Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Liang Yun Chen | -- | 4098 | 2023-04-11 12:35:58 | | | |
| 2 | Rita Xu | Meta information modification | 4098 | 2023-04-12 07:22:29 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chen, L.; Kao, T.; Chen, C.; Niaz, N.; Lee, H.; Chen, Y.; Kuo, C.; Shen, Y. Molecular Mechanisms of Exosomes. Encyclopedia. Available online: https://encyclopedia.pub/entry/42938 (accessed on 07 February 2026).
Chen L, Kao T, Chen C, Niaz N, Lee H, Chen Y, et al. Molecular Mechanisms of Exosomes. Encyclopedia. Available at: https://encyclopedia.pub/entry/42938. Accessed February 07, 2026.
Chen, Liang-Yun, Ting-Wan Kao, Chang-Cyuan Chen, Noreen Niaz, Hsin-Lun Lee, Yu-Hsin Chen, Chia-Chun Kuo, Yao-An Shen. "Molecular Mechanisms of Exosomes" Encyclopedia, https://encyclopedia.pub/entry/42938 (accessed February 07, 2026).
Chen, L., Kao, T., Chen, C., Niaz, N., Lee, H., Chen, Y., Kuo, C., & Shen, Y. (2023, April 11). Molecular Mechanisms of Exosomes. In Encyclopedia. https://encyclopedia.pub/entry/42938
Chen, Liang-Yun, et al. "Molecular Mechanisms of Exosomes." Encyclopedia. Web. 11 April, 2023.
Copy Citation
Exosomes are effective therapeutic vehicles that may transport their substances across cells. They are shown to possess the capacity to affect cell proliferation, migration, anti-apoptosis, anti-scarring, and angiogenesis, via the action of transporting molecular components. Possessing immense potential in regenerative medicine, exosomes, especially stem cell-derived exosomes, have the advantages of low immunogenicity, minimal invasiveness, and broad clinical applicability. Exosome biodistribution and pharmacokinetics may be altered, in response to recent advancements in technology, for the purpose of treating particular illnesses.
stem cell
exosome
stem cell-derived exosome
regenerative medicine
1. Introduction
Cell-based therapies, such as stem cell transplantation, have long been regarded as key players in the field of regenerative medicine. In the last decade, research into the mechanism of action of cell-based treatments has revealed that intercellular signaling mediates their therapeutic effects. In other words, the biologically active molecules generated by therapeutic cells, either stem cells or non-stem cells, are the actual driving force behind their effect. Because of this, a fresh page was turned in the history of cell-free therapy.
Cell-free therapy is a novel way to explore the therapeutic effects of cell-based therapy without the need for cellular transplantation. In cell-free therapy, cells are utilized as a source of biologically effective molecules, rather than as a direct therapeutic agent. Research and clinical studies on cell-free therapy have demonstrated better tolerance and comparable, or even superior, effects to cell-based therapies in several fields of applications, [1]. Cell-free therapies utilize products secreted by cells. Materials for cell-free therapy can encompass secretomes of cells and extracellular vesicles (EVs). It is clear that, biologically, there are three types of EVs, based on their distinct release mechanism, but there is no consensus on the criteria for categorizing these vesicles experimentally. It is known that microvesicles (MVs), or ectosomes, are formed by directly budding through the cell membrane, while exosomes, or multivesicular bodies emerge by directly budding into the endosomes. Depending on the multiple vesicle body type, content is either digested by lysosomes, or released as exosomes via cell membrane fusion. Thirdly, apoptotic bodies are formed from apoptosis. The formation of apoptotic bodies is thought to occur either through the separation of membrane blebs or through the formation of apoptopodia during apoptosis. It remains to be discovered how these different routes differ in terms of their courses and roles [2].
1.1. Characteristics of Exosomes
Exosomes are EVs between 30 and 160 nm in size that originate from the endosomal network of a cell. Exosomes are able to transport substances such as proteins, lipids, DNA, mRNA, miRNA, and long non-coding chain RNAs (lncRNA) to recipient cells. Depending on the cells of origin and environmental conditions, exosomes serve distinct functions, and are currently recognized as essential for cell–cell communication, regulation of cellular physiology, and reprogramming of cell behavior. In humans, exosomes have been identified in most tissues and body fluids, indicating their biological significance. Extensive research has been focused on the prospective use of exosomes in the treatment of numerous diseases throughout the past decade [3].
1.2. Current Application of Mesenchymal Stem Cell-Derived Exosomes
Among the various exosome-based therapeutics, mesenchymal stem cell-derived exosomes (MSC-Exos) have gained great attention due to their diverse applicability in regenerative medicine. As one of the multipotent progenitor cells, mesenchymal stem cells (MSCs) are capable of self-renewal and differentiating into numerous cell lineages, including adipocytes, chondrocytes, and osteoblasts. In addition, MSCs can be readily isolated, and exhibit relatively limited immunogenicity. These advantages have sparked the development of MSC-based therapeutics, which have been widely adopted in animal disease models and clinical trials in humans. Nonetheless, concerns about carcinogenesis and immunostimulation have impeded the practical deployment of MSC-based therapy, notwithstanding the fact that they are uncommon [4][5].
From this background, MSC-Exos emerged as a preferred therapeutic option, since they are free from the safety concerns ensuing from cell administration. Moreover, they can be readily prepared into off-the-shelf products after sterilization, which is unachievable with MSCs [6][7]. Aside from their favorable clinical applicability, the capacity of MSC-Exos to affect cell proliferation, migration, anti-apoptosis, anti-scarring, angiogenesis, chondrogenesis, neurogenesis, and immunomodulation has been evidenced by an accumulating amount of research [8][9][10]. In addition, the creation of an anti-inflammatory microenvironment by MSC-Exos enables accelerated tissue repair and regeneration [11]. Collectively, these biological capabilities empower MSC-Exos to become a promising therapeutic tool in regenerative medicine for a wide range of diseases.
However, although a substantial number of preclinical experiments have demonstrated the therapeutic effect of MSC-Exos in vitro and in animal models [12][13][14], ongoing clinical studies are few. In other words, exosomes are promising, but do not yet have a practical medicinal application. This is most likely due to their high cost, as a result of their disease-and/or patient-specific nature [15] and the fact that certain mechanisms behind their action remain hidden.
2. Molecular Mechanisms of Exosomes
2.1. Musculoskeletal Regeneration
With the aging of the global population, the incidence of musculoskeletal diseases is steadily increasing [16]. Exosomes have gained great attention in musculoskeletal research due to their regenerative potential and minimally invasive nature (Figure 1).
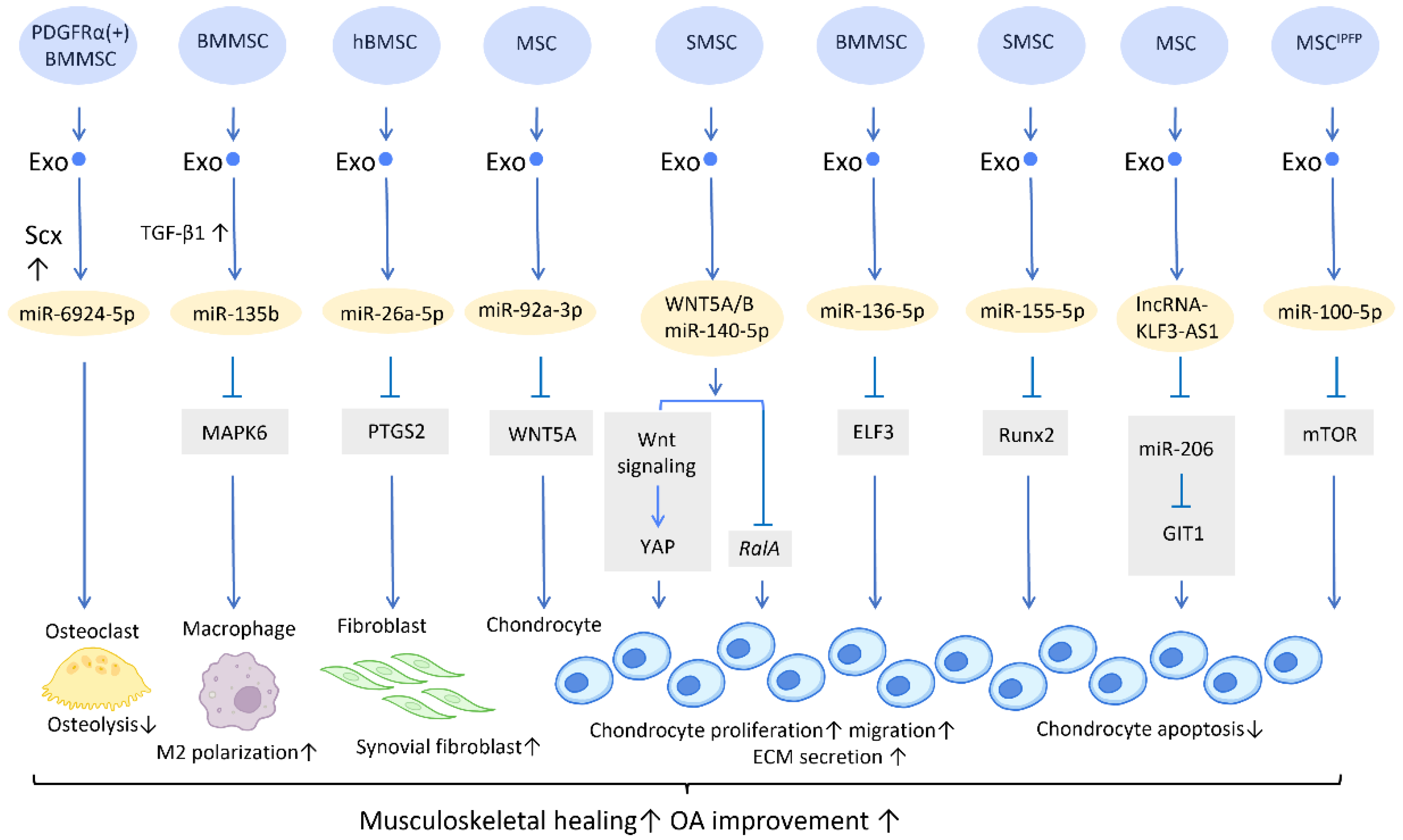
Figure 1. Molecular mechanisms of stem cell-derived exosomes in sport injuries and osteoarthritis. Stem cell-derived exosomes promote tissue healing, M2 polarization, fibroblast activation, chondrocyte proliferation and migration, and decreases osteolysis and chondrocyte apoptosis via transferring of diverse molecular components. An overall regenerative effect was demonstrated, which manifested as improved musculoskeletal healing and osteoarthritis recovery. Abbreviation: Scx: scleraxis; PTGS2: prostaglandin-endoperoxide synthase 2; YAP: Yes-associated protein; ELF3: E74-like factor 3; GIT1: G-protein-coupled receptor kinase interacting protein-1; hBMSC: human bone MSC; SMSC: synovial MSC; MSCIPFP: infrapatellar fat pad. ↑ represents upregulation; ↓ represents downregulation.
2.1.1. Sports Medicine and Musculoskeletal Rehabilitation
In contrast to the prevalent application of platelet-rich plasma (PRP) in clinical settings, the development of exosome-based therapies is still preliminary, and studies are largely preclinical. Recent research has gradually demonstrated the therapeutic efficacy of exosomes in musculoskeletal healing [17][18]. Exosomes derived from adipose-derived stem cells (ADSC-Exos), for example, have been shown to promote human rotator cuff healing by inhibiting muscle atrophy and degeneration, while also improving the histological properties of the torn tendon [19]. This could be accomplished by increasing AMP-activated protein kinase (AMPK) signaling, and thus inhibiting Wnt/β-catenin activity [20]. In addition, bone mesenchymal stem cells (BMMSCs)-derived exosomes (BMMSC-Exos) have been shown to stimulate the proliferation and differentiation of tendon stem/progenitor cells in vitro, increase the expression of mohawk, tenomodulin, and type I collagen, enhance the proliferation of local tendon stem/progenitor cells in vivo, and promote the mechanical properties of neotendons in the defect area of rat patellar tendons [21].
Furthermore, a potential treatment for tendon–bone regeneration was proposed, utilizing exosomes derived from genetically modified BMMSCs. Scleraxis is a transcription factor that is believed to play an active role in tendon–bone repair. A study by Feng et al. demonstrated that local injection of miR-6924-5p-rich exosomes, derived from scleraxis-overexpressing PDGFRα (+) BMMSCs, leads to reduced osteolysis and improved healing strength [22] (Figure 1).
2.1.2. Osteoarthritis
The treatment of osteoarthritis (OA) has been challenging due to the low potential for spontaneous healing of cartilage tissue [23].
In the application of exosomes in OA, several studies have proposed possible mechanisms behind its effect. It is well recognized that the proinflammatory cytokine IL-1β is one of the most influential players in cartilage destruction in OA [24]. BMMSC-Exo injection significantly reduces IL-1β-mediated suppression of chondrocyte proliferation, and thereby inhibits the downregulation of anabolic markers COL2A1 and ACAN, as well as blocks the overexpression of catabolic markers MMP13 and ADAMTS5. This treatment reduced both cartilage damage and knee pain in rats with OA [25]. In addition, exosomes derived from human embryonic stem cell-induced mesenchymal stem cells (ESC-MSCs) have shown therapeutic promise for the treatment of OA. In the presence of IL-1β, exosomes from ESC-MSCs were able to modulate chondrocytes by increasing the expression of the extracellular matrix protein collagen type II and decreasing the expression of the matrix degradation enzyme ADAMTS5. This effectively prevented cartilage destruction in the progression of OA [26]. A study has shown that high expression of miR-135b in TGF-β1-stimulated BMMSC-Exos (BMMSC-ExosTGF-β1) reduces pro-inflammatory factors and alleviates cartilage damage in OA. This is likely achieved by targeting MAPK6, which in turn boosts the M2 polarization of synovial macrophages [27] (Figure 1). Another study demonstrated that IL-1β-induced damage to synovial fibroblasts might be mitigated by exosomal miR-26a-5p, from human bone MSCs (hBMSCs), by hindering prostaglandin-endoperoxide synthase 2 (PTGS2) [28] (Figure 1).
WNT5A has a dual role, functioning in both chondrogenic differentiation and cartilage degradation, and it is involved in the destruction and degradation of cartilage in the formation of OA [29]. Exosomal miR-92a-3p, released from MSCs, precluded cartilage destruction in an OA mouse model by downregulating WNT5A expression [30] (Figure 1). Furthermore, WNT5A and WNT5B were substantially expressed in synovial MSC exosomes (SMSC-Exos). WNT5A and WNT5B carried by exosomes stimulated Yes-associated protein (YAP) activation via an alternative Wnt signaling pathway, which contributed to the enhancement of chondrocyte proliferation and migration. Overexpression of miR-140-5p could block the side effect of YAP by suppressing RalA, which rescued SOX9, aggrecan, and collagen type II expression and restored extracellular matrix (ECM) secretion [31] (Figure 1).
In the cartilage tissues of patients with traumatic OA, increased E74-like factor 3 (ELF3) expression and decreased miR-136-5p expression were found. Similar to the aforementioned miR-140-5p, exosomal miR-136-5p derived from BMMSCs was also shown to promote chondrocyte proliferation, migration, and ECM secretion by downregulating ELF3 expression, lessening MMP-1 levels, and intensifying collagen II, aggrecan, and SOX9 levels, thereby inhibiting cartilage degradation [32] (Figure 1). Furthermore, miR-155-5p-overexpressing SMSC-Exos might stimulate chondrocyte proliferation and migration while decreasing apoptosis, as well as increasing ECM secretion by targeting Runx2 to prevent OA [33] (Figure 1). Similarly, exosomal lncRNA-KLF3-AS1 generated from MSCs was reported to reduce chondrocyte apoptosis and stimulate chondrocyte proliferation, via sponging miR-206 to enhance G-protein-coupled receptor kinase interacting protein-1 (GIT1) expression, which attenuates IL-1β-induced chondrocyte injury [34] (Figure 1). Another study explored the effect of infrapatellar fat pad (IPFP) MSC-derived exosomes (MSCIPFP-Exos) in OA. MiR-100-5p-abundant MSCIPFP-Exos prevented damage to articular cartilage and lessened the severity of OA by hampering apoptosis of chondrocytes through the mTOR-autophagy signaling pathway [35] (Figure 1).
2.2. Wound Healing
Wound healing is a dynamic physiological process that may be roughly broken down into four stages: hemostasis, inflammation, proliferation, and remodeling. In this complicated process, numerous aspects, including angiogenesis, proliferation and differentiation of fibroblasts, and immunomodulation, play their roles in different stages of wound healing [36]. The effect of exosomes on the healing of wounds has long been a subject of significant research and clinical interest. Studies show that extracellular signal-regulated kinase, STAT-3, and protein kinase B are just a few of the pathways that exosomes can target. These pathways are essential for aiding in and speeding up wound healing via upstream effectors, such as insulin-like growth factor-1, hepatocyte growth factor, nerve growth factor, and stromal cell-derived factor. Low quantities of TGF-β have been discovered in MSC-Exos in the umbilical cord, and, when exosomes are further laden with TGF-β cargo, they promote the remodeling of the matrix and vascularization [37].
2.2.1. Stimulation of Angiogenesis
Neovascularization is a paramount process in wound healing and tissue regeneration (Figure 2). HiPSC-MSC-Exos were first found to exhibit the ability to stimulate angiogenesis in human umbilical vein endothelial cells [38] (Figure 2). Further research indicates plausible mechanisms behind this finding. UCMSC-derived exosomes (UCMSC-Exos) transmitted angiopoietin-2 (Ang-2) to human umbilical vein endothelial cells [39], thus enhancing their proliferative, migratory, and tube-forming capabilities. Additionally, another study reported that UCMSC-Exos also transmitted miR-21-3p to HUVEC, which increased angiogenesis and proliferation by inhibiting phosphatase and tensin homolog (PTEN), and sprouty homolog 1 (SPRY1) [40] (Figure 2). Exosomes derived from ADSCs-Exos can also trigger the proliferation and migration of vascular endothelial cells, thus promoting angiogenesis. In another study, it was found that transferring miR-125a from ADSC-Exos to endothelial cells increased angiogenesis by blocking the effect of delta-like 4 (DLL4), an inhibitor of angiogenesis [41] (Figure 2).
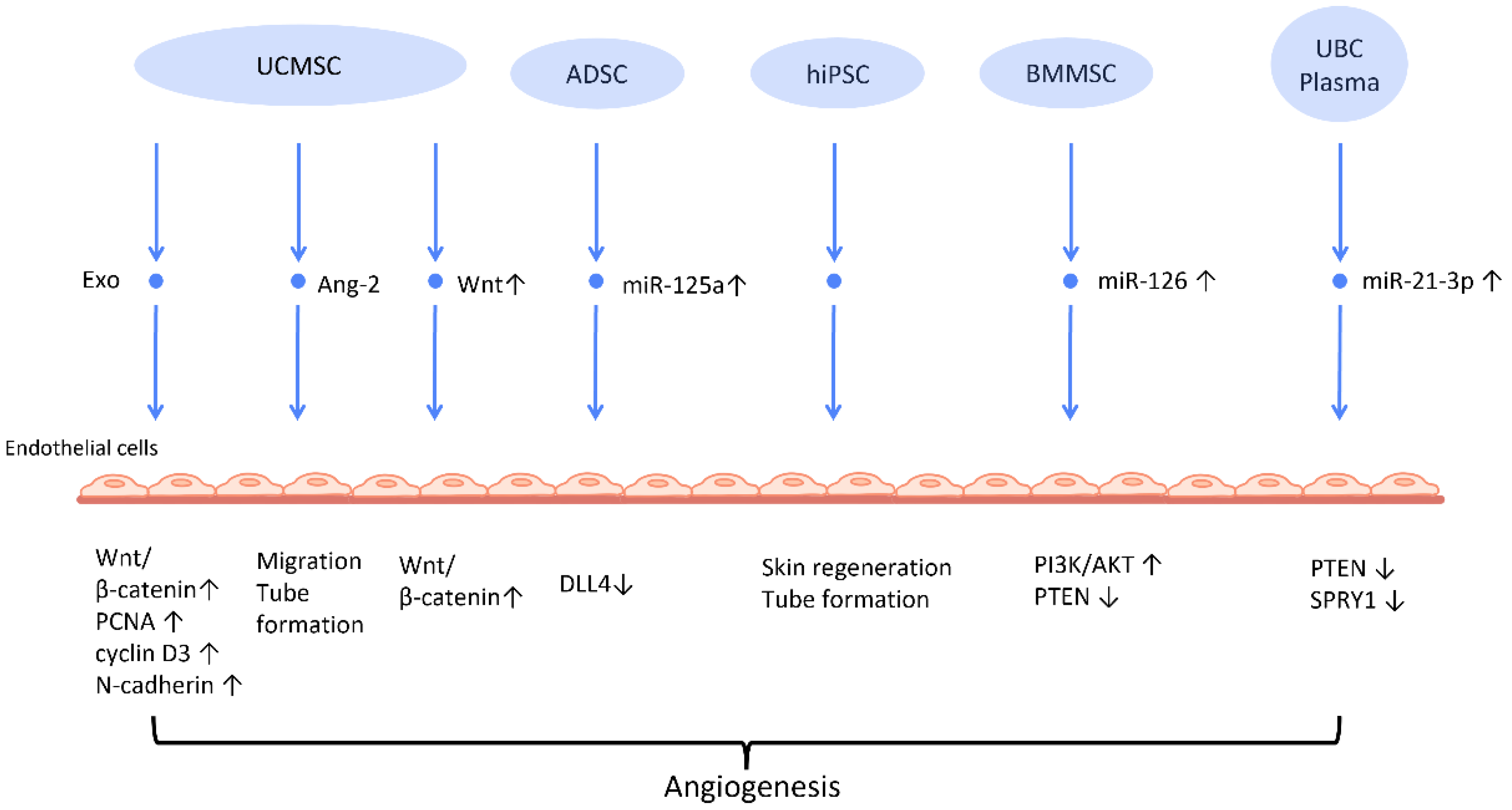
Figure 2. Molecular mechanisms of stem cell-derived exosomes in stimulation of angiogenesis. Stem cell-derived exosomes facilitate neovascularization by transmitting various angiogenic factors or miRNA to endothelial cells, leading to increased proliferation and migration of endothelial cells. Abbreviation: Exo: exosome; MSC: mesenchymal stem cell; UCMSC: human umbilical cord MSC; PCNA: proliferating cell nuclear antigen; ADSC: adipose-derived stem cells; DLL4: delta-like 4; hiPSC-MSC: human induced pluripotent stem cell-derived mesenchymal stem cells; BMSC: bone marrow-derived MSCs; Ang-2: angiopoietin-2; UBC: umbilical cord blood; PTEN: phosphatase and tensin homolog; SPRY1: sprouty homolog 1. ↑ represents upregulation; ↓ represents downregulation.
Another mechanism that has been often addressed in MSC-Exos is the Wnt/β-catenin signaling pathway, which plays a pivotal role in the proliferative phase of the wound healing process [42]. The transfer of Wnt4 from UCMSC-Exos to endothelial cells activated the Wnt/β-catenin pathway and increased the number of angiogenesis-related factors, including proliferating cell nuclear antigen (PCNA), cyclin D3, and N-cadherin, to promote angiogenesis for the healing of second-degree burn injuries [43]. In the rat skin burn model, UCMSC-Exos containing Wnt4 stimulated the Wnt/β-catenin pathway and the AKT pathway to increase angiogenesis and decrease heat stress-induced apoptosis, respectively [44]. ADSC-Exos hindered the H2O2-induced apoptosis of HaCaT cells via stimulating the Wnt/β-catenin signaling pathway, which may be advantageous for cutaneous wound healing [45].
The angiogenic efficacy of MSC-Exos has also been applied to diabetic wound healing, where hyperglycemia impairs angiogenesis and impedes the wound healing process [46]. ADSC-Exos that overexpressed nuclear factor-E2-related factor 2 (Nrf2), a transcription factor that favors anti-oxidative properties, not only promoted proliferation of endothelial progenitor cells and angiopoiesis, but also increased granulation tissue, growth factor levels, and vascularization in diabetic mouse wounds while decreasing inflammatory and oxidative stress-related proteins [47].
Recent investigations have found that exosomes derived from preconditioned MSC are more therapeutically efficacious than those obtained from MSC grown under standard conditions. By transferring miR-126, which downregulated PTEN and activated the PI3K/AKT signaling pathway [48], BMMSC-Exos with deferoxamine, a hypoxia-inducing agent, strengthened the proliferative, migratory, and proangiogenic capacity of human umbilical vein endothelial cell and diabetic wound healing.
2.2.2. Proliferation and Collagen Synthesis of Fibroblast
To establish an intact epidermal barrier, fibroblasts proliferate and produce ECM, which includes fibronectin, type 1 collagen, and type 3 collagen. Accumulating evidence suggests that MSC-Exos may be absorbed by fibroblasts and thereby stimulate collagen production (Figure 3). HiPSC-MSC-Exos increased the production, migration, and secretion of type 1 collagen, type 3 collagen, and elastin by human dermal fibroblasts (DF) [38]. ADSC-Exos transferred to DF enhanced collagen-related proteins, including type 1 collagen, type 3 collagen, MMP1, bFGF, and TGF-β1, and activated PI3K/Akt signaling to maximize collagen deposition [49] (Figure 3). In addition, exosomes produced from fetal dermal MSC-derived exosomes (FDMSC-Exos) augmented the secretion, migration, and proliferation of adult DF via activation of the Notch signaling pathway [50] (Figure 3).
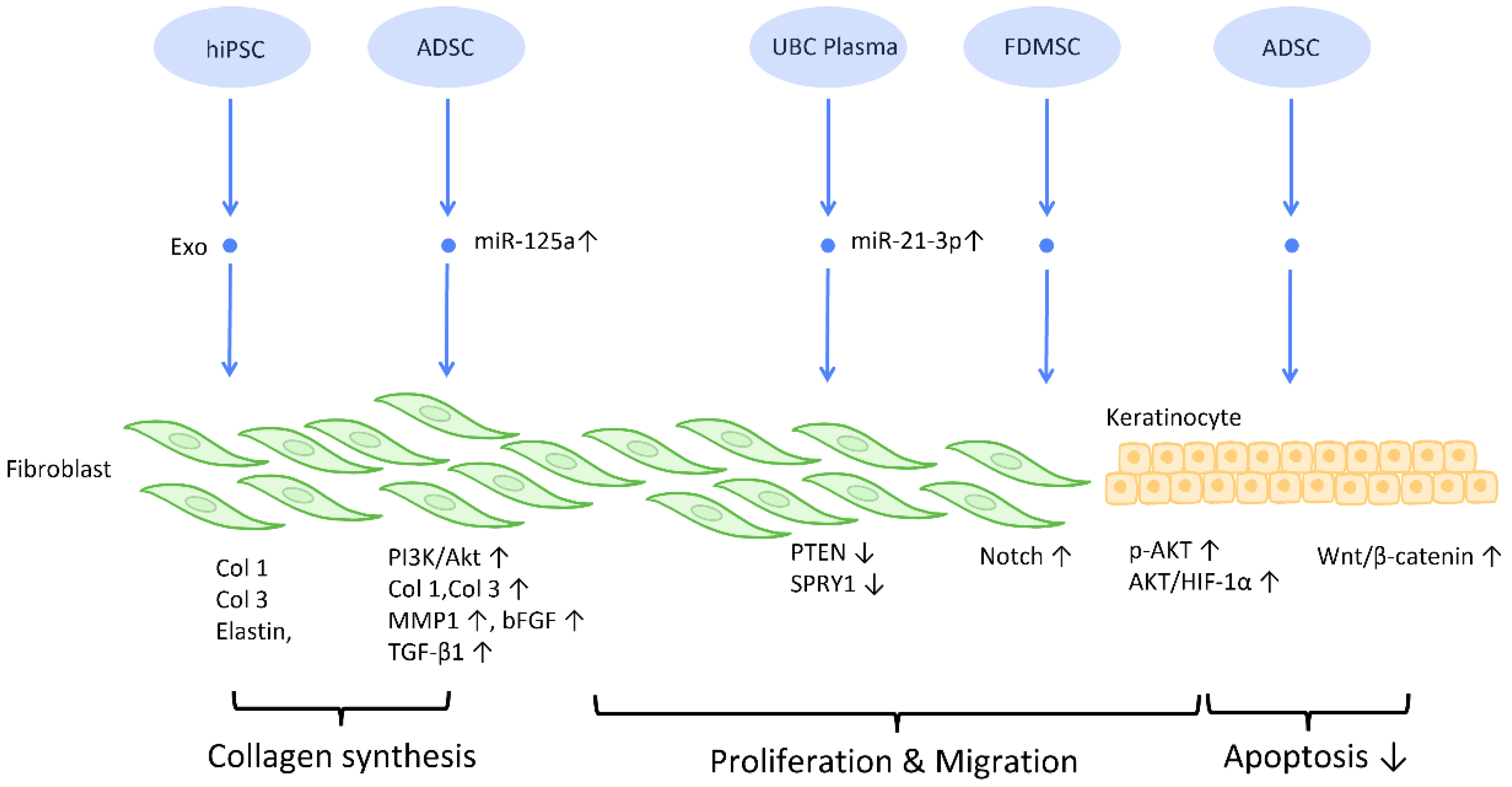
Figure 3. Molecular mechanisms of stem cell-derived exosomes in regulation of fibroblast and keratinocyte. Stem cell-derived exosomes enhance the proliferation and collagen synthesis of fibroblasts, thereby improving the healing process. ADSCs are also shown to accelerate keratinocyte migration and proliferation, which contributes to augmented healing activity. Abbreviation: Exo: exosome; Col: collagen; MSC: mesenchymal stem cell; ADSC: adipose-derived stem cell; miR: micro-RNA; TGFβ: transforming growth factor-β; PI3K/Akt: phosphatidyl-inositol 3-kinase/serine-threonine kinase; MMP: matrix metalloproteinase. ↑ represents upregulation; ↓ represents downregulation.
2.2.3. Inhibition of Scar Formation
In the process of wound healing and tissue repair, the excessive accumulation of myofibroblasts and deposition of collagen results in aberrant scar formation. By controlling fibroblast transition and ECM remodeling, administrations of stem cell-generated exosomes have the potential to limit scar formation, and even accomplish scar eradication, striking a balance between therapeutic effects and aesthetic concerns (Figure 4) [51].
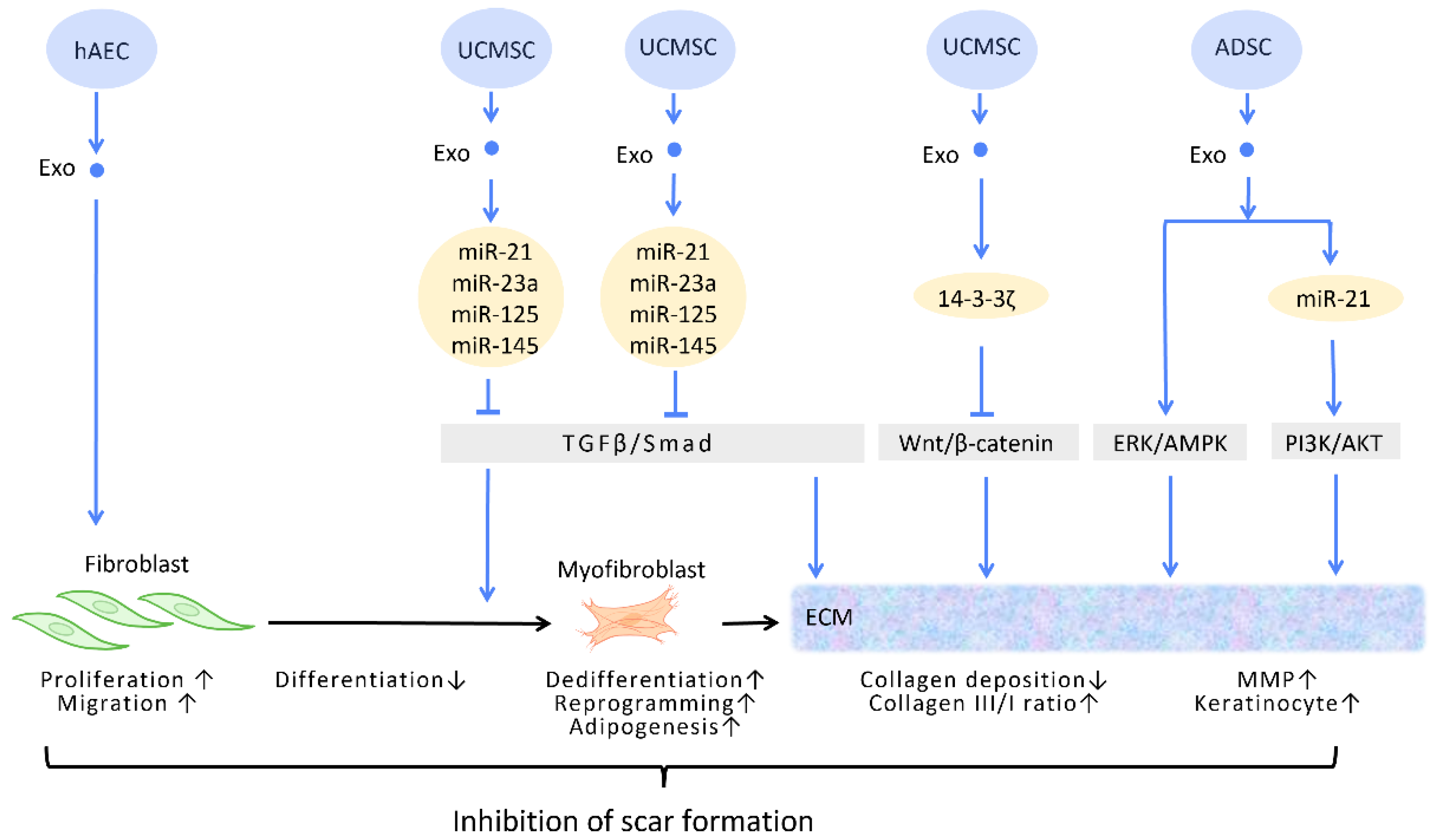
Figure 4. Molecular mechanisms of stem cell-derived exosomes in inhibition of scar formation and promotion of ECM remodeling. In the process of tissue repair, excessive accumulation of myofibroblasts and deposition of collagen result in aberrant scar formation. By controlling fibroblast transition and ECM remodeling, administrations of stem cell-generated exosomes have the potential to limit scar formation. Abbreviation: ECM: extracellular matrix; ERK: extracellular signal-regulated kinase; MAPK: mitogen-activated protein kinase; PI3K/Akt: phosphatidyl-inositol 3-kinase/serine-threonine kinase; MMP: matrix metalloproteinase. ↑ represents upregulation; ↓ represents downregulation.
In the wound healing microenvironment, macrophages secrete TGF-β1, which initiates the differentiation of fibroblasts into myofibroblasts. Disrupting the TGF-β/Smad pathway to prevent TGF-β1-induced differentiation of fibroblasts into myofibroblasts is a straightforward strategy for minimizing fibrosis and scarring under these circumstances [52] (Figure 4). In this context, evidence suggested that UCMSC-Exos, enriched in miR-21, miR-23a, miR-125b, and miR-145, inhibited TGF-β/Smad2 and myofibroblast formation [53] (Figure 4).
2.2.4. Extracellular Matrix Remodeling
Accumulating evidence has suggested that exosomes are able to remodel and adjust the protein composition of the ECM, modifying the ECM into a more preferable condition throughout the process of wound healing [54].
The ECM is composed of a variety of proteins, the most prevalent of which is collagen. Collagen possesses dual roles during different stages of wound healing. During the early phase of wound healing, stimulation of collagen synthesis is essential for restoration of wound strength, but excessive collagen during the late phase of wound healing results in scar formation [55]. It has been reported that, aside from promoting the formation of myofibroblasts, the aforementioned TGF-β/Smad pathway is also involved in the regulation of collagen synthesis. The upregulation of the TGF-β/Smad pathway intensifies the expression of the COLI2 gene during the early phase of wound healing but decreases collagen I deposition during the late phase [53]. Through the targeting of this pathway, UCMSC-Exos promote favorable ECM composition throughout the healing process [56] (Figure 4).
Zhang et al. demonstrated that UCMSC-Exos transport 14-3-3ζ proteins and diminishes collagen deposition. 14-3-3ζ proteins facilitate the binding between YAP and phosphorylation-large tumor suppressor kinase, resulting in YAP phosphorylation and the blockage of Wnt/β-catenin signaling, which causes skin fibrosis during the remodeling phase of healing [10] (Figure 4).
Matrix metalloproteinases (MMPs) are components of the ECM that degrade excessive collagen. It has been suggested that ADSC-Exos trigger the ERK/MAPK pathway and augment the expression of MMP-3 [57] (Figure 4). Furthermore, Yang et al. revealed that miR-21 in ADSC-Exos regulates the PI3K/AKT pathway and elevates MMP-9 production, which accelerates HaCaT keratinocyte migration and proliferation [51][58] (Figure 4).
2.3. Female Infertility
Impaired fertility is manifested as a declining pregnancy rate and poor pregnancy outcomes [59]. By definition, infertility is the failure to establish a pregnancy within 12 months of regular sexual intercourse [60]. Female reproductive disorders pose great threats to women’s health and eventually contribute to infertility [61]. Stem cell-derived exosomes used in cell-free treatment have been shown to enhance the ovarian and uterine environments through their capacity for regeneration, which could potentially reverse female infertility.
2.3.1. Promoting Follicular Development
Several reproductive diseases are associated with disrupted follicle development. While ovarian aging is linked to a drop in the number and quality of oocytes, premature ovarian insufficiency, also known as premature ovarian failure, is a disease characterized by senescence of the ovaries in women under the age of 40 [60]. The resulting follicular malfunction often manifests clinically as amenorrhea, estrogen deficiency, and hypergonadotropism [62]. In addition, polycystic ovary syndrome (PCOS) is another common disease with ovulatory dysfunction among reproductive-aged females [63].
The successful maturation of oocytes, which is pivotal for ovulation and pregnancy, is intimately connected with hormone regulation, granulosa function, and the ovarian environment [64]. Since the number of oocytes defines the reserve of primordial follicles [65], restoring the impaired follicular development is the key to improving ovulatory failure caused by ovarian reproductive disorders. Premature ovarian insufficiency is the most frequently adopted model for this line of research. In the vast majority of studies, anti-apoptosis and adjustment of sex hormone levels are hypothesized to be the underlying mechanisms of exosome therapy.
Several researchers have delved into the molecular pathway of how stem cell-derived exosomes exert their ability to inhibit apoptosis through their cargo (Figure 5). Human ADSC-Exos were found to repress apoptosis of human granulosa cells by regulating the SMAD pathway, which elevates mRNA and protein expression of SMAD2, SMAD3, and SMAD5 to a normal level [66] (Figure 5). Furthermore, exosomes derived from ADSC transfected with miR-323-3p eliminate the pro-apoptotic action of programmed cell death protein 4 (PDCD4) in PCOS cumulus cells [63] (Figure 5). Exosomes derived from amniotic fluid stem cells (AFSC-Exo) deliver miR-10a and reduce follicular atresia by targeting Bim and downregulating the apoptotic factor Casp9 [67] (Figure 5). In parallel, exosomes collected from BMMSC-Exos, carrying miR-644-5p, impede apoptosis of ovarian GCs through targeting p53 [68] (Figure 5), the upstream of Bim. Meanwhile, BMMSC-Exos are capable of activating the PI3K/AKT pathway in granulosa cells by suppressing PTEN, an inhibitor of this signaling pathway [69] (Figure 5). On the other hand, UCMSC-Exos highly express miR-17-5p, inhibiting sirtuin 7 (SIRT7) and its downstream genes PARP1, γH2AX, and XRCC6. This results in a decreased accumulation of reactive oxygen species (ROS) in granulosa cells, which has an anti-apoptotic effect [70] (Figure 5).
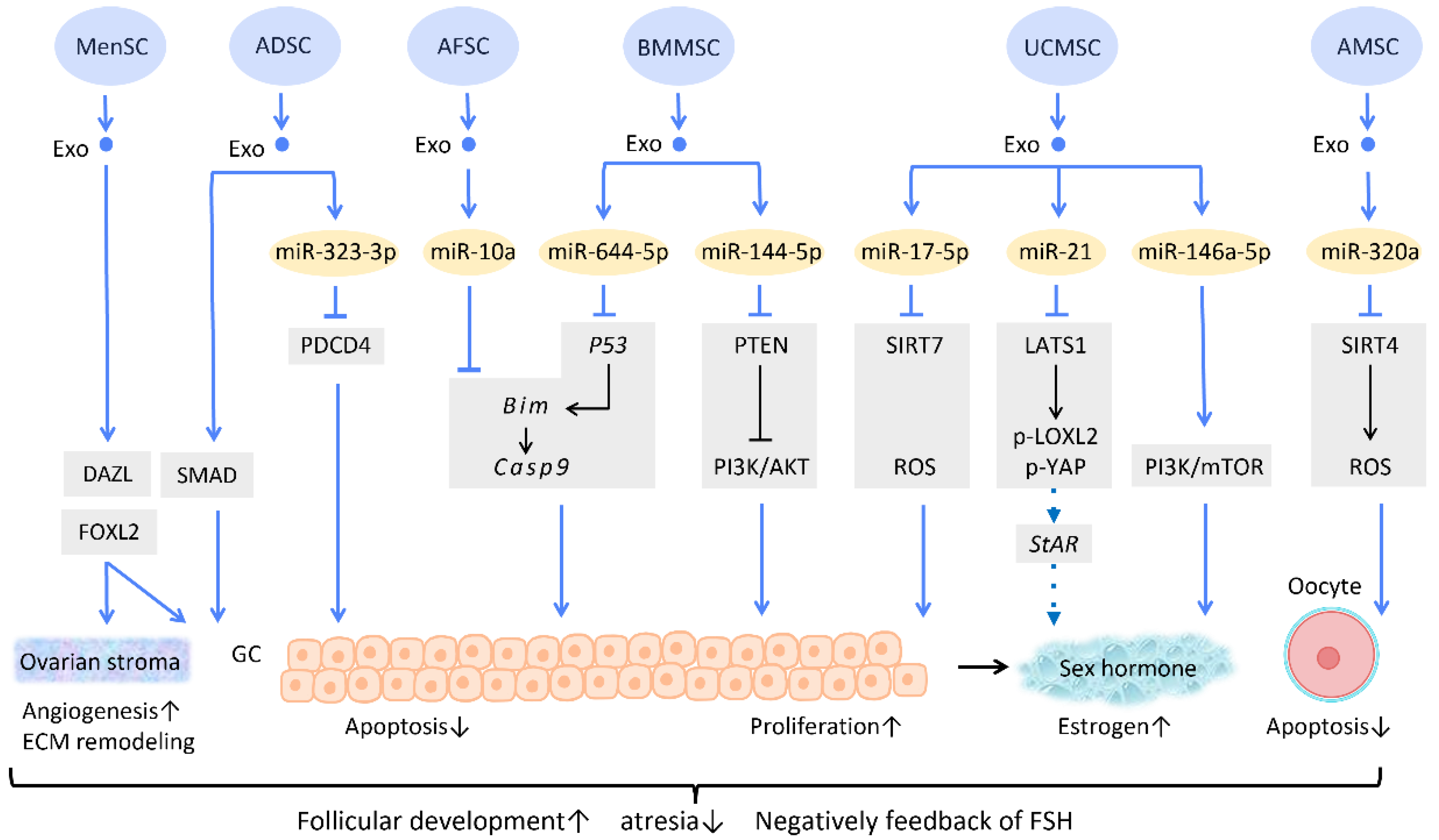
Figure 5. Molecular mechanisms of stem cell-derived exosomes in follicular development. Follicular malfunction is the critical reason of ovarian reproductive disorders. With the help of stem cell-derived exosomes, follicular growth and sex hormone levels could be restored, and ovarian microenvironment could be rejuvenated. Abbreviation: Exo: exosome; SC: stem cell/stromal cell; MSC: mesenchymal stem cell; MenSC: menstrual blood-derived SC; ADSC: adipose-derived stem cell; AFSC: amniotic fluid stem cell-derived; BMMSC: bone marrow-derived MSC; UCMSC: umbilical cord MSC; AMSC: amniotic MSC; miR: micro-RNA; DAZL: deleted in azoospermia like; FOXL2: forkhead box L2; PDCD4: programmed cell death protein 4; PI3K/Akt: phosphatidyl-inositol 3-kinase/serine-threonine kinase; SIRT: sirtuin; ROS: reactive oxygen species; LATS1: large tumor suppressor 1; LOXL2: lysyl oxidase-like 2; YAP: Yes-associated protein; StAR: steroidogenic acute regulatory gene; GC: granulosa cell; ECM: extracellular matrix. ↑ represents upregulation; ↓ represents downregulation.
Regarding regulation of sex hormone levels, one study revealed that exosomal miR-21 is involved in enhancing estrogen secretion in hUCMSCs (Figure 5). Further effort was made to clarify the molecular mechanism underlying this observation. Exosomal miR-21 was found to downregulate large tumor suppressor 1 (LATS1), which belongs to the Hippo pathway, thereby mitigating phosphorylation of lysyl oxidase-like 2 (LOXL2) and YAP. In this way, YAP protein could bind to the promotor of the steroidogenic acute regulatory (StAR) gene, thereby elevating StAR expression and promoting estrogen secretion in ovarian granulosa cells [71] (Figure 5).
Aside from acting on granulosa cells, exosomes also have an effect on oocytes (Figure 5). For instance, the mechanism of miR-320a, from human amniotic MSC-derived exosome (AMSC-Exo), inhibits oocyte apoptosis by suppressing SIRT4 signaling and reducing ROS levels [72] (Figure 5), an effect which is similar to that of miR-17-5p from BMMSC-Exos. In the model of age-related fertile retardation, UCMSC-Exos carries miR-146a-5p, which triggers the PI3K/mTOR pathway, improving oocyte quality and accelerating follicular development [65] (Figure 5). By means of promoting follicular development and obliterating atresia, stem cell-derived exosomes restore ovarian function and negatively feedback on follicle-stimulating hormone (FSH) levels.
In addition to the above-mentioned mechanisms, several studies indicate that exosome therapy can adjust ovarian stroma into a more favorable microenvironment. Zhang et al. showed that exosomes from menstrual blood-derived stromal cells not only stimulated the expression of early follicle markers Deleted in Azoospermia-like (DAZL) and Forkhead Box L2 (FOXL2), but also regulated the composition of the ECM. Enhanced expression of collagen IV, FN1, and laminin in the ovarian stroma was noted; the latter two were also observed in granulosa cells, and are involved in follicular growth [64] (Figure 5).
2.3.2. Regeneration of Damaged Endometrium
Being the implant sites of blastocytes, endometrium undoubtedly plays a crucial role in female pregnancy. Asherman’s syndrome, also called intrauterine adhesion (IUA), is characterized by poor glandular epithelial growth and vascular development from scarring, and fibrosis primarily caused by artificial trauma, repeated endometritis, and infection [59][73][74]. Endometrial fibrosis causes a loss of regeneration competence in functional endometrium, which eventually leads to secondary infertility, the most common type of infertility worldwide [60][75].
Recent research has found that the administration of stem cell-derived exosomes has similar effects to PRP in endometrial fibrosis and AS models, including promoting cellular proliferation, differentiation, functional recovery, angiogenesis, anti-inflammation, and anti-fibrosis. Saribas et al. utilized uterus-derived MSCs-exosomes (uMSC-Exo) in AS models and found increased proliferation, vascularization, and reduced fibrosis in uterine tissue. The effect was confirmed by significantly elevated proliferating cell nuclear antigen (PCNA), VEGFR-1, MMP-2, MMP-9, and declined TIMP-2 [76] (Figure 6). Concerning specific molecular interactions between exosomes and endometrial cells, Shao et al. established a negative regulatory relationship between lncRNAs and miRNAs. They elucidated that ADSC-Exos transfer lncRNA-MIAT by targeting miR-150-5p in endometrial epithelial cells, and alleviate fibrosis of endometrial tissue with a higher expression of cytokeratin 19 and a lower expression of fibrosis markers α-SMA and TGFβR1 [75] (Figure 6).
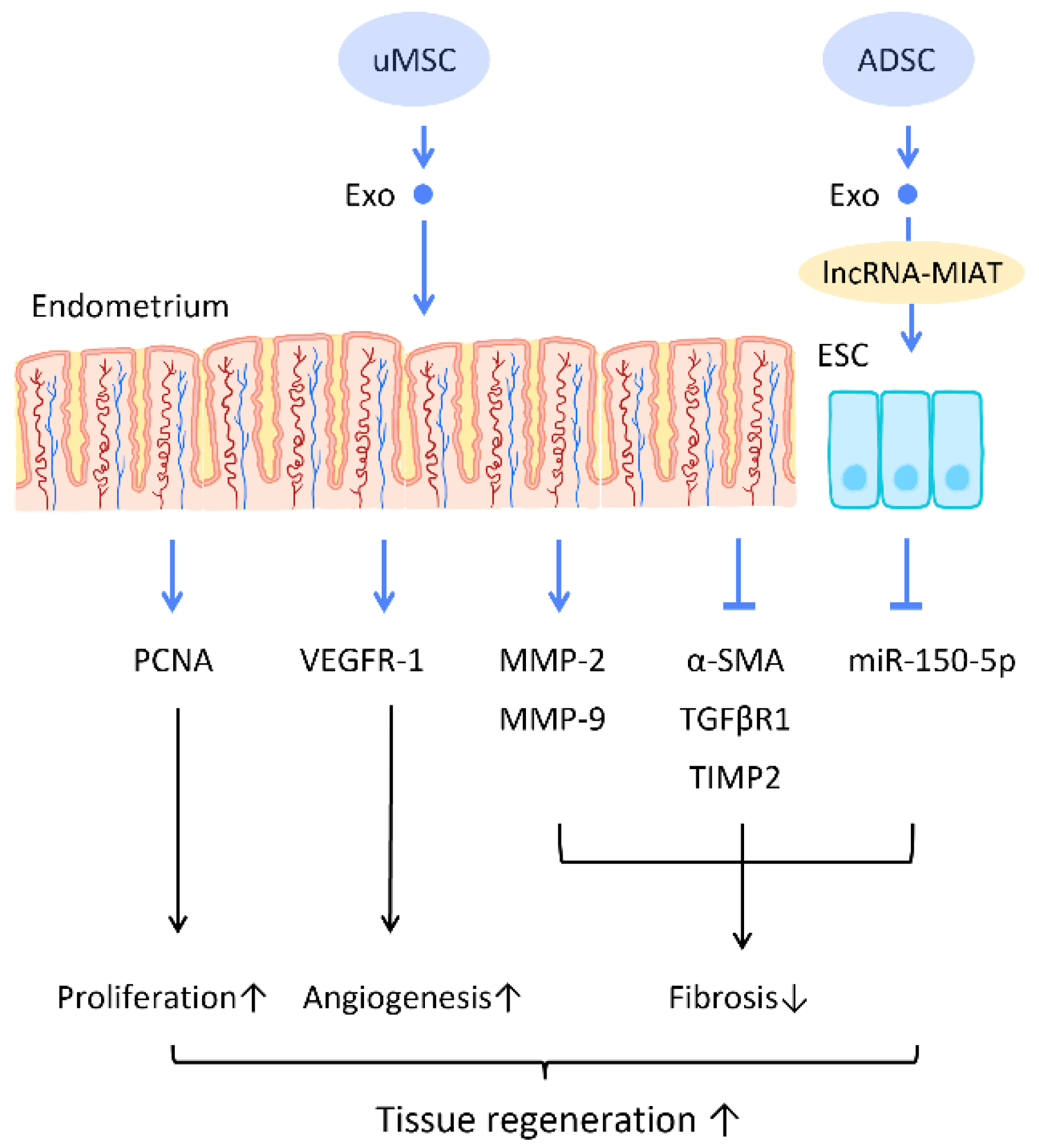
Figure 6. Molecular mechanisms of stem cell-derived exosomes regeneration of damaged endometrium. The administration of stem cell-derived exosomes is beneficial in female pregnancy, which alleviates endometrial fibrosis and enhances the regeneration competence in endometrial tissues. Abbreviation: Exo: exosome; MSC: mesenchymal stem cell; uMSC: uterus-derived SC; ADSC: adipose-derived stem cell; lncRNA: long noncoding chain RNA; VEGF: vascular endothelial growth factor; VEGFR: VEGF receptor; MMP: matrix metalloproteinase; TIMP: tissue inhibitor of metalloproteinase; α-SMA: alpha smooth muscle actin; miR: micro-RNA. ↑ represents upregulation; ↓ represents downregulation.
References
- Shao, L.; Zhang, Y.; Lan, B.; Wang, J.; Zhang, Z.; Zhang, L.; Xiao, P.; Meng, Q.; Geng, Y.-J.; Yu, X.-Y.; et al. MiRNA-sequence indicates that mesenchymal stem cells and exosomes have similar mechanism to enhance cardiac repair. BioMed Res. Int. 2017, 2017, 4150705.
- Ibrahim, S.A.; Khan, Y.S. Histology, Extracellular Vesicles. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022.
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977.
- Herberts, C.A.; Kwa, M.S.; Hermsen, H.P. Risk factors in the development of stem cell therapy. J. Transl. Med. 2011, 9, 29.
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22.
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19.
- Ha, D.H.; Kim, H.K.; Lee, J.; Kwon, H.H.; Park, G.H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 2020, 9, 1157.
- Lai, R.C.; Yeo, R.W.; Lim, S.K. Mesenchymal stem cell exosomes. Semin. Cell Dev. Biol. 2015, 40, 82–88.
- Pashoutan Sarvar, D.; Shamsasenjan, K.; Akbarzadehlaleh, P. Mesenchymal Stem Cell-Derived Exosomes: New Opportunity in Cell-Free Therapy. Adv. Pharm. Bull. 2016, 6, 293–299.
- Wu, P.; Zhang, B.; Shi, H.; Qian, H.; Xu, W. MSC-exosome: A novel cell-free therapy for cutaneous regeneration. Cytotherapy 2018, 20, 291–301.
- Harrell, C.R.; Miloradovic, D.; Sadikot, R.; Fellabaum, C.; Markovic, B.S.; Miloradovic, D.; Acovic, A.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular and Cellular Mechanisms Responsible for Beneficial Effects of Mesenchymal Stem Cell-Derived Product “Exo-d-MAPPS” in Attenuation of Chronic Airway Inflammation. Anal. Cell Pathol. (Amst.) 2020, 2020, 3153891.
- Cao, J.Y.; Wang, B.; Tang, T.T.; Wen, Y.; Li, Z.L.; Feng, S.T.; Wu, M.; Liu, D.; Yin, D.; Ma, K.L.; et al. Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury. Theranostics 2021, 11, 5248–5266.
- Liu, H.; Liang, Z.; Wang, F.; Zhou, C.; Zheng, X.; Hu, T.; He, X.; Wu, X.; Lan, P. Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight 2019, 4, e131273.
- Ouyang, X.; Han, X.; Chen, Z.; Fang, J.; Huang, X.; Wei, H. MSC-derived exosomes ameliorate erectile dysfunction by alleviation of corpus cavernosum smooth muscle apoptosis in a rat model of cavernous nerve injury. Stem Cell Res. Ther. 2018, 9, 246.
- Lee, B.C.; Kang, I.; Yu, K.R. Therapeutic Features and Updated Clinical Trials of Mesenchymal Stem Cell (MSC)-Derived Exosomes. J. Clin. Med. 2021, 10, 711.
- Blyth, F.M.; Briggs, A.M.; Schneider, C.H.; Hoy, D.G.; March, L.M. The Global Burden of Musculoskeletal Pain-Where to From Here? Am. J. Public Health 2019, 109, 35–40.
- Kim, Y.G.; Choi, J.; Kim, K. Mesenchymal Stem Cell-Derived Exosomes for Effective Cartilage Tissue Repair and Treatment of Osteoarthritis. Biotechnol. J. 2020, 15, e2000082.
- Ke, Z.; Zhu, J. Stem-Cell Derived Exosomes for the Treatment of Osteoarthritis. Curr. Stem Cell Res. Ther. 2020, 15, 597–601.
- Wang, C.; Song, W.; Chen, B.; Liu, X.; He, Y. Exosomes Isolated From Adipose-Derived Stem Cells: A New Cell-Free Approach to Prevent the Muscle Degeneration Associated With Torn Rotator Cuffs. Am. J. Sport. Med. 2019, 47, 3247–3255.
- Zhang, X.; Cai, Z.; Wu, M.; Huangfu, X.; Li, J.; Liu, X. Adipose Stem Cell-Derived Exosomes Recover Impaired Matrix Metabolism of Torn Human Rotator Cuff Tendons by Maintaining Tissue Homeostasis. Am. J. Sport. Med. 2021, 49, 899–908.
- Yu, H.; Cheng, J.; Shi, W.; Ren, B.; Zhao, F.; Shi, Y.; Yang, P.; Duan, X.; Zhang, J.; Fu, X.; et al. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater. 2020, 106, 328–341.
- Feng, W.; Jin, Q.; Ming-Yu, Y.; Yang, H.; Xu, T.; You-Xing, S.; Xu-Ting, B.; Wan, C.; Yun-Jiao, W.; Huan, W.; et al. MiR-6924-5p-rich exosomes derived from genetically modified Scleraxis-overexpressing PDGFRα(+) BMMSCs as novel nanotherapeutics for treating osteolysis during tendon-bone healing and improving healing strength. Biomaterials 2021, 279, 121242.
- Ding, S.L.; Liu, X.; Zhao, X.Y.; Wang, K.T.; Xiong, W.; Gao, Z.L.; Sun, C.Y.; Jia, M.X.; Li, C.; Gu, Q.; et al. Microcarriers in application for cartilage tissue engineering: Recent progress and challenges. Bioact. Mater. 2022, 17, 81–108.
- Yi, H.; Zhang, W.; Cui, Z.M.; Cui, S.Y.; Fan, J.B.; Zhu, X.H.; Liu, W. Resveratrol alleviates the interleukin-1β-induced chondrocytes injury through the NF-κB signaling pathway. J. Orthop. Surg. Res. 2020, 15, 424.
- He, L.; He, T.; Xing, J.; Zhou, Q.; Fan, L.; Liu, C.; Chen, Y.; Wu, D.; Tian, Z.; Liu, B.; et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res. Ther. 2020, 11, 276.
- Wang, Y.; Yu, D.; Liu, Z.; Zhou, F.; Dai, J.; Wu, B.; Zhou, J.; Heng, B.C.; Zou, X.H.; Ouyang, H.; et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 2017, 8, 189.
- Wang, R.; Xu, B. TGF-β1-modified MSC-derived exosomal miR-135b attenuates cartilage injury via promoting M2 synovial macrophage polarization by targeting MAPK6. Cell Tissue Res. 2021, 384, 113–127.
- Jin, Z.; Ren, J.; Qi, S. Human bone mesenchymal stem cells-derived exosomes overexpressing microRNA-26a-5p alleviate osteoarthritis via down-regulation of PTGS2. Int. Immunopharmacol. 2020, 78, 105946.
- Huang, G.; Chubinskaya, S.; Liao, W.; Loeser, R.F. Wnt5a induces catabolic signaling and matrix metalloproteinase production in human articular chondrocytes. Osteoarthr. Cartil. 2017, 25, 1505–1515.
- Mao, G.; Zhang, Z.; Hu, S.; Zhang, Z.; Chang, Z.; Huang, Z.; Liao, W.; Kang, Y. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther. 2018, 9, 247.
- Tao, S.C.; Yuan, T.; Zhang, Y.L.; Yin, W.J.; Guo, S.C.; Zhang, C.Q. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics 2017, 7, 180–195.
- Chen, X.; Shi, Y.; Xue, P.; Ma, X.; Li, J.; Zhang, J. Mesenchymal stem cell-derived exosomal microRNA-136-5p inhibits chondrocyte degeneration in traumatic osteoarthritis by targeting ELF3. Arthritis Res. Ther. 2020, 22, 256.
- Wang, Z.; Yan, K.; Ge, G.; Zhang, D.; Bai, J.; Guo, X.; Zhou, J.; Xu, T.; Xu, M.; Long, X.; et al. Exosomes derived from miR-155-5p-overexpressing synovial mesenchymal stem cells prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes. Cell Biol. Toxicol. 2021, 37, 85–96.
- Liu, Y.; Lin, L.; Zou, R.; Wen, C.; Wang, Z.; Lin, F. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle 2018, 17, 2411–2422.
- Wu, J.; Kuang, L.; Chen, C.; Yang, J.; Zeng, W.N.; Li, T.; Chen, H.; Huang, S.; Fu, Z.; Li, J.; et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials 2019, 206, 87–100.
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J Dent Res 2010, 89, 219–229.
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959.
- Zhang, J.; Guan, J.; Niu, X.; Hu, G.; Guo, S.; Li, Q.; Xie, Z.; Zhang, C.; Wang, Y. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J. Transl. Med. 2015, 13, 49.
- Liu, J.; Yan, Z.; Yang, F.; Huang, Y.; Yu, Y.; Zhou, L.; Sun, Z.; Cui, D.; Yan, Y. Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells Accelerate Cutaneous Wound Healing by Enhancing Angiogenesis through Delivering Angiopoietin-2. Stem Cell Rev. Rep. 2021, 17, 305–317.
- Hu, Y.; Rao, S.S.; Wang, Z.X.; Cao, J.; Tan, Y.J.; Luo, J.; Li, H.M.; Zhang, W.S.; Chen, C.Y.; Xie, H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 169–184.
- Liang, X.; Zhang, L.; Wang, S.; Han, Q.; Zhao, R.C. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J. Cell Sci. 2016, 129, 2182–2189.
- Zeng, Q.L.; Liu, D.W. Mesenchymal stem cell-derived exosomes: An emerging therapeutic strategy for normal and chronic wound healing. World J. Clin. Cases 2021, 9, 6218–6233.
- Zhang, B.; Wu, X.; Zhang, X.; Sun, Y.; Yan, Y.; Shi, H.; Zhu, Y.; Wu, L.; Pan, Z.; Zhu, W.; et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl. Med. 2015, 4, 513–522.
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells 2015, 33, 2158–2168.
- Ma, T.; Fu, B.; Yang, X.; Xiao, Y.; Pan, M. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. J. Cell Biochem. 2019, 120, 10847–10854.
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610.
- Li, X.; Xie, X.; Lian, W.; Shi, R.; Han, S.; Zhang, H.; Lu, L.; Li, M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp. Mol. Med. 2018, 50, 1–14.
- Ding, J.; Wang, X.; Chen, B.; Zhang, J.; Xu, J. Exosomes Derived from Human Bone Marrow Mesenchymal Stem Cells Stimulated by Deferoxamine Accelerate Cutaneous Wound Healing by Promoting Angiogenesis. Biomed. Res. Int. 2019, 2019, 9742765.
- Zhang, W.; Bai, X.; Zhao, B.; Li, Y.; Zhang, Y.; Li, Z.; Wang, X.; Luo, L.; Han, F.; Zhang, J.; et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp. Cell Res. 2018, 370, 333–342.
- Wang, X.; Jiao, Y.; Pan, Y.; Zhang, L.; Gong, H.; Qi, Y.; Wang, M.; Gong, H.; Shao, M.; Wang, X.; et al. Fetal Dermal Mesenchymal Stem Cell-Derived Exosomes Accelerate Cutaneous Wound Healing by Activating Notch Signaling. Stem Cells Int. 2019, 2019, 2402916.
- Xiong, M.; Zhang, Q.; Hu, W.; Zhao, C.; Lv, W.; Yi, Y.; Wu, Y.; Wu, M. Exosomes From Adipose-Derived Stem Cells: The Emerging Roles and Applications in Tissue Regeneration of Plastic and Cosmetic Surgery. Front. Cell Dev. Biol. 2020, 8, 574223.
- Zhang, T.; Wang, X.F.; Wang, Z.C.; Lou, D.; Fang, Q.Q.; Hu, Y.Y.; Zhao, W.Y.; Zhang, L.Y.; Wu, L.H.; Tan, W.Q. Current potential therapeutic strategies targeting the TGF-β/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed. Pharm. 2020, 129, 110287.
- Fang, S.; Xu, C.; Zhang, Y.; Xue, C.; Yang, C.; Bi, H.; Qian, X.; Wu, M.; Ji, K.; Zhao, Y.; et al. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-β/SMAD2 Pathway During Wound Healing. Stem Cells Transl. Med. 2016, 5, 1425–1439.
- Diegelmann, R.F. Analysis of collagen synthesis. Methods Mol. Med. 2003, 78, 349–358.
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63.
- Farghali, H.A.; AbdElKader, N.A.; Khattab, M.S.; AbuBakr, H.O. Evaluation of subcutaneous infiltration of autologous platelet-rich plasma on skin-wound healing in dogs. Biosci. Rep. 2017, 37, BSR20160503.
- Wang, L.; Hu, L.; Zhou, X.; Xiong, Z.; Zhang, C.; Shehada, H.M.A.; Hu, B.; Song, J.; Chen, L. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci. Rep. 2017, 7, 13321.
- Yang, C.; Luo, L.; Bai, X.; Shen, K.; Liu, K.; Wang, J.; Hu, D. Highly-expressed micoRNA-21 in adipose derived stem cell exosomes can enhance the migration and proliferation of the HaCaT cells by increasing the MMP-9 expression through the PI3K/AKT pathway. Arch. Biochem. Biophys. 2020, 681, 108259.
- Kim, J.H.; Park, M.; Paek, J.Y.; Lee, W.S.; Song, H.; Lyu, S.W. Intrauterine Infusion of Human Platelet-Rich Plasma Improves Endometrial Regeneration and Pregnancy Outcomes in a Murine Model of Asherman’s Syndrome. Front. Physiol. 2020, 11, 105.
- Vander Borght, M.; Wyns, C. Fertility and infertility: Definition and epidemiology. Clin. Biochem. 2018, 62, 2–10.
- Liao, Z.; Liu, C.; Wang, L.; Sui, C.; Zhang, H. Therapeutic Role of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Female Reproductive Diseases. Front. Endocrinol. (Lausanne) 2021, 12, 665645.
- Esfandyari, S.; Elkafas, H.; Chugh, R.M.; Park, H.S.; Navarro, A.; Al-Hendy, A. Exosomes as Biomarkers for Female Reproductive Diseases Diagnosis and Therapy. Int. J. Mol. Sci. 2021, 22, 2165.
- Zhao, Y.; Tao, M.; Wei, M.; Du, S.; Wang, H.; Wang, X. Mesenchymal stem cells derived exosomal miR-323-3p promotes proliferation and inhibits apoptosis of cumulus cells in polycystic ovary syndrome (PCOS). Artif. Cells Nanomed. Biotechnol. 2019, 47, 3804–3813.
- Zadehmodarres, S.; Salehpour, S.; Saharkhiz, N.; Nazari, L. Treatment of thin endometrium with autologous platelet-rich plasma: A pilot study. JBRA Assist. Reprod. 2017, 21, 54–56.
- Yang, W.; Zhang, J.; Xu, B.; He, Y.; Liu, W.; Li, J.; Zhang, S.; Lin, X.; Su, D.; Wu, T.; et al. HucMSC-Derived Exosomes Mitigate the Age-Related Retardation of Fertility in Female Mice. Mol. Ther. 2020, 28, 1200–1213.
- Huang, B.; Lu, J.; Ding, C.; Zou, Q.; Wang, W.; Li, H. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. Stem Cell Res. Ther. 2018, 9, 216.
- Xiao, G.Y.; Cheng, C.C.; Chiang, Y.S.; Cheng, W.T.; Liu, I.H.; Wu, S.C. Exosomal miR-10a derived from amniotic fluid stem cells preserves ovarian follicles after chemotherapy. Sci. Rep. 2016, 6, 23120.
- Sun, B.; Ma, Y.; Wang, F.; Hu, L.; Sun, Y. miR-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis. Stem Cell Res. Ther. 2019, 10, 360.
- Yang, M.; Lin, L.; Sha, C.; Li, T.; Zhao, D.; Wei, H.; Chen, Q.; Liu, Y.; Chen, X.; Xu, W.; et al. Bone marrow mesenchymal stem cell-derived exosomal miR-144-5p improves rat ovarian function after chemotherapy-induced ovarian failure by targeting PTEN. Lab. Investig. 2020, 100, 342–352.
- Ding, C.; Zhu, L.; Shen, H.; Lu, J.; Zou, Q.; Huang, C.; Li, H.; Huang, B. Exosomal miRNA-17-5p derived from human umbilical cord mesenchymal stem cells improves ovarian function in premature ovarian insufficiency by regulating SIRT7. Stem Cells 2020, 38, 1137–1148.
- Cai, J.H.; Sun, Y.T.; Bao, S. HucMSCs-exosomes containing miR-21 promoted estrogen production in ovarian granulosa cells via LATS1-mediated phosphorylation of LOXL2 and YAP. Gen. Comp. Endocrinol. 2022, 321–322, 114015.
- Ding, C.; Qian, C.; Hou, S.; Lu, J.; Zou, Q.; Li, H.; Huang, B. Exosomal miRNA-320a Is Released from hAMSCs and Regulates SIRT4 to Prevent Reactive Oxygen Species Generation in POI. Mol. Ther. Nucleic Acids 2020, 21, 37–50.
- Cheng, Y.H.; Tsai, N.C.; Chen, Y.J.; Weng, P.L.; Chang, Y.C.; Cheng, J.H.; Ko, J.Y.; Kang, H.Y.; Lan, K.C. Extracorporeal Shock Wave Therapy Combined with Platelet-Rich Plasma during Preventive and Therapeutic Stages of Intrauterine Adhesion in a Rat Model. Biomedicines 2022, 10, 476.
- Jang, H.Y.; Myoung, S.M.; Choe, J.M.; Kim, T.; Cheon, Y.P.; Kim, Y.M.; Park, H. Effects of Autologous Platelet-Rich Plasma on Regeneration of Damaged Endometrium in Female Rats. Yonsei Med. J. 2017, 58, 1195–1203.
- Shao, X.; Qin, J.; Wan, C.; Cheng, J.; Wang, L.; Ai, G.; Cheng, Z.; Tong, X. ADSC Exosomes Mediate lncRNA-MIAT Alleviation of Endometrial Fibrosis by Regulating miR-150-5p. Front. Genet. 2021, 12, 679643.
- Saribas, G.S.; Ozogul, C.; Tiryaki, M.; Alpaslan Pinarli, F.; Hamdemir Kilic, S. Effects of uterus derived mesenchymal stem cells and their exosomes on asherman’s syndrome. Acta Histochem. 2020, 122, 151465.
More
Information
Subjects:
Nanoscience & Nanotechnology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
12 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




