
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hichem Moulahoum | -- | 2924 | 2023-04-11 20:56:17 | | | |
| 2 | Lindsay Dong | Meta information modification | 2924 | 2023-04-13 07:40:25 | | |
Video Upload Options
The impressive development in biosensing devices has led to the creation of man-made implantable devices that are temporarily or permanently introduced into the human body, and thus, diminishing the pain and discomfort of the person. Biosensors in the in vivo environment are required to have specific features, including biocompatibility (minimal immune response or biofouling), biodegradability, reliability, high accuracy, and miniaturization (flexible, stretchable, lightweight, and ultra-thin). However, the performance of implantable biosensors is limited by body responses and insufficient power supplies (due to minimized batteries/electronics and data transmission without wires).
1. Introduction
2. Implantable Biosensors and Their Design
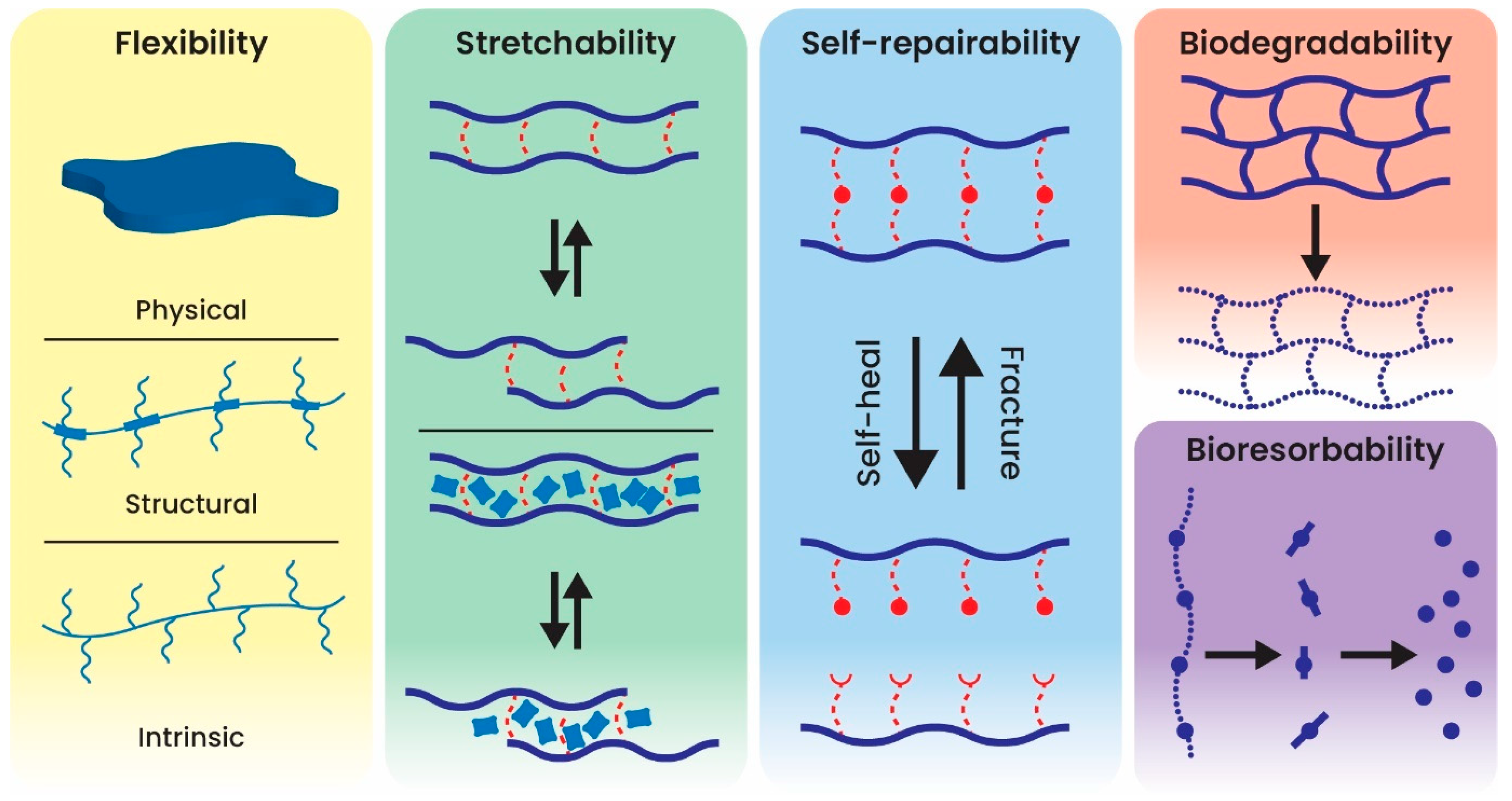
3. Classification of Implantable Biosensors Based on Material Design
3.1. Electrochemical Active-Based Implantable Biosensors
3.2. Nanomaterial-Based Implantable Biosensors
3.3. Fiber-Based Implantable Biosensors
3.4. Polymer-Based Implantable Biosensors
4. Coating Implantable Biosensors
5. Challenges
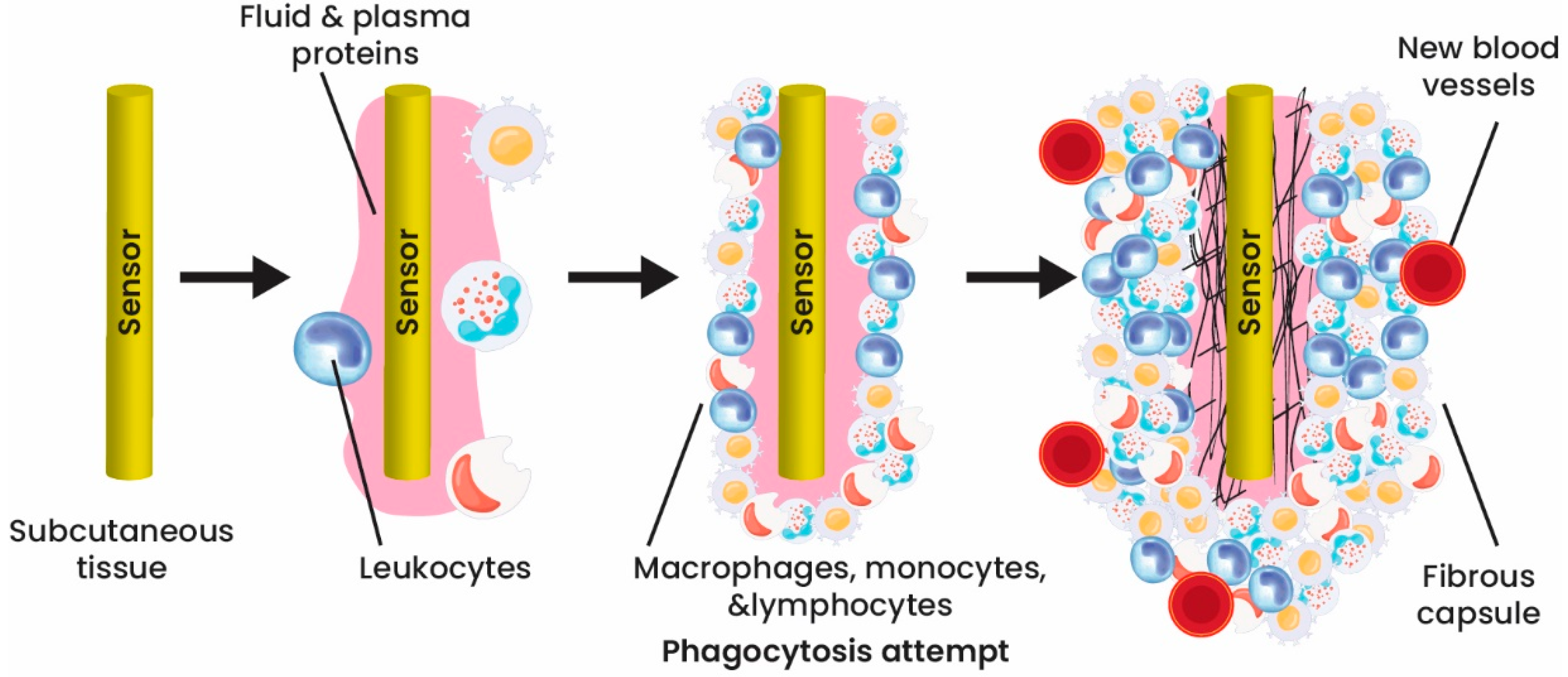
Creating implantable biosensors comes with significant obstacles, including the foreign-body response, stability, and biosensor response, as well as the need for continuous monitoring, power supply, and data transmission. Overcoming these obstacles requires meeting specific criteria, such as utilizing more adaptable and biocompatible biomaterials, achieving miniaturization, and ensuring reliability. The implementation of these design parameters is essential in the development of implantable biosensors [46][47].
Various concerns should be considered and thought of in advance to design and then apply an ideal implantable biosensor. As aforementioned, biocompatibility of the design materials to avoid any unfavorable reactions in the body is the first and most crucial factor [48]. With developing science, many implantable sensors have shown minor cell injury. However, the materials used in long-term working biosensors (years or even a lifetime) must be biodegradable and biocompatible. For short-term strategies (e.g., digestible biosensors), high biocompatibility is demanded for both the instrument and the degraded products.
Besides biocompatibility, the device should show lasting stability, accuracy, selectivity, miniaturization, downscaled power, and portability. The label-free electrochemical implantable biosensors with the promising features cited earlier have gotten tremendous attention in this field. Integrating nanomaterials and nanotechnology in the biosensor field could improve biosensors’ performance and functionality and solve the main problematic issues in designing biosensors [49].
References
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45.
- Boutry, C.M.; Beker, L.; Kaizawa, Y.; Vassos, C.; Tran, H.; Hinckley, A.C.; Pfattner, R.; Niu, S.; Li, J.; Claverie, J.; et al. Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat. Biomed. Eng. 2019, 3, 47–57.
- Lee, J.; Cho, H.R.; Cha, G.D.; Seo, H.; Lee, S.; Park, C.K.; Kim, J.W.; Qiao, S.; Wang, L.; Kang, D.; et al. Flexible, sticky, and biodegradable wireless device for drug delivery to brain tumors. Nat. Commun. 2019, 10, 5205.
- Xiao, X.; Wang, J.; Liu, C.; Carlisle, J.A.; Mech, B.; Greenberg, R.; Guven, D.; Freda, R.; Humayun, M.S.; Weiland, J.; et al. In vitro and in vivo evaluation of ultrananocrystalline diamond for coating of implantable retinal microchips. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 77, 273–281.
- Ben-Menachem, E.; Manon-Espaillat, R.; Ristanovic, R.; Wilder, B.J.; Stefan, H.; Mirza, W.; Tarver, W.B.; Wernicke, J.F. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia 1994, 35, 616–626.
- Cribier, A.; Eltchaninoff, H.; Bash, A.; Borenstein, N.; Tron, C.; Bauer, F.; Derumeaux, G.; Anselme, F.; Laborde, F.; Leon, M.B. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: First human case description. Circulation 2002, 106, 3006–3008.
- Kujawa, S.G.; Liberman, M.C. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci. 2009, 29, 14077–14085.
- Rose, E.A.; Gelijns, A.C.; Moskowitz, A.J.; Heitjan, D.F.; Stevenson, L.W.; Dembitsky, W.; Long, J.W.; Ascheim, D.D.; Tierney, A.R.; Levitan, R.G.; et al. Long-term use of a left ventricular assist device for end-stage heart failure. N. Engl. J. Med. 2001, 345, 1435–1443.
- Sanders, G.D.; Hlatky, M.A.; Owens, D.K. Cost-effectiveness of implantable cardioverter-defibrillators. N. Engl. J. Med. 2005, 353, 1471–1480.
- Song, E.; Li, J.; Won, S.M.; Bai, W.; Rogers, J.A. Materials for flexible bioelectronic systems as chronic neural interfaces. Nat. Mater. 2020, 19, 590–603.
- Byun, S.H.; Sim, J.Y.; Zhou, Z.; Lee, J.; Qazi, R.; Walicki, M.C.; Parker, K.E.; Haney, M.P.; Choi, S.H.; Shon, A.; et al. Mechanically transformative electronics, sensors, and implantable devices. Sci. Adv. 2019, 5, eaay0418.
- Lim, H.R.; Kim, H.S.; Qazi, R.; Kwon, Y.T.; Jeong, J.W.; Yeo, W.H. Advanced Soft Materials, Sensor Integrations, and Applications of Wearable Flexible Hybrid Electronics in Healthcare, Energy, and Environment. Adv. Mater. 2020, 32, e1901924.
- Kang, J.; Son, D.; Wang, G.N.; Liu, Y.; Lopez, J.; Kim, Y.; Oh, J.Y.; Katsumata, T.; Mun, J.; Lee, Y.; et al. Tough and Water-Insensitive Self-Healing Elastomer for Robust Electronic Skin. Adv. Mater. 2018, 30, e1706846.
- Kang, J.; Tok, J.B.H.; Bao, Z.A. Self-healing soft electronics. Nat. Electron. 2019, 2, 144–150.
- Liu, Y.; Li, J.; Song, S.; Kang, J.; Tsao, Y.; Chen, S.; Mottini, V.; McConnell, K.; Xu, W.; Zheng, Y.Q.; et al. Morphing electronics enable neuromodulation in growing tissue. Nat. Biotechnol. 2020, 38, 1031–1036.
- Jeong, J.W.; McCall, J.G.; Shin, G.; Zhang, Y.; Al-Hasani, R.; Kim, M.; Li, S.; Sim, J.Y.; Jang, K.I.; Shi, Y.; et al. Wireless Optofluidic Systems for Programmable In Vivo Pharmacology and Optogenetics. Cell 2015, 162, 662–674.
- Kang, S.K.; Murphy, R.K.; Hwang, S.W.; Lee, S.M.; Harburg, D.V.; Krueger, N.A.; Shin, J.; Gamble, P.; Cheng, H.; Yu, S.; et al. Bioresorbable silicon electronic sensors for the brain. Nature 2016, 530, 71–76.
- Nelson, B.D.; Karipott, S.S.; Wang, Y.; Ong, K.G. Wireless Technologies for Implantable Devices. Sensors 2020, 20, 4604.
- Guillou, P.J.; Quirke, P.; Thorpe, H.; Walker, J.; Jayne, D.G.; Smith, A.M.; Heath, R.M.; Brown, J.M.; for the MRC CLASICC Trial Group. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): Multicentre, randomised controlled trial. Lancet 2005, 365, 1718–1726.
- Liu, J.; Fu, T.M.; Cheng, Z.; Hong, G.; Zhou, T.; Jin, L.; Duvvuri, M.; Jiang, Z.; Kruskal, P.; Xie, C.; et al. Syringe-injectable electronics. Nat. Nanotechnol. 2015, 10, 629–636.
- Montgomery, M.; Ahadian, S.; Davenport Huyer, L.; Lo Rito, M.; Civitarese, R.A.; Vanderlaan, R.D.; Wu, J.; Reis, L.A.; Momen, A.; Akbari, S.; et al. Flexible shape-memory scaffold for minimally invasive delivery of functional tissues. Nat. Mater. 2017, 16, 1038–1046.
- Qazi, R.; Gomez, A.M.; Castro, D.C.; Zou, Z.; Sim, J.Y.; Xiong, Y.; Abdo, J.; Kim, C.Y.; Anderson, A.; Lohner, F.; et al. Wireless optofluidic brain probes for chronic neuropharmacology and photostimulation. Nat. Biomed. Eng. 2019, 3, 655–669.
- Whyte, W.; Roche, E.T.; Varela, C.E.; Mendez, K.; Islam, S.; O’Neill, H.; Weafer, F.; Shirazi, R.N.; Weaver, J.C.; Vasilyev, N.V.; et al. Sustained release of targeted cardiac therapy with a replenishable implanted epicardial reservoir. Nat. Biomed. Eng. 2018, 2, 416–428.
- Lu, T.; Ji, S.; Jin, W.; Yang, Q.; Luo, Q.; Ren, T.L. Biocompatible and Long-Term Monitoring Strategies of Wearable, Ingestible and Implantable Biosensors: Reform the Next Generation Healthcare. Sensors 2023, 23, 2991.
- Mendelson, Y. Biomedical Sensors. In Introduction to Biomedical Engineering; Enderle, J.D., Bronzino, J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 609–666.
- Long, N.; Yu, B.; Moussy, Y.; Moussy, F. Strategies for testing long-term transcutaneous amperometric glucose sensors. Diabetes Technol. Ther. 2005, 7, 927–936.
- Frost, M.C.; Meyerhoff, M.E. Implantable chemical sensors for real-time clinical monitoring: Progress and challenges. Curr. Opin. Chem. Biol. 2002, 6, 633–641.
- Zhang, X. Real time and in vivo monitoring of nitric oxide by electrochemical sensors—From dream to reality. Front. Biosci. 2004, 9, 3434–3446.
- Zhou, D.D.; Greenberg, R.J. Microsensors and microbiosensors for retinal implants. Front. Biosci.-Landmark 2005, 10, 166–179.
- Gerritsen, M.; Kros, A.; Sprakel, V.; Lutterman, J.A.; Nolte, R.J.; Jansen, J.A. Biocompatibility evaluation of sol-gel coatings for subcutaneously implantable glucose sensors. Biomaterials 2000, 21, 71–78.
- Lee, S.; Ozlu, B.; Eom, T.; Martin, D.C.; Shim, B.S. Electrically conducting polymers for bio-interfacing electronics: From neural and cardiac interfaces to bone and artificial tissue biomaterials. Biosens. Bioelectron. 2020, 170, 112620.
- Ramanavicius, S.; Ramanavicius, A. Conducting Polymers in the Design of Biosensors and Biofuel Cells. Polymers 2021, 13, 49.
- Sharkawy, A.A.; Klitzman, B.; Truskey, G.A.; Reichert, W.M. Engineering the tissue which encapsulates subcutaneous implants. III. Effective tissue response times. J. Biomed. Mater. Res. 1998, 40, 598–605.
- Updike, S.J.; Shults, M.; Rhodes, R. Principles of long-term fully implanted sensors with emphasis on radiotelemetric monitoring of blood glucose from inside a subcutaneous foreign body capsule (FBC). In Biosensors in the Body: Continuous In Vivo Monitoring; Wiley: Hoboken, NJ, USA, 1997; pp. 117–137.
- Srinivasan, S.; Sawyer, P.N. Role of surface charge of the blood vessel wall, blood cells, and prosthetic materials in intravascular thrombosis. J. Colloid Interface Sci. 1970, 32, 456–463.
- Ainslie, K.M.; Desai, T.A. Microfabricated implants for applications in therapeutic delivery, tissue engineering, and biosensing. Lab Chip 2008, 8, 1864–1878.
- Popat, K.C.; Eltgroth, M.; Latempa, T.J.; Grimes, C.A.; Desai, T.A. Decreased Staphylococcus epidermis adhesion and increased osteoblast functionality on antibiotic-loaded titania nanotubes. Biomaterials 2007, 28, 4880–4888.
- Martin, F.; Walczak, R.; Boiarski, A.; Cohen, M.; West, T.; Cosentino, C.; Shapiro, J.; Ferrari, M. Tailoring width of microfabricated nanochannels to solute size can be used to control diffusion kinetics. J. Control. Release 2005, 102, 123–133.
- Yang, X.; Zhou, T.; Zwang, T.J.; Hong, G.; Zhao, Y.; Viveros, R.D.; Fu, T.M.; Gao, T.; Lieber, C.M. Bioinspired neuron-like electronics. Nat. Mater. 2019, 18, 510–517.
- Wang, L.; Xie, S.; Wang, Z.; Liu, F.; Yang, Y.; Tang, C.; Wu, X.; Liu, P.; Li, Y.; Saiyin, H.; et al. Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers. Nat. Biomed. Eng. 2020, 4, 159–171.
- Curry, E.J.; Le, T.T.; Das, R.; Ke, K.; Santorella, E.M.; Paul, D.; Chorsi, M.T.; Tran, K.T.M.; Baroody, J.; Borges, E.R.; et al. Biodegradable nanofiber-based piezoelectric transducer. Proc. Natl. Acad. Sci. USA 2020, 117, 214–220.
- Morais, J.M.; Papadimitrakopoulos, F.; Burgess, D.J. Biomaterials/tissue interactions: Possible solutions to overcome foreign body response. AAPS J. 2010, 12, 188–196.
- Quinn, C.P.; Pathak, C.P.; Heller, A.; Hubbell, J.A. Photo-crosslinked copolymers of 2-hydroxyethyl methacrylate, poly(ethylene glycol) tetra-acrylate and ethylene dimethacrylate for improving biocompatibility of biosensors. Biomaterials 1995, 16, 389–396.
- Wisniewski, N.; Reichert, M. Methods for reducing biosensor membrane biofouling. Colloids Surf. B Biointerfaces 2000, 18, 197–219.
- Norton, L.W.; Koschwanez, H.E.; Wisniewski, N.A.; Klitzman, B.; Reichert, W.M. Vascular endothelial growth factor and dexamethasone release from nonfouling sensor coatings affect the foreign body response. J. Biomed. Mater. Res. A 2007, 81, 858–869.
- Webb, R.C.; Bonifas, A.P.; Behnaz, A.; Zhang, Y.; Yu, K.J.; Cheng, H.; Shi, M.; Bian, Z.; Liu, Z.; Kim, Y.S.; et al. Ultrathin conformal devices for precise and continuous thermal characterization of human skin. Nat. Mater. 2013, 12, 938–944.
- Rebelo, R.; Barbosa, A.I.; Correlo, V.M.; Reis, R.L. An Outlook on Implantable Biosensors for Personalized Medicine. Engineering 2021, 7, 1696–1699.
- Sadik, O.A.; Aluoch, A.O.; Zhou, A. Status of biomolecular recognition using electrochemical techniques. Biosens. Bioelectron. 2009, 24, 2749–2765.
- Erdem, A.; Karadeniz, H.; Caliskan, A. Single-Walled Carbon Nanotubes Modified Graphite Electrodes for Electrochemical Monitoring of Nucleic Acids and Biomolecular Interactions. Electroanalysis 2009, 21, 464–471.





