Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Christopher R. M. Asquith | -- | 1577 | 2023-04-11 13:21:46 | | | |
| 2 | Sirius Huang | Meta information modification | 1577 | 2023-04-12 02:31:17 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kalogirou, A.S.; Oh, H.J.; Asquith, C.R.M. Synthesis of the 1,2,3-Dithiazole Scaffold. Encyclopedia. Available online: https://encyclopedia.pub/entry/42944 (accessed on 08 February 2026).
Kalogirou AS, Oh HJ, Asquith CRM. Synthesis of the 1,2,3-Dithiazole Scaffold. Encyclopedia. Available at: https://encyclopedia.pub/entry/42944. Accessed February 08, 2026.
Kalogirou, Andreas S., Hans J. Oh, Christopher R. M. Asquith. "Synthesis of the 1,2,3-Dithiazole Scaffold" Encyclopedia, https://encyclopedia.pub/entry/42944 (accessed February 08, 2026).
Kalogirou, A.S., Oh, H.J., & Asquith, C.R.M. (2023, April 11). Synthesis of the 1,2,3-Dithiazole Scaffold. In Encyclopedia. https://encyclopedia.pub/entry/42944
Kalogirou, Andreas S., et al. "Synthesis of the 1,2,3-Dithiazole Scaffold." Encyclopedia. Web. 11 April, 2023.
Copy Citation
The 1,2,3-dithiazole is an underappreciated scaffold in medicinal chemistry despite possessing a wide variety of nascent pharmacological activities. The scaffold has a potential wealth of opportunities within these activities and further afield. The synthesis of Appel salt 1 acted as a catalyst to the field and granted access to many 1,2,3-dithiazole derivatives, and to other heterocycles incorporating sulfur and nitrogen atoms.
appel salt
1,2,3-dithiazole
disulfide bridge
herbicidal
antifungal
antibacterial
antiviral
anticancer
1. Introduction
The 1,2,3-dithiazole core is a five membered heterocycle containing two sulfur atoms and one nitrogen atom. Despite the fact that the 1,2,3-dithiazole is not present in nature, similar to many other heterocycles, it does have a broad range of interesting biological activities. The 1,2,3-dithiazole moiety was first synthesized in 1957 by G. Schindler et al. [1]. This was followed two decades later by a report by J. E. Moore on behalf of Chevron Research Co. where it showcased antifungal and herbicidal activity [2][3]. In 1985, Appel et al. reported the synthesis of 4,5-dichloro-1,2,3-dithiazolium chloride 1 (Appel’s salt), a precursor which allowed access to the 1,2,3-dithiazole core within a single step [4][5].
The synthesis of Appel salt 1 acted as a catalyst to the field and granted access to many 1,2,3-dithiazole derivatives, and to other heterocycles incorporating sulfur and nitrogen atoms [6][7][8][9][10][11]. The subsequent synthetic reports focused on transformations on the C5 position [6][7][8][9][10][11]. However, one of the key synthetic interests beyond expanding the scope of 5-substituted 1,2,3-dithiazoles was the limited reactivity of the C4 position. Several different approaches were used to address this C4 reactively issue, including intramolecular cyclization [6] using a multi-step oxime pathway [12][13], or more recently, direct reactions [14], all of which expanded the chemical space around the 1,2,3-dithiazole. Some of these approaches have been covered in past reviews around the chemistry of 1,2,3-dithiazoles [6][7][8][9][10][11] (Figure 1).
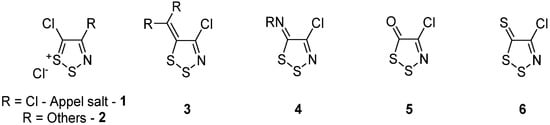
Figure 1. Appel salt (1) and other general 1,2,3-dithazoles structures 2–6.
2. 1,2,3-Dithiazoles Synthesis Overview
2.1. Early Years before Appel Salt
Early work on the synthesis of 1,2,3-dithiazoles used cyanothioformamides as starting materials. Treatment of a variety of arylcyanothioformamides 7 with sulfur dichloride at 0–25 °C gave a number of N-aryl-5H-1,2,3-dithiazol-5-imines 4 (Scheme 1) [2]. The initial reaction yielded the corresponding hydrochloride salts, which could be converted to the free base by refluxing in a toluene solution.

Scheme 1. Synthesis of N-aryl-5H-1,2,3-dithiazol-5-imines 4 from arylcyanothioformamides 7.
Interestingly, the N-aryl-5H-1,2,3-dithiazol-5-imines 4 can be degraded to the respective cyanothioformamides 7 by thiophilic ring cleavage after reaction with triphenylphosphine or sodium hydroxide [4][15], oxidative ring cleavage after reaction with m-CPBA [16], or by reductive ring cleavage after reaction with sodium cyanoborohydride [17] (Scheme 2).
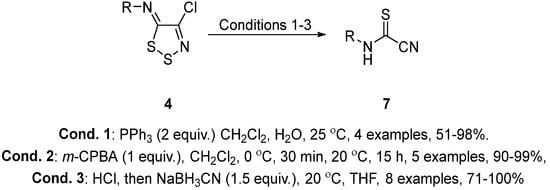
Scheme 2. Degradation of N-aryl-5H-1,2,3-dithiazol-5-imines 4 to cyanothioformamides 7.
2.2. Discovery of Appel Salt and Applications
A significant discovery in the chemistry of 1,2,3-dithiazoles was the synthesis of 4,5-dichloro-1,2,3-dithiazolium chloride 1 (Appel salt) by Appel et al. in 1985, which was readily prepared from chloroacetonitrile and disulfur dichloride [4][18] (Scheme 3). Appel salt 1 was subsequently used as an important reagent for the preparation of other 4-chloro-5H-1,2,3-dithiazoles with the most reactive site being the electrophilic C-5 position [4][9][16].
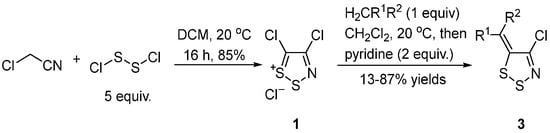
Scheme 3. Synthesis of Appel salt and transformation to 4-chloro-5H-1,2,3-dithiazolylidenes 3.
Appel salt 1 can condense with active methylenes, such as acetonitrile derivatives [4][19][20], diketones, ketoesters, and others [21], to give 4-chloro-5H-1,2,3-dithiazolylidenes 3 (Scheme 3).
The condensation of Appel salt with hydrogen sulfide [4] afforded dithiazole-5-thione 6 in 69% yield (Scheme 4). The reaction with oxygen nucleophiles are also common with NaNO3 [4], sulfoxides [22], or formic acid [23] all acting as the source of oxygen to give 4-chloro-5H-1,2,3-dithiazol-5-one 5 in good yields (Scheme 4). Furthermore, the reaction with other carboxylic acids [24] at −78 °C and subsequent treatment with alcohols gave esters 8 in medium to good yields (Scheme 4).
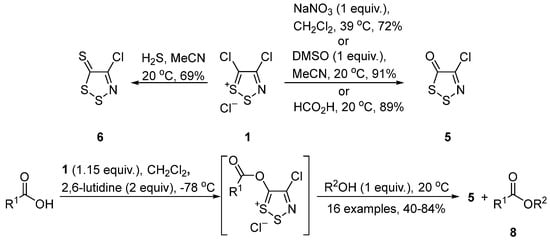
Scheme 4. Reactions of Appel salt 1 with oxygen and sulfur nucleophiles.
The condensation of Appel salt 1 with primary anilines is well studied [4][5][25][26] and typically occurs by treatment with 1 equiv. of the aniline in the presence of pyridine (2 equiv.) as the base to give, in most cases, good yields of N-aryl-5H-1,2,3-dithiazol-5-imines 4 (Scheme 5).
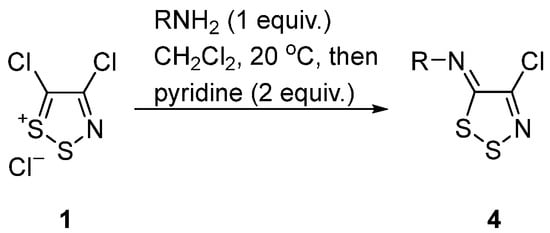
Scheme 5. Synthesis of N-aryl-5H-1,2,3-dithiazol-5-imines 4 from Appel salt 1.
Some limitations of this chemistry appear when using heterocyclic arylamines, such as aminopyridines. A recent study by Koutentis et al. highlighted that the reactions of the three isomeric aminopyridines with Appel salt 1 gave very different yields based on the position of the amino group. The 2-, 3- and 4-aminopyridines gave 69%, 24%, and 1% yields of the desired 1,2,3-dithiazole, respectively [27] (Scheme 6). Koutentis et al. suggested the low yield of 4-aminopyridine is likely attributed to the reduced nucleophilicity of the primary amine due to a contribution of its zwitterionic resonance form. The low reactivity of the amine leads to complex reaction mixtures due to side reactions.
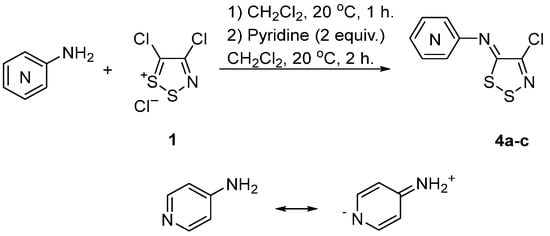
Scheme 6. Reaction of Appel salt 1 with aminopyridines.
2.3. Reactivity of C-4 and the Displacement of the Chloride
The less reactive C4 chlorine of neutral 5H-1,2,3-dithiazoles cannot be directly substituted by nucleophiles. However, utilizing an ANRORC-(Addition of the Nucleophile, Ring Opening, and Ring Closure)-style mechanism, nucleophilic substitution can occur on the C4 chlorine of the 1,2,3-dithazole. An example of this is where the N-Aryl-5H-1,2,3-dithiazol-5-imines 4 react with an excess of dialkylamines to give 4-aminodithiazoles 9 in variable yields (Scheme 7). The reaction was found to proceed via an ANRORC-style mechanism [28][29] involving ring opening by nucleophilic attack on the S2 position to yield disulfides 10 and subsequent recyclization after amine addition on the cyano group [30]. In another report by Koutentis et al. [14], DABCO was reacted with neutral 5H-1,2,3-dithiazoles 4–6 to give N-(2-chloroethyl)piperazines 11 in good yields (Scheme 7). The chloroethyl group originating from chloride attack on the intermediate quaternary ammonium salt formed by the displacement of the C4 chloride by DABCO.
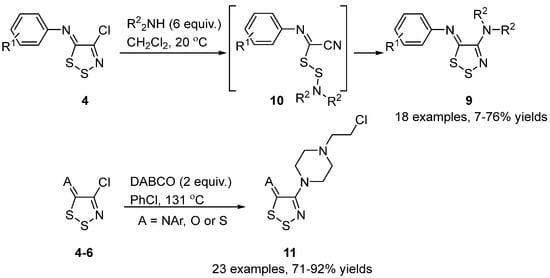
Scheme 7. Displacement of the C4 chlorine of neutral 5H-1,2,3-dithiazoles.
2.4. Alternatives beyond Appel Salt Chemistry
A different way to access both monocyclic and ring fused 1,2,3-dithiazoles is by the reaction of oximes with disulfur dichloride. An example of the synthesis of a ring fused dithiazole is the reaction of benzoindenone oxime 12 to give dithiazole 13 in 81% yield [31][32] (Scheme 8). Acetophenone oximes 14 were reacted with disulfur dichloride to yield dithiazolium chlorides 2, which were subsequently converted to either imines 15, thiones 16, or ketone 17 [13] (Scheme 8). Insights in the mechanism of the oxime to dithiazole transformation were given by Hafner et al. [12], who isolated the dithiazole N-oxide, which is the intermediate in this reaction.
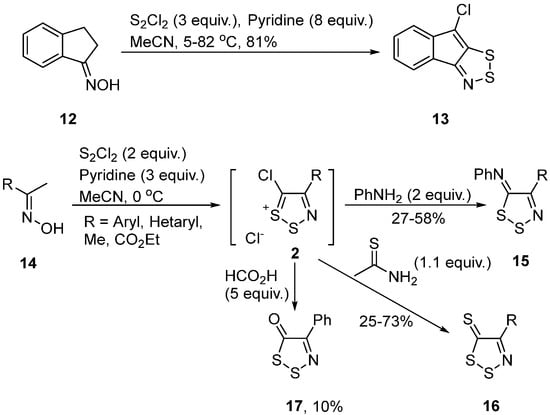
Scheme 8. Synthesis of 1,2,3-dithiazoles from oximes.
2.5. Reactivity of 1,2,3-Dithiazoles
Neutral 1,2,3-dithiazoles can also be transformed to a plethora of other heterocycles, often substituted by a cyano group originating from the imidoyl chloride of the starting material using thermal or reactions with thiophiles. An interesting example of an ANRORC-style mechanism leading to a ring transformation was the reaction of (Z)-N-(4-chloro-5H-1,2,3-dithiazol-5-ylidene)-1H-pyrazol-5-amines 4d with diethylamine that results in disulfide intermediates 18. Subsequent treatment with concentrated sulfuric acid gave 1,2,4-dithiazines 19 in good yields [33] (Scheme 9).
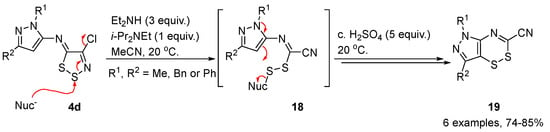
Scheme 9. Synthesis of 1,2,4-dithiazines 19.
In another example, the pyrazoleimino dithiazoles 20 were converted to 4-methoxy-pyrazolo[3,4-d]pyrimidines 21 in medium to good yields by treatment with sodium methoxide in methanol [34] (Scheme 10). The transformation occurs after addition of the methoxide on the nitrile followed by cyclisation onto the dithiazole C5 position that fragments losing S2 and chloride to give the final pyrimidine 21.
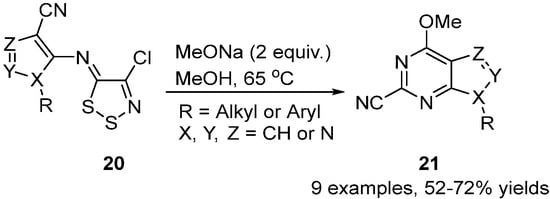
Scheme 10. Synthesis of 4-methoxy-pyrazolo[3,4-d]pyrimidines 21.
A similar example of ring transformations is that of 2-aminobenzyl alcohol dithiazole-imines 4e to 1,3-benzoxazines 22 and 1,3-benzothiazines 23 [35]. Treatment of imines 4e with sodium hydride in THF gave mixtures of benzoxazines 22 and benzothiazines 23, with the former as the main products (Scheme 11). The formation of the former involves deprotonation of the alcohol and cyclisation of the alkoxide onto the dithiazole C5 position. Subsequent fragmentation with loss of S2 and chloride gave the final benzoxazine 22. Alternatively, treatment of imines 4e with Ph3P gave exclusively benzothiazines 23 in good yields (Scheme 11). Thiophilic attack on S1 ring opens the dithiazole ring and a second attack by Ph3P gives the intermediate alkene 24 that cyclizes to benzothiazine 23.
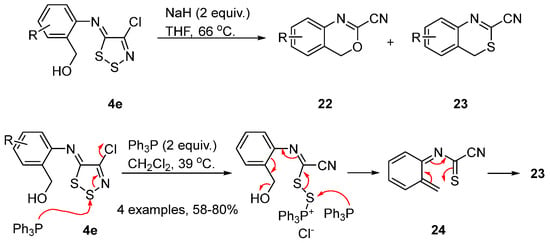
Scheme 11. Synthesis of 1,3-benzoxazines 22 and 1,3-benzothiazines 23.
1,2,3-Dithiazole derivatives can also be converted to mercaptoacetonitriles by the removal of the S1 atom. One example of this are the 3-(1,2,3-dithiazolylidene)indololin-2-ones 25 reacting with sodium hydride (2 equiv.) to yield the mercaptoacetonitrile products 26 in medium to good yields [36] (Scheme 12).
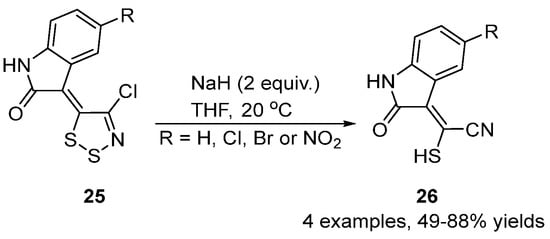
Scheme 12. Conversion of dithiazoles 25 to mercaptoacetonitriles 26.
Perhaps the most unstable 1,2,3-dithiazole is Appel salt itself, which, while relatively stable at ca. 20 °C under a desiccant, in its absence, Appel salt has a tendency to react with moisture. One study by Koutentis et al. revealed that simple stirring in wet MeCN gave elemental sulfur, dithiazole-5-thione 6, dithiazol-5-one 5, and thiazol-5-one 27 [37] (Scheme 13), assisting other scientists working with Appel salt, to identify these products. Interestingly, other dithiazolium salts have also been prepared with increased stability and lower sensitivity to moisture. A series of perchlorate salts of 1,2,3-dithiazoles were prepared by the anion exchange with perchloric acid allowing for more detailed characterization and study of the 1,2,3-dithiazole [18].
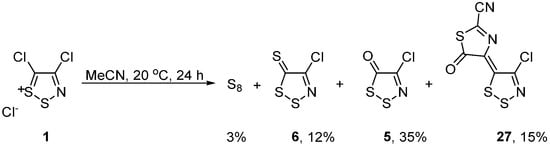
Scheme 13. Degradation of Appel salt 1 in wet MeCN.
In another study by Rakitin et al., 4-substituted 5H-1,2,3-dithiazoles 16 and 17 were converted to 1,2,5-thiadiazoles 28 and 29 by treatment with primary amines [38] (Scheme 14). Mechanistically, the reaction occurs by addition of the amine to the C5 position followed by ring opening of the C-S bond and subsequent ring closing by loss of hydrogen sulfide.
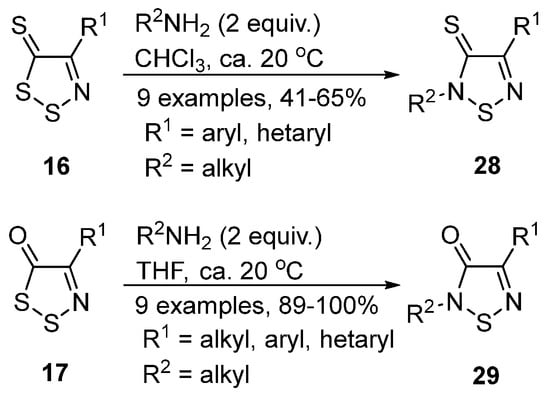
Scheme 14. Transformation of dithiazoles 16–17 to thiadiazines 28–29.
To summarize, 1,2,3-dithiazoles can be converted to other heterocyclic or ring opened derivatives. The six most common mechanisms involved in the transformations of 1,2,3-dithiazoles to other systems are shown below (Scheme 15). These mechanisms begin via a nucleophile assisted ring opening of the dithiazole to disulfide intermediates that then can react either intermolecular or intramolecular with other nucleophiles via the six paths presented.
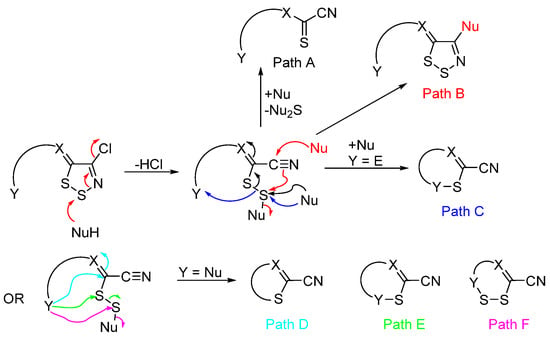
Scheme 15. Overview of the mechanisms of the reactions of 1,2,3-dithiazoles.
References
- Wannagat, U.; Schindler, G. Reaktionen des Schwefeldichlorids mit Pyridin und verwandten Verbindungen. Angew. Chem. 1957, 69, 784-784.
- Moore, J.E. (Chevron Research Co.) Certain 4-Halo-5-aryl-1,2,3-dithiazole Compounds and their Preparation. U.S. Patent Application No. US4059590, 22 November 1977.
- Moore, J.E. (Chevron Research Co.) Method for control of fungi using 4-halo-5-aryl-1,2,3,-dithiazoles. U.S. Patent Application No. US4119722 A, 10 October 1978.
- Appel, R.; Janssen, H.; Siray, M.; Knoch, F. Synthese und Reaktionen des 4,5-Dichlor-1,2,3-dithiazolium-chlorids. Chem. Ber. 1985, 118, 1632–1643.
- Cuadro, A.M.; Alvarez-Buila, J. 4,5-Dichloro-1,2,3-dithiazolium chloride (Appel’s Salt): Reactions with N-nucleophiles. Tetrahedron 1994, 50, 10037–10046.
- Rees, C.W. Polysulfur-nitrogen Heterocyclic Chemistry. J. Heterocycl. Chem. 1992, 29, 639–651.
- Rakitin, O.A. 1,2-Oxa/thia-3-azoles. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Zhdankin, V.V., Eds.; Elsevier: Oxford, UK, 2008; Volume 6, Chapter 6.01; pp. 1–36.
- Navo, C.D.; Peccati, F.; Mazo, N.; Núñez-Franco, R.; Jiménez-Osés, G. 1,2-Oxa/thia-3-azoles. In Comprehensive Heterocyclic Chemistry IV; Black, D.S., Cossy, J., Stevens, C.V., Eds.; Elsevier: Oxford, UK, 2022; Volume 6, Chapter 6.01; pp. 1–55.
- Konstantinova, L.S.; Rakitin, O.A. Synthesis and properties of 1,2,3-dithiazoles. Russ. Chem. Rev. 2008, 77, 521–546.
- Kim, K. Recent Advances in 1,2,3-Dithiazole Chemistry. Phosphorus Sulfur Silicon Relat. Elem. 1997, 120, 229–244.
- Kim, K. Synthesis and Reactions of 1,2,3-Dithiazoles. Sulfur Rep. 1998, 21, 147–207.
- Hafner, K.; Stowasser, B.; Sturm, V. Synthesis and properties of 4,6-Di-t-Butyl-Cyclopenta-1,2-Dithiole and its 3-aza-derivative. Tetrahedron Lett. 1985, 26, 189–192.
- Konstantinova, L.S.; Bol’shakov, O.I.; Obruchnikova, N.V.; Laborie, H.; Tanga, A.; Sopéna, V.; Lanneluc, I.; Picot, L.; Sablé, S.; Thiéry, V.; et al. One-pot synthesis of 5-phenylimino, 5-thieno or 5-oxo-1,2,3-dithiazoles and evaluation of their antimicrobial and antitumor activity. Bioorg. Med. Chem. Lett. 2009, 19, 136–141.
- Koyioni, M.; Manoli, M.; Koutentis, P.A. The Reaction of DABCO with 4-Chloro-5H-1,2,3-dithiazoles: Synthesis and Chemistry of 4--5H-1,2,3-dithiazoles. J. Org. Chem. 2016, 81, 615–631.
- Besson, T.; Emayan, K.; Rees, C.W. 1,2,3-Dithiazoles and new routes to 3,l-benzoxazin-4-ones, 3,l -benzothiazin-4-ones and N-arylcyanothioformamides. J. Chem. Soc. Perkin Trans. 1995, 1, 2097–2102.
- Besson, T.; Rees, C.W. Some chemistry of 4,5-dichloro-l,2,3-dithiazolium chloride and its derivatives. J. Chem. Soc. Perkin Trans. 1995, 1, 1659–1662.
- Lee, H.; Kim, K. A new procedure to N-Arylcyanothioformamides from 5-arylimino-4-chloro-5H-1,2,3-dithiazoles. Bull. Korean Chem. Soc. 1992, 13, 107–108.
- Koutentis, P.A. The Preparation and Characterization of 5-Substituted-4-chloro-1,2,3-dithiazolium Salts and their Conversion into 4-Substituted-3-chloro-1,2,5-thiadiazoles. Molecules 2005, 10, 346–359.
- Clarke, D.; Emayan, K.; Rees, C.W. New synthesis of isothiazoles from primary enamines. J. Chem. Soc. Perkin Trans. I 1998, 1, 77–82.
- Kalogirou, A.S.; Christoforou, I.C.; Ioannidou, H.A.; Manos, M.J.; Koutentis, P.A. Ring transformation of (4-chloro-5H-1,2,3-dithiazol-5-ylidene)acetonitriles to 3-haloisothiazole-5-carbonitriles. RSC Adv. 2014, 4, 7735–7748.
- Moon-Kook, J.; Kim, K. Synthesis of new 5-alkylidene-4-chloro-5H-1,2,3-dithiazoles and their stereochemistry. Tetrahedron 1999, 55, 9651–9667.
- Kalogirou, A.S.; Koutentis, P.A. The reaction of 4,5-dichloro-1,2,3-dithiazolium chloride with DMSO: An improved synthesis of 4-chloro-1,2,3-dithiazol-5H-one. Tetrahedron 2009, 65, 6855–6858.
- Kalogirou, A.S.; Koutentis, P.A. A qualitative comparison of the reactivities of 3,4,4,5-tetrachloro-4H-1,2,6-thiadiazine and 4,5-dichloro-1,2,3-dithiazolium chloride. Molecules 2015, 20, 14576–14594.
- Folmer, J.J.; Weinreb, S.M. Generation of esters from carboxylic acids using Appel’s salt (4,5-dichloro-1,2,3-dithiazolium chloride). Tetrahedron Lett. 1993, 34, 2737–2740.
- Cottenceau, G.; Besson, T.; Gautier, V.; Rees, C.W.; Pons, A.-M. Antibacterial Evaluation of Novel N-Arylimino-1,2,3-dithiazoles and N-Arylcyanothioformamides. Bioorg. Med. Chem. Lett. 1996, 6, 529–532.
- Laitinen, T.; Meili, T.; Koyioni, M.; Koutentis, P.A.; Poso, A.; Hofmann-Lehmann, R.; Asquith, C.R.M. Synthesis and evaluation of 1,2,3-dithiazole inhibitors of the nucleocapsid protein of feline immunodeficiency virus (FIV) as a model for HIV infection. Bioorg. Med. Chem. 2022, 68, 116834.
- Koutentis, P.A.; Koyioni, M.; Michaelidou, S.S. Synthesis of azines. Molecules 2011, 16, 8992–9002.
- Van der Plas, H.C. Chapter II SN(ANRORC) Reactions in Azines, Containing an “Outside” Leaving Group. Adv. Heterocycl. Chem. 1999, 74, 9–86.
- Van der Plas, H.C. Chapter III SN(ANRORC) Reactions in Azaheterocycles Containing an “Inside” Leaving Group. Adv. Heterocycl. Chem. 1999, 74, 87–151.
- Lee, H.; Kim, K.; Whang, D.; Kim, K. Novel Synthesis of 5-(arylimino)-4-(dialkylamino)-5H-l,2,3-dithiazoles and the mechanism of their formation. J. Org. Chem. 1994, 59, 6179–6183.
- Konstantinova, L.S.; Baranovsky, I.V.; Irtegova, I.G.; Bagryanskaya, I.Y.; Shundrin, L.A.; Zibarev, A.V.; Rakitin, O.A. Fused 1,2,3-dithiazoles: Convenient synthesis, structural characterization, and electrochemical properties. Molecules 2016, 21, 596.
- Plater, M.J.; Rees, C.W.; Roe, D.G.; Torroba, T. Cyclopenta-1,2,3-dithiazoles and related compounds. J. Chem. Soc. Chem. Commun. 1993, 7, 293–294.
- Koyioni, M.; Manoli, M.; Manos, M.J.; Koutentis, P.A. Reinvestigating the Reaction of 1H-Pyrazol-5-amines with 4,5-Dichloro-1,2,3-dithiazolium Chloride: A Route to Pyrazolo isothiazoles and Pyrazolothiazoles. J. Org. Chem. 2014, 79, 4025–4037.
- Baraldi, P.G.; Pavani, M.G.; Nuñez, M.C.; Brigidi, P.; Vitali, B.; Gambari, R.; Romagnoli, R. Antimicrobial and antitumor activity of N-heteroimmine-1,2,3-dithiazoles and their transformation in triazolo-, imidazo-, and pyrazolopirimidines. Bioorg. Med. Chem. 2002, 10, 449–456.
- Besson, T.; Guillaumet, G.; Lamazzi, C.; Rees, C.W. Synthesis of 3,1-Benzoxazines, 3,1-Benzothiazines and 3,1-Benzoxazepines via N-Arylimino-1,2,3-dithiazoles. Synlett 1997, 6, 704–706.
- Letribot, B.; Delatouche, R.; Rouillard, H.; Bonnet, A.; Chérouvrier, J.-R.; Domon, L.; Besson, T.; Thiéry, V. Synthesis of 2-Mercapto-(2-Oxoindolin-3-Ylidene)Acetonitriles from 3-(4-Chloro-5H-1,2,3-Dithiazol-5-Ylidene)Indolin-2-ones. Molecules 2018, 23, 1390.
- Kalogirou, A.S.; Koutentis, P.A. The degradation of 4,5-dichloro-1,2,3-dithiazolium chloride in wet solvents. Tetrahedron 2009, 65, 6859–6862.
- Konstantinova, L.S.; Bol’shakov, O.I.; Obruchnikova, N.V.; Golova, S.P.; Nelyubina, Y.V.; Lyssenko, K.A.; Rakitin, O.A. Reactions of 4-substituted 5H-1,2,3-dithiazoles with primary and secondary amines: Fast and convenient synthesis of 1,2,5-thiadiazoles, 2-iminothioacetamides and 2-oxoacetamides. Tetrahedron 2010, 66, 4330–4338.
More
Information
Subjects:
Chemistry, Organic
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.3K
Entry Collection:
Organic Synthesis
Revisions:
2 times
(View History)
Update Date:
12 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




