of the tank [17].
- (7)
-
Potential safety hazard:Foam in the lubricating oil increases the volume of the lubricating oil, and lubricating oil may overflow from the oil tank, resulting in oil loss, fire and other unsafe factors [18].
3. Defoaming Mechanism
4. Defoaming Methods
There are many defoaming methods. Generally, they could be divided into physical defoaming methods and chemical defoaming methods, as shown in Figure 2. However, there are two more implications, i.e., bubble suppression and bubble breaking [21], as shown in Figure 3. To suppress bubbles is to prevent the generation of bubbles, that is, nip in the bud; to destroy bubbles is to eliminate the bubbles that have been created, that is, suit the remedy to the case. Among them, the method of adding defoaming agents belongs to chemical methods.
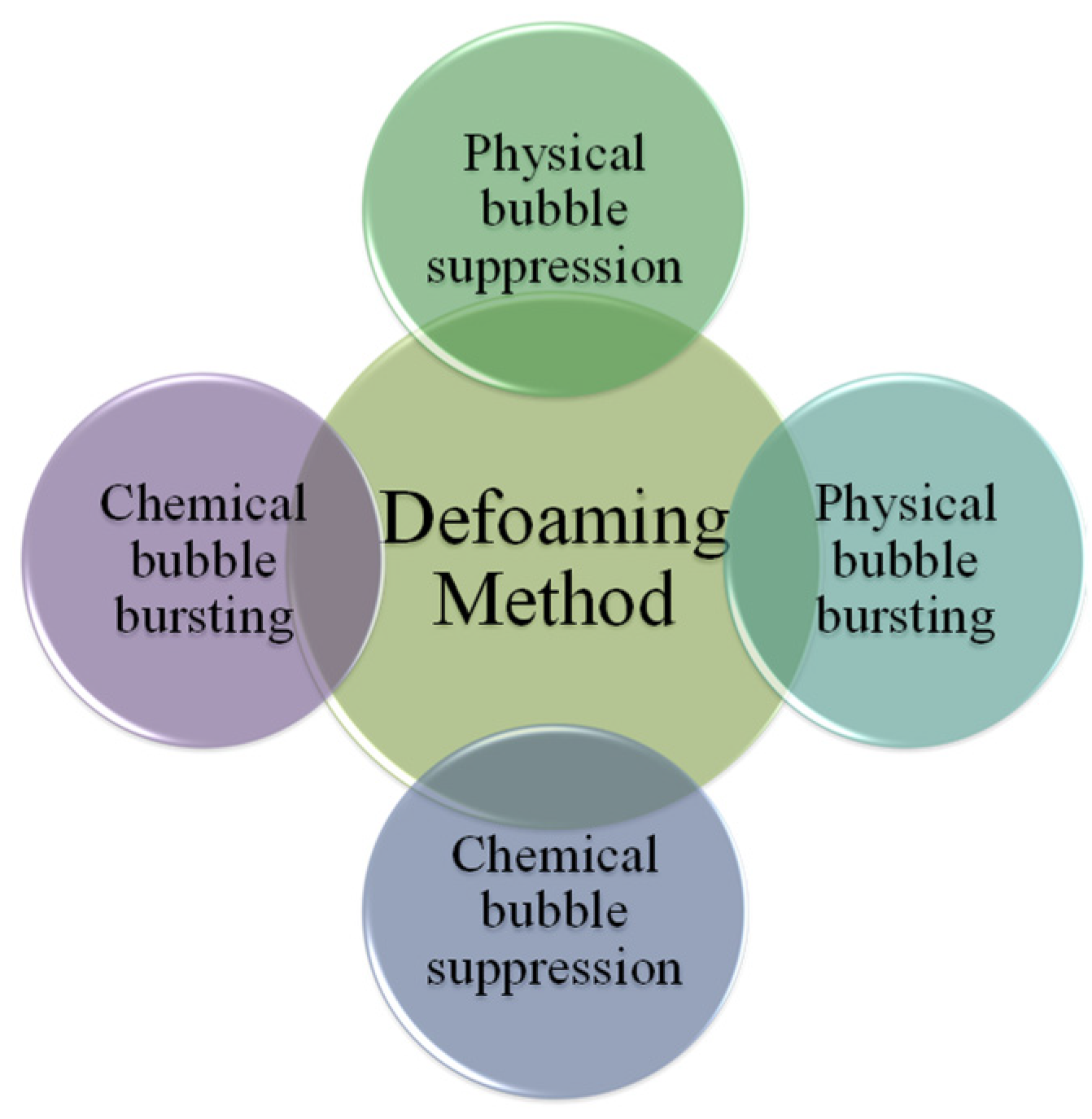
Figure 2. Defoaming methods.
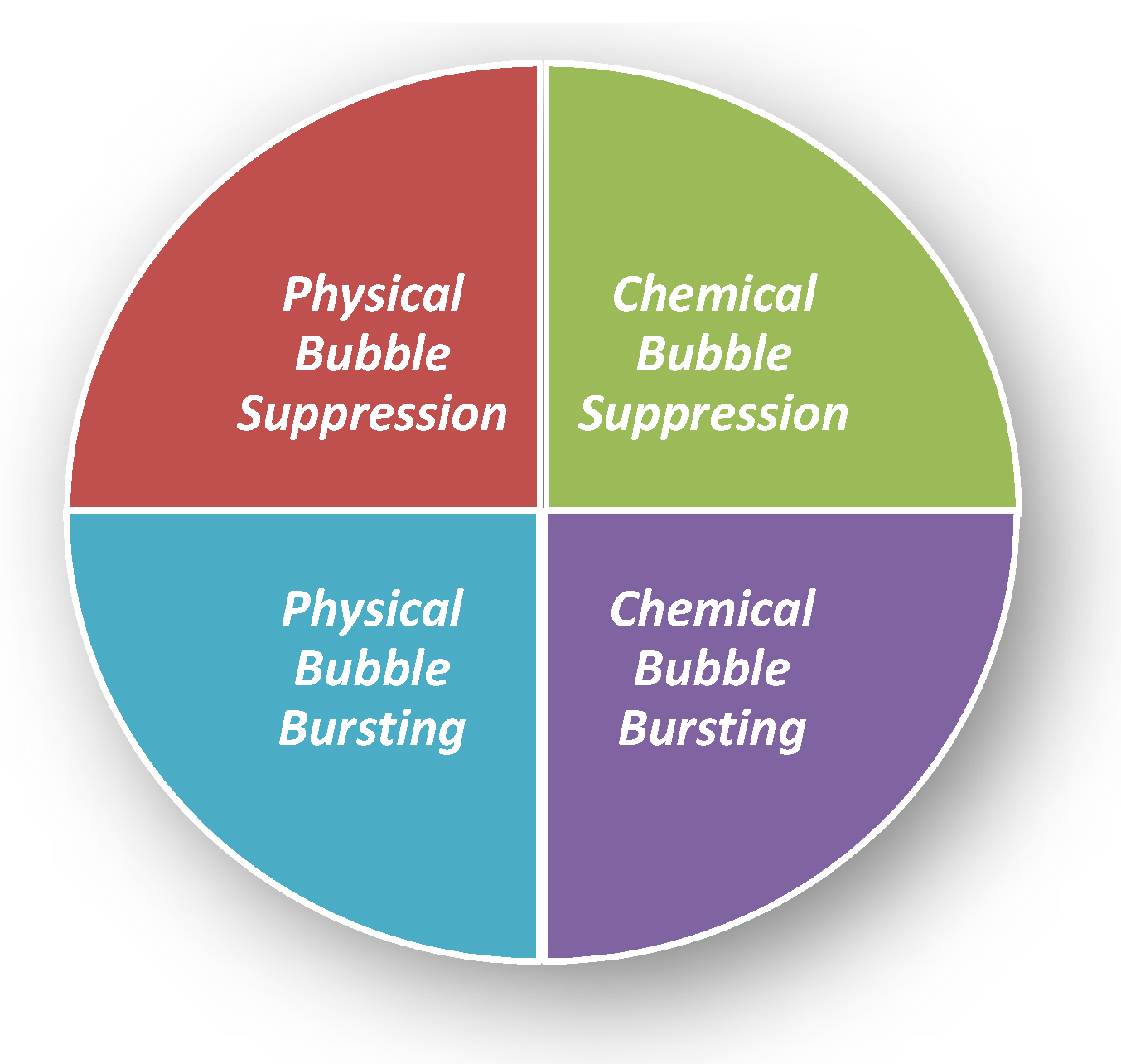
Figure 3. Four kinds of defoaming methods. Physical Bubble Suppression: Temperature change [22]; filtering to remove floating materials [21]; making the vessel open, to remove the mechanical foaming factors (to avoid violent boiling, oscillation, decompression, splashing). Physical Bubble Bursting: Temperature change [22] (freezing, heating [23], evaporation, drying); pressure change [24] (ultrasonic wave [25] and air injection); liquid injection; stirring and tapping with a hydrophobic metal mesh. Chemical Bubble Suppression: Adding defoaming agents; adding defoaming gases; using low foaming surfactants; adding electrolytes; adding substances that eliminate foam stability [21]. Chemical Bubble Bursting: Addition of electrolytes or by electrolysis to weaken the repulsion of the double electrical layers, adding substances discharging liquid ([21]; salting out [29].
4.1. Physical Defoaming
4.1.1. Physical Bubble Suppression
4.1.2. Physical Bubble Bursting
These physical methods all promote the rate of gas transmission at both ends of the liquid film and the discharge of the bubble film to varying degrees, making the stabilization factor of the foam lower than the decay factor, thus gradually reducing the amount of foam. However, the common disadvantage of these methods is that their use is strongly constrained by environmental factors and the defoaming rate is not high; the advantages are environmental protection and high reusability [26].
4.2. Chemical Defoaming
4.2.1. Chemical Bubble Suppression
Adding defoaming agents; adding defoaming gases; using low-foaming surfactants; removing foaming substances by using adsorption, precipitation and chemical reactions; adjusting pH [27] and HLB; coating the entire vessel wall with adsorbent agents (to prevent violent boiling); adding substances that increase the solubility of foaming substances; adding electrolytes; adding substances that eliminate foam stability [21] are chemical bubble suppression methods.
4.2.2. Chemical Bubble Bursting
Adding defoaming agents [28]; using adsorption, dissolution, dilution and chemical reaction to remove foaming substances; contacting with volatile gases; adjustment of pH [27] and HLB by the addition of acid and base; removing dispersive bubbles by defoaming agents [23]; addition of electrolytes or by electrolysis to weaken the repulsion of the double electrical layers, adding substances discharging liquid [21]; salting out [29] are chemical bubble bursting methods.
These chemical methods have some shortcomings, such as the uncertainty of foaming substance’s composition, insolubility and harm to system equipment [26]. Nowadays, the most widely used defoaming method is adding a defoaming agent. The biggest advantage of this method lies in high defoaming efficiency and convenient use, but finding a suitable and efficient defoaming agent is the key.
4.3. Defoaming Agent
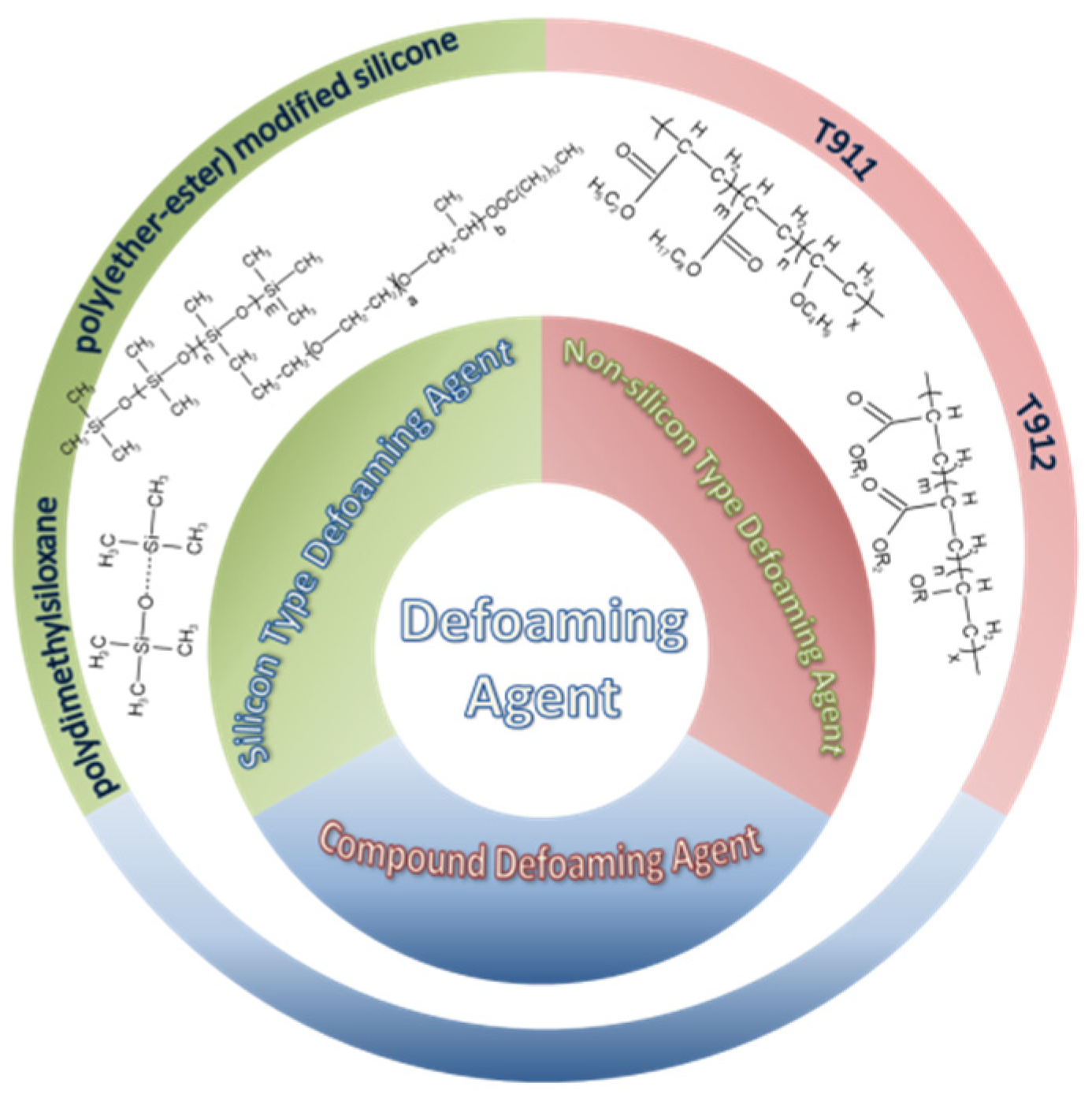
4.3.1. Silicone-Type Defoaming Agent
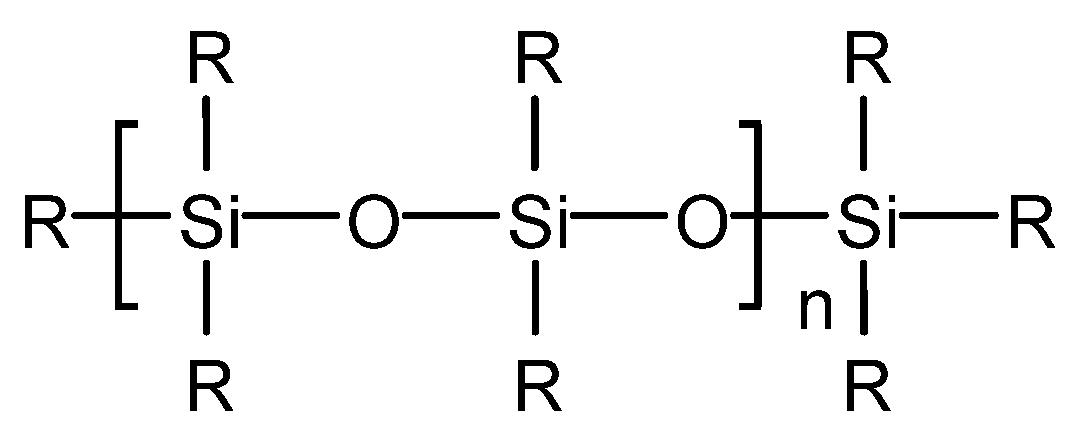
In addition, T901 is sensitive to blending technology, is prone to settling and accumulating in lubricating oil and has poor defoaming performance after storage. Zhang Liang et al. [31] have proved through experiments that with the increase of T901 addition, fine particles of the defoaming agent gather into droplets, which damages the surface tension system of the lubricating oil itself, resulting in undesirable phenomena such as the decrease of defoaming performance and the increase of turbidity of the lubricating oil.
Polyether-modified polysiloxane defoaming agent [32] is also a hot research topic in recent years. The main focus is on introducing polyether segments into the polysiloxane chain through block copolymerization or graft copolymerization. The hydrophilic polyether chain segments endow it with water solubility, and the hydrophobic polysiloxane chain segments endow it with low surface tension [33]. This kind of defoaming agent has the advantages of both polyether and silicone defoaming agent. Therefore, it has the characteristics of low surface tension, rapid defoaming, long effective foam suppression time, no toxicity and harm, good stability, low cost, lower dosage, wide application and so on. It is also the most ideal new variety in silicone and has good development prospects.
Yinchen Dou et al. synthesized a poly (ether-ester)-modified silicone defoaming agent [34]. The defoaming performance of the product was tested, and it was concluded that the surface tension is 28.6 mN/m when the mass concentration of poly (ether-ester)-modified silicone solution is 0.3 g/L. The defoaming time of poly (ether-ester)-modified silicone defoaming agent is 5 s, which is superior to that of a polyether defoaming agent GPE and a silicone defoaming agent X-100F and inferior to that of a silicone defoaming agent SAG. Its foam inhibition height is 300 mL, which is superior to that of a polyether defoaming agent GPE and a silicone defoaming agent X-100F and is equal to that of a silicone defoaming agent SAG. Poly (ether-ester)-modified silicone can rapidly reduce the surface tension at a low mass concentration and has excellent surface performance. Its structure diagram is shown in Figure 6. For the determination of surface tension, the poly (ether-ester)-modified silicone oil was prepared into different concentrations of an aqueous solution at room temperature and measured by a HARKE-A surface tensiometer. For the determination of defoaming performance, the foaming solution was prepared according to GB/T26527-2011 “silicone defoaming agent”, the defoaming performance was determined, and the foaming force and defoaming performance were measured by a cyclic bubbling meter.
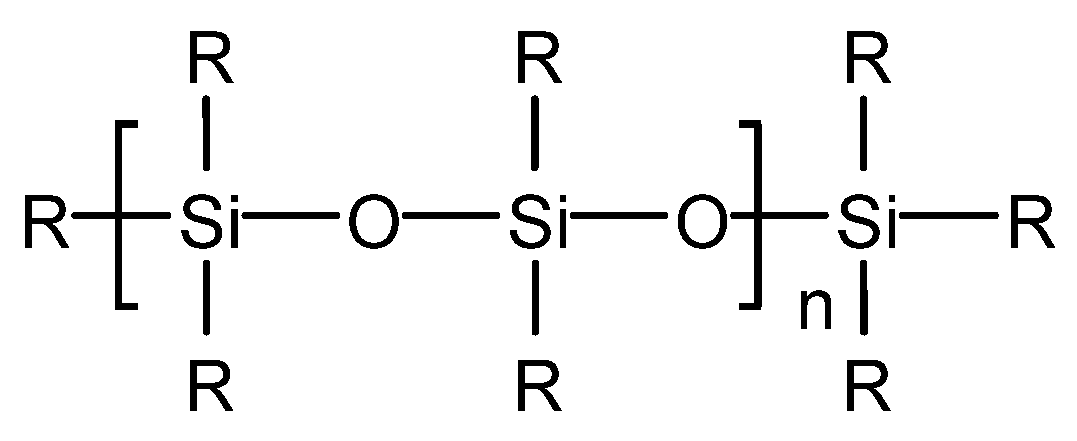
Figure 6. Structure of poly (ether-ester)-modified silicone.
Qiufeng An et al. [35] prepared hydroxyl-capped polyoxypropylene polyoxyethylene oxypropyl-b-polydimethylsiloxane (polyether-b-polysiloxane for short), which is denoted as PESO, and its structure is shown in Figure 7. PESO, dimethyl silicone oil, hydroxyl silicone oil and silicone rubber complex and hydrophobic silica white were added into the three-necked flask equipped with a stirrer, a reflux condenser and a thermometer according to the metering ratio, stirred, heated and warmed up to the set reaction temperature for 30 min. Then, an emulsifier was added and mixed evenly. Finally, deionized water was added while stirring until the solid mass fraction was 45% and a milky white homogeneous liquid could be obtained, namely a nano-effective polyether silicone defoaming agent. The nano-effective polyether silicone defoaming agent has fast foam-bursting speed, relatively long-lasting foam inhibition time, and its performance is close to the level of similar samples.
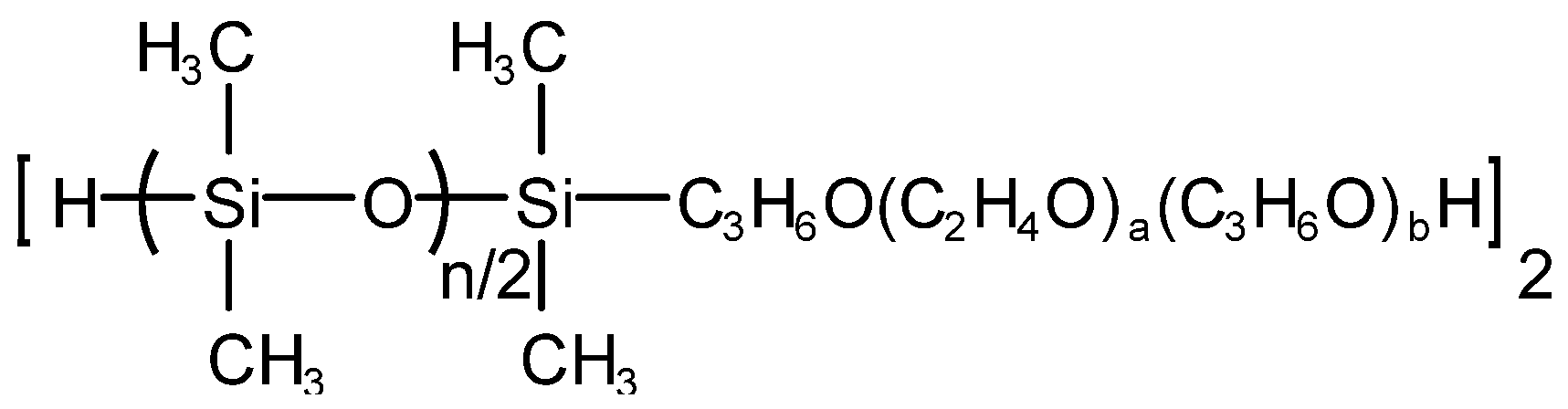
Figure 7. Structure of PESO.
Yan Hu et al. [36] synthesized a low-silicone defoaming agent with a variety of polyether-modified polysiloxanes as the main body (named polyether-modified polysiloxane defoaming agent) and used a refinery residual oil from CNOOC (China National Offshore Oil Corporation) as the foaming fluid to simulate the delayed coking foaming process in the laboratory. The performance was evaluated by comparing it with that of many different types of delayed coking defoaming agents on the market.
4.3.2. Non-Silicone-Type Defoaming Agent
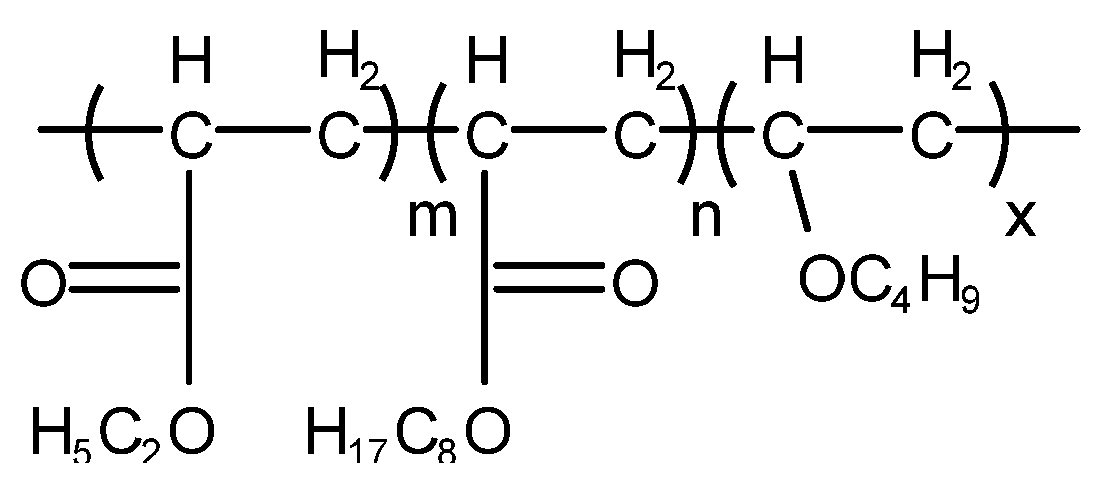
HongZhou等人[40]开发了一种新型非有机硅消泡剂AR-1101,其效果与NF消泡剂相似。公式如图 10 所示。AR-1101外观为乳白色粘稠液体,非离子,固含量在40%以上,pH6-8,无腐蚀性。AR-1101是一种新型多组分非有机硅消泡剂,具有良好的稳定性和持久的消泡效果。
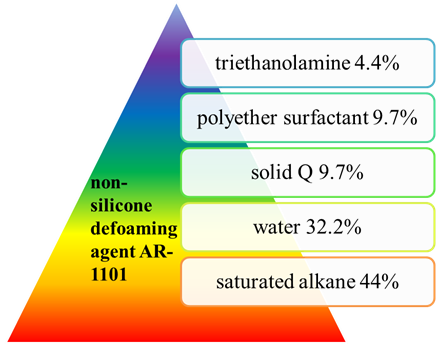
图 10. 制备非有机硅消泡剂的原料AR-1101。
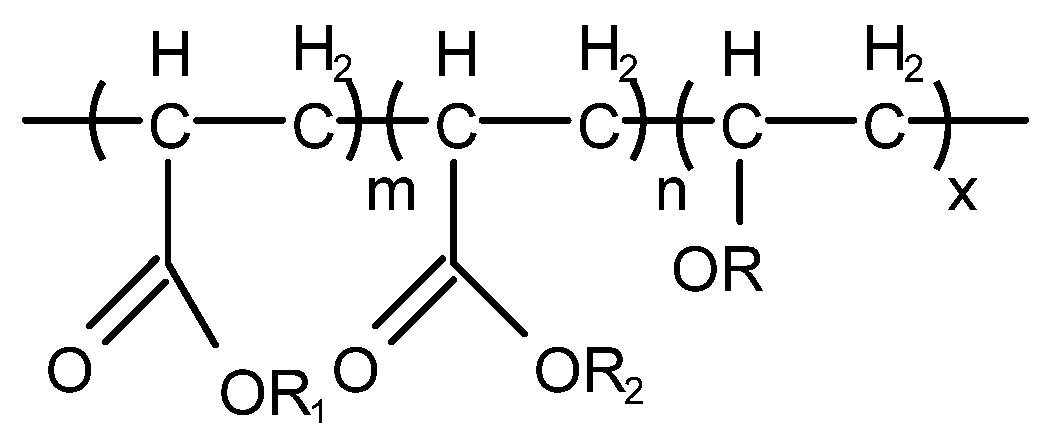
Jing Xiong等[41]发明了一种三元共聚物型非有机硅消泡剂,其分子式如图11所示。消泡性能按GB/T12579-2002《润滑油发泡特性的测定》进行。
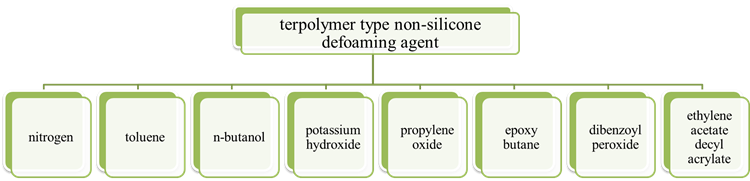
图 11. 用于制备三元共聚物型非有机硅消泡剂的原料。
陈玉娟等[42]发明了一种非有机硅消泡剂及其制备方法。以植物油及其衍生物为载体,以二氧化硅和脂肪酸金属皂为主要消泡物质。为了解决这个问题,本发明引入了一种氢化蓖麻油物质。一方面,通过特殊工艺,保证了消泡性能,同时保证了产品的稳定性。通过特殊工艺和氢化蓖麻油的二次引入,进一步提高了产品的消泡性能。此外,引入具有特殊结构(如图12所示)的蓖麻油聚氧乙烯聚氧内酯油酸酯,确保了产品的良好相容性。

图 12. 蓖麻油聚氧乙烯聚氧内酯油酸酯的结构(R选自H,-OC(CH2)7CH=CH(CH2)7CH3至少有一个 R 基团是 –OC(CH2)7CH=CH(CH2)7CH3,a + b + c = 0–40 和 d + e + f = 0–20。
4.3.3. Compound Defoaming Agent
Yunfang Cao [46] produced a novel 410 compound defoaming agent that was mixed with silicone defoaming agent methylsilicone oil (T901) and non-silicone defoaming agent acrylate ether copolymer (T912). 410 is an effective compound defoaming agent that is suitable for internal combustion engine oil. The recommended dosage is 10–1200 μg/g. When using, the oil is heated to 60 ± 5 °C under mechanical stirring conditions, the 410 compound defoaming agent is added directly and slowly to the oil according to the required amount and stirred evenly. With adding 0.02% of the 410 compound defoaming agent, the foaming characteristic was 10/0 mL/mL, which proved its good defoaming property (Table 1).
Wei Xu et al. invented a variety of compound defoaming agents for lubricating oil [47], including compound defoaming agents 1, 2, 3, 4 and 5 (Table 1).
Table 1. Defoaming performance parameters of some typical defoaming agents.
|
Defoaming Agents |
Dosage |
Foaming Characteristics (Foam Tendency/Foam Stability) (24 °C, mL/mL) |
Oil for Test |
Data Source |
|
|
silicone-type defoaming agent |
Polydimethylsiloxane |
0 |
650/600 |
TBN25 marine medium-speed oil |
[12] [48] [49] [50] |
|
(T901) |
0.03% |
570/470 |
|||
|
Non-silicone-type defoaming agent |
Acrylate ether copolymer |
0 |
435/20 |
Medium extreme-pressure gear oil |
[51] |
|
T911 |
0.03%~0.1% |
0/0 |
[52] |
||
|
Acrylate ether copolymer |
0 |
650/600 |
TBN25 marine medium-speed oil |
[51] |
|
|
T912 |
0.14% |
570/280 |
[50] |
||
|
2-EHA/VAC copolymer high-efficiency defoaming agent |
0 |
600/520 |
Cold heading gear oil |
[43] |
|
|
0.05% |
0/0 |
||||
|
T921 |
0 |
/ |
Advanced anti-wear hydraulic oil |
[52] |
|
|
0.005%~0.1% |
5/0 |
||||
|
Compound defoaming agent |
T922 |
0 |
620/560 |
Shanghai 4040 medium-speed engine oil |
[45] |
|
0.1% |
355/0 |
||||
|
T923 |
0 |
620/560 |
Shanghai 4040 medium-speed engine oil |
[45] |
|
|
0.05% |
10/0 |
||||
|
410 |
0 |
570/530 |
Diesel engine three-generation oil |
[46] |
|
|
0.02% |
10/0 |
||||
|
412 |
0 |
650/600 |
TBN25 marine medium-speed oil |
[50] |
|
|
0.1% |
10/0 |
||||
|
Compound defoaming agent 1 |
0 |
240/30 (150 °C) |
Internal combustion engine oil |
[47] |
|
|
0.005% |
70/0 (150 °C) |
SL5W-30 |
|||
|
Compound defoaming agent 2 |
0 |
210/0 (150 °C) |
Internal combustion engine oil |
[47] |
|
|
0.01% |
70/0 (150 °C) |
SM5W-30 |
|||
|
Compound defoaming agent 3 |
0 |
210/0 (150 °C) |
Internal combustion engine oil |
[47] |
|
|
0.005% |
40/0 (150 °C) |
SM5W-30 |
|||
|
Compound defoaming agent 4 |
0 |
210/0 (150 °C) |
Internal combustion engine oil |
[47] |
|
|
0.005% |
30/0 (150 °C) |
SM5W-30 |
|||
|
Compound defoaming agent 5 |
0 |
190/10 (150 °C) |
Continuously variable transmission oil |
[47] |
|
|
0.005% |
50/0 (150 °C) |
CVTF |
|||
- Foam Resistance Parameters of Defoaming Agent
The defoaming parameters of three kinds of defoaming agents are listed in this paper, as shown in Table 1.
6. Conclusions and Outlook
The application of silicone-based compounds in the separation tank can inhibit the formation of foam but can then cause serious catalyst deactivation in the later stage of the refining process [53]. Therefore, silicone defoaming agents have a good ability to eliminate foam, but the effect of long-term foam inhibition is poor. The initial foam elimination effect of non-silicone defoaming agents is not as good as that of silicone defoaming agents, but their defoaming ability is stable and does not decrease significantly after long-term storage. Compound defoaming agents with good bubble suppression and bubble bursting effects, high dispersibility and good durability will occupy the dominant position in the market to replace single defoaming agents with poor performance and unstable chemical properties [54]. However, the application of compound defoaming agents is a new, and their application scope and methods need to be further studied [55]. The synergistic defoaming effect of the mixture of insoluble hydrophobic particles and hydrophobic oil (filled defoaming agent) dispersed in aqueous media has been well confirmed in the patent literature in the early 1950s. These mixed defoaming agents are very effective at low concentrations (10–1000 ppm) and are widely used [56].
However, there is little information in the public literature on the inactivation of other types of defoaming agents [57]. In the future, research on polyether-modified polysiloxane defoaming agents can be carried out from the following aspects [58]: (1) Optimizing the structure of polyether-modified polysiloxane from the perspective of molecular design, and preparing polyether-modified polysiloxane with high yield, good performance, strong stability, and environmental protection by adjusting the amount and arrangement formula of ethylene oxide and propylene oxide in the polyether chain segment, the type of polyether end group and the structure of hydrogen-containing silicone oil. (2) Introducing some functional groups to impart other properties to polyether-modified polysiloxanes, suitable for some special foaming systems. (3) For -Si-C- polyether-modified polysiloxanes, seeking low-cost catalysts to reduce production costs; For -Si-O-C- polyether-modified polysiloxanes, seeking suitable additives to reduce the hydrolysis rate of the product and extend the product’s shelf life. (4) Continued exploring of the defoaming mechanism of this type of defoaming agent and optimizing the molecular structure of polyether-modified polysiloxane and composite additives based on the mechanism.
references
[1]
[2]
[3]
[4]
[5]
[6]
[7]
[8]
[9]
[10]
[11]
[12]
[13]
[14]
[15]
[16]
[17]
[18]
[19]
[20]
[21]
[22]
[23]
[24]
[25]
[26]
[27]
[28]
[29]
[30]
[31]
[32]
[33]
[34]
[35]
[36]
[37]
[38]
[39]
[40]
[41]
[42]
[43]
[44]
[45]
[46]
[47]
[48]
[49]
[50]
[51]
[52]
[53]
[54]
[55]
[56]
[57]
[58]
This entry is adapted from the peer-reviewed paper 10.3390/molecules28073152
