Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Gašper Tonin | -- | 3077 | 2023-04-10 21:18:46 | | | |
| 2 | Rita Xu | Meta information modification | 3077 | 2023-04-11 07:57:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Tonin, G.; Klen, J. Eptifibatide. Encyclopedia. Available online: https://encyclopedia.pub/entry/42909 (accessed on 07 February 2026).
Tonin G, Klen J. Eptifibatide. Encyclopedia. Available at: https://encyclopedia.pub/entry/42909. Accessed February 07, 2026.
Tonin, Gašper, Jasna Klen. "Eptifibatide" Encyclopedia, https://encyclopedia.pub/entry/42909 (accessed February 07, 2026).
Tonin, G., & Klen, J. (2023, April 10). Eptifibatide. In Encyclopedia. https://encyclopedia.pub/entry/42909
Tonin, Gašper and Jasna Klen. "Eptifibatide." Encyclopedia. Web. 10 April, 2023.
Copy Citation
Eptifibatide is a glycoprotein IIb/IIIa inhibitor that blocks different pathways in platelet activation and aggregation.
therapeutic peptides
glycoprotein IIb/IIIa inhibitors
antiplatelet drug
septic shock
percutaneous coronary intervention
acute coronary syndrome
ischemic stroke
carotid stenting
intracranial aneurysm stenting
cardiogenic shock
1. Introduction
Peptide-based therapies are an emerging class of medications [1][2]. Following the rapid evolution of cutting-edge production, modification, and analytical technologies, peptide medication development has advanced significantly in recent years. These technological developments have helped to reduce the inherent limitations of peptides. As a result, a wide variety of natural and engineered peptides have been created, studied, and used for a range of medicinal purposes [3][4][5][6][7][8][9][10][11][12][13][14]. In the last two decades, more than 25 peptide drugs have been approved for clinical use, and over 150 peptides are in active development today [4][5].
Therapeutic peptides are notably utilized in the treatment of cardiovascular diseases, which are the leading cause of death and morbidity among non-communicable diseases [15]. Several important peptide drugs have been discovered in this field, primarily targeting hypertension, vascular function, and manifestations of coronary artery disease, such as acute coronary syndromes (ACS) [16][17][18][19].
2. Eptifibatide
Eptifibatide (also known as Integrilin, Intrifiban, SB-1, or Sch-60936; DrugBank accession number: DB00063) is a heptapeptide derived from a disintegrin protein in the rattlesnake venom. It reversibly inhibits the GPIIb/IIIa, preventing platelet aggregation and activation [20][21][22][23].
2.1. Associated Drug Classes
Eptifibatide belongs functionally to the GPIIb/IIIa inhibitors (GPI) and, with respect to its structure and origin, to the family of disintegrin proteins [21]. This section briefly presents the pharmacological basis of eptifibatide classification.
2.1.1. GPIIb/IIIa Inhibitors
Several drugs affect GPIIb/IIIa, but three GPI are the most prominent, namely eptifibatide (Integrilin®), tirofiban (Aggrastat®), and abciximab (ReoPro®), which are all administered intravenously. In addition, some active oral peptide agents targeting GPIIb/IIIa, such as orbofiban, xemilofiban, sibrafiban, and roxifiban, have been tested. However, these have not shown encouraging outcomes and have been linked to an increase in mortality, warranting the termination of many clinical trials [20][24][25][26][27][28][29][30].
2.1.2. Snake Venom and Disintegrin Peptide Family
With several derivative drugs in clinical or research use, snake venoms are an attractive natural source for drug discovery and development [31][32]. They consist of a wide array of molecules, most of which are bioactive and have toxic effects on muscles, neurons, the heart, or other organ cells. Nevertheless, snake venom has been employed in medicine since ancient times, notably in traditional Chinese medicine. Moreover, in the 17th century, the Italian Felice Fontana demonstrated the effect of snake venom on human blood [33]. Several toxins are now recognized as valuable therapeutic agents or diagnostic tools [22][31]. The United States Food and Drug Administration (FDA) has already approved numerous snake venom-derived drugs, including Integrilin® (eptifibatide), Captopril® (enalapril), Aggrastat® (tirofiban), Reptilase® (batroxobin), and Exanta® (ximelagatran). In addition, many medications are now at the preclinical and clinical stages of testing for therapeutic use [22].
Pharmacologically, snake venom consists of various substances with numerous effects. Some of them are peptides that mainly target (in an enzymatic or non-enzymatic way) membrane receptors, enzymes, ion channels, and elements of the hemostatic system [22].
Disintegrins are a class of small cysteine-rich peptides that target integrins and are found in different species of snake venom. They carry the KTS, MGD, RTS, VGD, KGD, WGD, or RGD amino acid motifs recognized by integrins. It is important to understand that proper cysteine bridges are essential for protein folding and conformational exposure of the binding motif, composed of three amino acids. As different motif exposure translates to different effects on different integrin protein types, these short peptides are involved in several processes. For example, they take part in the regulation of angiogenesis, platelet aggregation, apoptosis, cell migration, invasion, adhesion, and proliferation [22][32].
Disintegrins may be classified into four groups [32]:
-
Approximately 41–51 amino acid long peptides with four cysteine bridges (echistatin and obtustatin);
-
Approximately 70 amino acid long peptides with six cysteine bridges (barbourin, flavoviridin, and atrolysin);
-
Approximately 84 amino acid long peptides with seven cysteine bridges (bitistatin);
-
Macromolecular complexes of usually noncovalently bound homodimers or heterodimers, which are 67 amino acids long and have 10 cysteines incorporated into the structure.
Eptifibatide is a disintegrin type of peptide that mimics a portion of barbourin, a toxic peptide found in the venom of the Southeastern pygmy rattlesnake (Sistrurus miliarius barbouri) [32]. Barbourin is selective for GPIIb/IIIa, despite other disintegrins having nonspecific affinities for different integrins. This nonspecific binding of other disintegrins is mediated by the RGD motif, whereas barbourin derives its favorable selectivity from the unique KGD motif in which lysine is replaced by arginine. In eptifibatide, the KGD motif is preserved but modified [34]. In the Protein Data Bank (PDB), there are currently three models of the GPIIb/IIIa complexes with eptifibatide (PDB ID: 7THO, 2VDN, and 7U60) [35][36][37].
2.2. Biochemical Structure
Eptifibatide is a cyclic peptide derived from the disintegrin family protein, barbourin [32]. Its molecular formula is C35H49N11O9S2, and its molecular mass is 832.0 g/mol [38]. Both the structure and the amino acids sequence are presented in Figure 1.
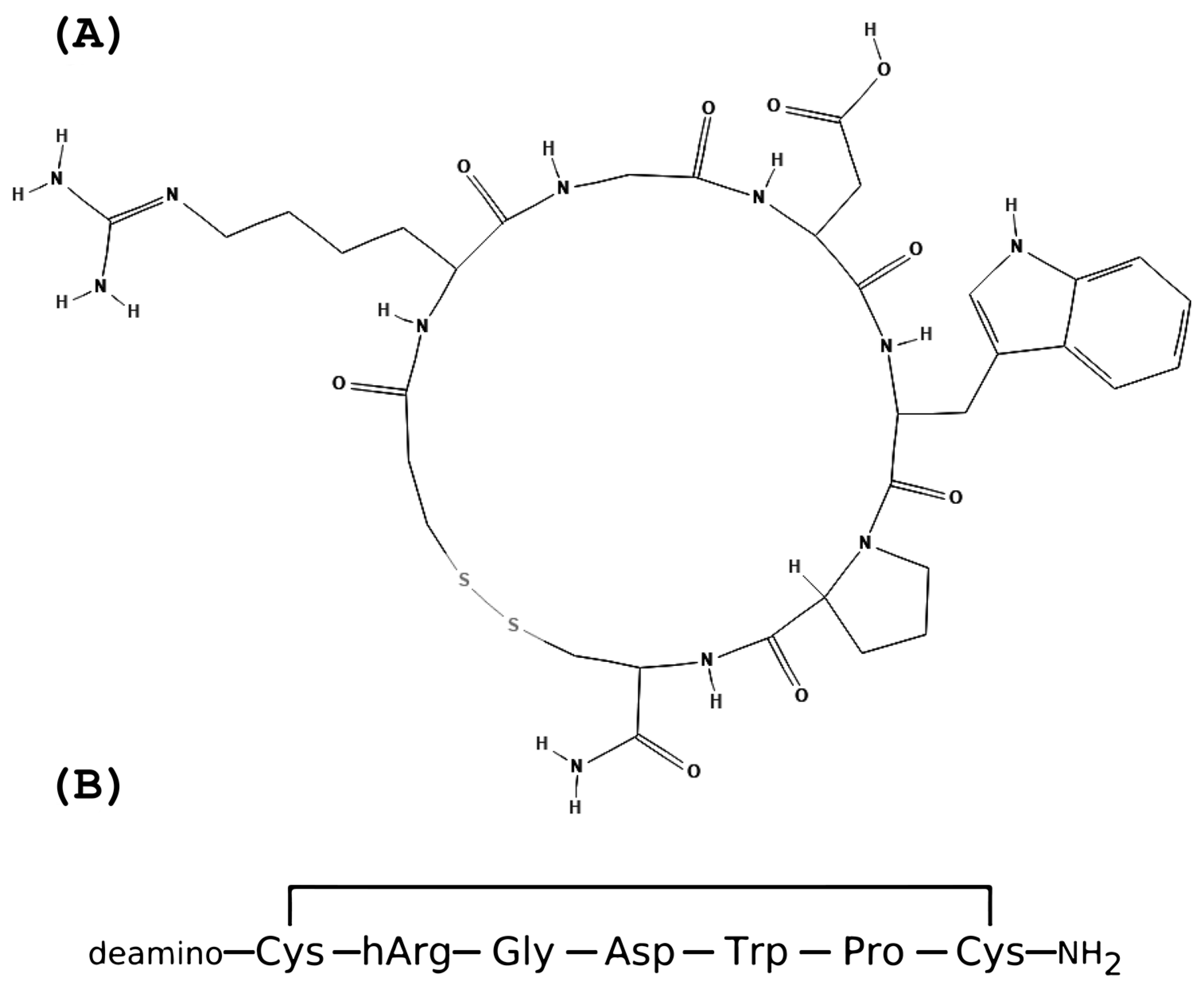
Figure 1. The eptifibatide’s structure and amino acid sequence. (A) Eptifibatide structure; (B) Eptifibatide amino acids sequence.
It was derived by determining the minimum active sequence (MAS) of this component of snake venom. A minimum active sequence (MAS) is the shortest amino acid sequence derived from an endogenous peptide, still retaining its potency or binding affinity to its target [21]. The process of truncation is used to remove biologically redundant amino acid residues from the protein. The endogenous peptides are “trimmed” in such a way as to reach a more economical protein size that can be widely synthesized without compromising its effect on biological targets. The cys-rich endogenous disintegrin protein from snake venom contains 73 amino acids (UniProt code: P22827), and a length of seven amino acids was achieved for eptifibatide by the process of truncation [21][34][39]. The small size of the peptide explains its low immunogenicity, which is an essential factor in repetitive administration and the use of the drug on patients with an unknown history [34].
Moreover, in developing eptifibatide, researchers had to overcome the drawbacks of peptide drugs, especially their low in vivo stability and membrane impermeability [3]. Since the direct action of eptifibatide is thought to be limited only to the extracellular domain of GPIIb/IIIa on platelets, the drawback of membrane impermeability was eliminated. To increase the stability of eptifibatide, the peptide was cyclized using a disulfide bridge between the captopropionyl residue (des-amino-cysteinyl) and the cysteine. The cyclic structure increases the bioavailability of the drug and its resistance to plasma proteases [34]. Besides these modifications, the peptide undergoes guanylation at the Lys side chain and deamination at the N-terminus (among other modifications), giving it a highly potent therapeutic value [21][40].
2.3. Pharmacodynamics
Eptifibatide competes in a dose-dependent manner with fibrinogen for the GPIIb/IIIa. It is a specific inhibitor of the GPIIb/IIIa receptor, which limits the pharmacological effect of platelets and their precursors [34]. The treatment objective is to achieve 80% inhibition of platelet aggregation depending on the dose and concentration of medication. This proposition has been demonstrated ex vivo with adenosine diphosphate (ADP) and other agonists that induce platelet aggregation. The immediate effect of eptifibatide can be observed after an intravenous injection; when a continuous infusion is subsequently administered, this treatment can successfully inhibit more than 80% of ADP-induced platelet aggregation ex vivo with normal calcium levels in the majority of patients [41]. Furthermore, the eptifibatide effect can be quickly stopped, since the drug rapidly dissociates from GPIIb/IIIa, and after 4 h, platelet functions return to baseline and are swiftly cleared from plasma [34].
2.4. Pharmacokinetics
Intravenous administration of therapeutic peptides has the advantage of avoiding pre-systemic metabolism by the liver and gastrointestinal enzymes, resulting in complete systemic availability. For bolus doses of 90 to 250 µg/kg and infusion rates of 0.5 to 3.0 µg/min, the pharmacokinetics of eptifibatide are linear and proportional to the dose. When infused at 2.0 µg/kg/min, the mean steady-state plasma concentration of eptifibatide in patients with coronary artery disease is 1.5 to 2.2 µg/mL. Plasma concentrations in this range can be attained rapidly if a bolus of 180 µg/kg is used prior to the infusion [41]. The onset of action is rapid, with inhibition of platelet aggregation occurring 15 min after a bolus. The binding proportion of eptifibatide to human plasma proteins is approximately 25% [41].
The pharmacokinetics of peptides are characterized by their typically short half-life in the bloodstream, which results from cleavage by proteases and peptidases. A short elimination half-life for endogenous peptides is desirable for regulating their concentrations and function. The eptifibatide plasma elimination half-life is 2.5 h [2]. Peptides have a molecular weight between 1 and 10 kDa; therefore, the primary absorption process is diffusion-driven uptake into blood. On the other hand, eptifibatide has a molecular weight of 800 D. The volume of distribution for eptifibatide is 0.2 to 0.3 L/kg.
Eptifibatide is not known to be metabolized by uridine-5-diphosphate glucuronosyltransferase enzymes or cytochrome P450 (CYP) but is deaminated by metabolic enzymes. Furthermore, kidney clearance accounts for approximately 50% of total body clearance; therefore, deaminated eptifibatide and polar metabolites are excreted in the urine [41]. Hepatic metabolism is not the primary route of elimination for most peptides, but it can play an essential role in the metabolism of some peptide drugs [2].
2.5. Clinical Applications
Eptifibatide is an antiplatelet agent; therefore, it is used in diseases in which thrombus formation is a critical part of pathogenesis or complications. As with any antithrombotic treatment, consideration should be given to the trade-off between the risk of ischemic injury and bleeding when administering eptifibatide [42]. The FDA indicates its use for the treatment of ACS and in percutaneous coronary intervention (PCI) [43]. However, research over the past decade has also sought to evaluate the role of eptifibatide in ischemic stroke, stenting of carotid and intracranial aneurysms, and septic shock [43][44][45][46][47][48].
2.5.1. Acute Coronary Syndromes: Angina Pectoris, STEMI, and NSTEMI
ACS is a manifestation of coronary heart disease associated with an abrupt reduction in the blood supply to the heart. Underlying factors contributing to the disease are smoking, hyperlipidemia, obesity, diabetes, etc. The syndromes comprise different clinical presentations, including unstable angina pectoris, non-ST elevation myocardial infarction (NSTEMI), and ST-elevation myocardial infarction (STEMI). Despite different presentations, all syndromes usually present with chest discomfort at rest [49][50][51]. In most cases, the pathophysiological basis of these diseases is the rupture of an atherosclerotic plaque in a cardiac vessel causing platelet aggregation and thrombus formation, which in turn restricts the blood flow to the heart tissue, resulting in cardiac ischemia [49][50].
Angina Pectoris and non-ST Elevation Myocardial Infarction
Eptifibatide is indicated for the prevention of myocardial infarction in unstable angina and NSTEMI in both drug-treated and PCI patients [43][52]. The PURSUIT trial (1998) showed a reduction in endpoint mortality and a beneficial effect in preventing nonfatal myocardial infarction in these patients [52]. In NSTEMI, a combination of loading dose by aspirin and maintenance treatment by eptifibatide could be used [42]. Aspirin inhibits thromboxane A2 production and therefore prevents platelet aggregation. Although both drugs affect the platelets, their mechanisms of work are different, potentiating their effect.
ST-Elevated Myocardial Infarction
A meta-analysis by Karathanos et al. in 2019 showed that routine use of GPIs in patients with STEMI was associated with reduced mortality, which was probably the consequence of a reduction in recurrent ischemic events. Despite the promising result, it should be noted that these results are largely based on studies before dual antiplatelet therapy with prasugrel/ticagrelor was routinely used, as is the case today [53]. Although less convincing, eptifibatide can also improve myocardial perfusion in STEMI, as shown in the TITAN-TIMI 34 trial [43][54]. Nevertheless, more recent studies have shown that prehospital administration of GPI in STEMI has not shown benefits and even increases the bleeding risk compared to routine use in a catheterization laboratory [55][56][57]. Although eptifibatide has not been tested in a randomized trial, the European Society of Cardiology guidelines (ESC) suggest it as bail-out therapy in high-risk patients (slow flow or no flow with occlusion of the stent, high thrombus burden, etc.) but not as a routine drug for primary PCI [57].
A meta-analysis by Saleiro et al. in 2020 has shown that the use of GPIs as an adjunct to standard therapy may be beneficial in myocardial infarction that results in cardiogenic shock. In this study, the use of GPIs was associated with better outcomes, namely short-term and long-term survival. Moreover, it did not increase the risk of bleeding in the treated patients [58]. Other newer studies have shown similar results [58][59][60].
2.5.2. Percutaneous Coronary Intervention
PCI is a non-surgical but invasive procedure in which a catheter is used to insert a stent into narrowed or occluded coronary arteries, improving the blood supply. It is the preferred method of treatment for ACS [61][62]. As the pretreatment in patients undergoing PCI, the ESC guidelines propose using a combination of eptifibatide and unfractionated heparin, as both anticoagulation and platelet inhibition are important in the pathogenesis-based therapy of NSTEMI [42]. Guidelines from the American College of Cardiology (ACC) and the American Heart Association (AHA) similarly suggest the use of eptifibatide as an initial antiplatelet therapy in patients with high-risk features [63]. Heparin dosages of 50–70 IU/kg i.v. should be used if administered in this combination [42].
Although the FDA has approved using eptifibatide for patients undergoing PCI (including stenting), data for using eptifibatide in the periinterventional treatment of NSTEMI are limited and partially outdated. A major trial that has shown the benefits of eptifibatide use in PCI was the IMPACT-II trial, published in 1997 [43][64]. Most research on the use of eptifibatide in PCI pretreatment predates routine dual antiplatelet treatment (DAPT) [65][66][67]. In periinterventional antiplatelet treatment, oral P2Y12 receptor inhibitors were found to be as effective as GPI and are recommended for routine use [42][43][65][66][67]. Therefore, little to no evidence exists to support the use of eptifibatide in patients who will undergo coronary angiography and are receiving DAPT [65][66][67]. On the other hand, eptifibatide may be considered when facing high-risk PCI patients (slow flow or no-flow with the closure of the stent, high thrombus burden, etc.), patients who did not receive pretreatment with P2Y12 receptor inhibitors, and patients with thrombotic complications [42][67][68]. The intracoronary administration of the drug is comparable to intravenous use [69][70].
2.5.3. Bridging Strategy for Patients Undergoing Surgery after Coronary Stent Insertion
Postoperative bleeding prevention after cardiac surgery is crucial to decreasing morbidity and mortality. Since i.v. antiplatelet medications, such as eptifibatide, are quickly cleared from the system and their antiplatelet effect can be quickly reversed, they are used before cardiac and noncardiac surgery as a substitution for oral P2Y12 inhibitors [71]. A 2022 meta-analysis by Wu et al. showed that eptifibatide might be safe and effective when used as a bridging strategy for patients undergoing coronary stent implantation requiring surgery. The GPI might be used without an increased bleeding risk when temporarily discontinuing DAPT. Nevertheless, further randomized studies are needed to substantiate this claim [72][73]. In a 2019 study by Van Tuyl et al., eptifibatide was shown to be an effective choice for these patients and was even preferred over abciximab [71].
Another drug that is often compared to eptifibatide in bridging strategies is cangrelor. Cangrelor is a reversible P2Y12 receptor inhibitor that prevents ADP-induced platelet aggregation and activation. It should be considered in patients with renal insufficiency, as clearance of eptifibatide is influenced by renal function [71]. Moreover, a study by Yun et al. from 2019 showed that cangrelor and eptifibatide were similar in terms of overall bleeding events and major inpatient cardiac adverse events [74].
2.5.4. Ischemic Stroke and Carotid and Intracranial Aneurysm Stenting
The CLEAR trial from 2008 showed that eptifibatide is beneficial in preventing intracerebral hemorrhage in patients with acute ischemic stroke if administered with TPA [43][43][44]. Nevertheless, a 2022 meta-analysis by Liu et al. showed that adding eptifibatide to routine thrombolysis or thrombectomy treatment did not improve functional outcomes, favorable outcomes, or the National Institutes of Health Stroke Scale (NIHSS) score. Moreover, it might be associated with an increase in fatal ICH three months after AIS [75]. Another meta-analysis by Zhu et al. in 2020 showed that eptifibatide might be promising when used at a reduced dose (a low dose was also used in the CLEAR study); thus, more randomized trials with different doses are needed to evaluate the role of eptifibatide in the treatment of acute ischemic stroke [43][44][45]. A newer retrospective case-control study by Luo et al. (2022) compared routine therapy and treatment with an additional low dose of eptifibatide. Although the study reported no significant differences in NIHSS or adverse events, an analysis of the subgroups showed that eptifibatide is a safe and effective treatment when small artery occlusion is involved [46]. Similarly, a trial by Rana et al. from 2022 showed that using eptifibatide during endovascular therapy in large vessel occlusion is associated with a higher rate of hemorrhages and no benefits to the NIHSS or 90-day mortality [76]. On the other hand, a matched-control analysis by Ma et al. in 2022 showed that the use of eptifibatide was safe and effective in patients undergoing mechanical thrombectomy after ischemic stroke, because the rate of successful recanalization was significantly higher in the intervention group (91.3% versus 81.5%; p = 0.043) and the 3-month outcome on the modified Rankin Scale showed good results (53.1% versus 33.3%; p = 0.016) [77]. As shown, the existing evidence in this research area is not yet conclusive, and further studies are needed to evaluate the use of eptifibatide in the treatment of ischemic stroke.
Antiplatelet agents are administered to prevent one of the most critical complications of stenting, namely stent thrombosis. In a study by Osteraas et al., the use of eptifibatide (as a bolus followed by infusion for 24 h after stent placement) was shown to be associated with a lower risk of symptomatic intracranial hemorrhage after carotid stenting [78]. Another study by Horev et al. from 2021 similarly reported a reduced number of complications when using eptifibatide immediately after carotid stenting [79].
Eptifibatide has also been explored as a potential antiplatelet therapy in the stenting of intracranial aneurysms. A 2022 study by Aouni et al. compared three antiplatelet agents (ticagrelor, eptifibatide, and cangrelor) in the stent-assisted endovascular treatment of unruptured intracranial aneurysms and found no significant differences between them [48].
2.5.5. Septic Shock
One of the main mechanisms in septic shock is the activation of the endothelium and platelets. This activation subsequently leads to generalized microvascular damage, microthrombi, capillary leaks, and coagulopathy caused by widespread consumption of coagulation factors [47][80]. It is noteworthy that research on the use of eptifibatide in septic shock is extremely limited, as to our knowledge, only one study has been performed. The 2019 randomized and placebo-controlled double-blind trial by Berthelsen et al. showed the benefits in improving Sequential Organ Failure Assessment (SOFA) and reducing platelet consumption, fibrinolytic biomarkers, and endothelial damage when a combination of the synthetic analogs of prostacyclin, iloprost, and eptifibatide was used in patients with septic shock [47]. Further research in this area is needed to elucidate the role of eptifibatide.
References
- Vlieghe, P.; Lisowski, V.; Martinez, J.; Khrestchatisky, M. Synthetic therapeutic peptides: Science and market. Drug Discov. Today 2010, 15, 40–56.
- Diao, L.; Meibohm, B. Pharmacokinetics and pharmacokinetic-pharmacodynamic correlations of therapeutic peptides. Clin. Pharm. 2013, 52, 855–868.
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target Ther. 2022, 7, 48.
- Henninot, A.; Collins, J.C.; Nuss, J.M. The current state of peptide drug discovery: Back to the future? J. Med. Chem. 2018, 61, 1382–1414.
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorganic Med. Chem. 2018, 26, 2700–2707.
- Angell, Y.; Holford, M.; Moos, W.H. Building on success: A bright future for peptide therapeutics. Protein Pept. Lett. 2018, 25, 1044–1050.
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325.
- Fisher, E.; Pavlenko, K.; Vlasov, A.; Ramenskaya, G. Peptide-based therapeutics for oncology. Pharm. Med. 2019, 33, 9–20.
- Sloan, L.A. Review of Glucagon-Like Peptide-1 receptor agonists for the treatment of type 2 diabetes mellitus in patients with chronic kidney disease and their renal effects. J. Diabetes 2019, 11, 938–948.
- Peterson, S.C.; Barry, A.R. Effect of Glucagon-like Peptide-1 receptor agonists on all-cause mortality and cardiovascular outcomes: A meta-analysis. Curr. Diabetes Rev. 2018, 14, 273–279.
- Li, X.-F.; Liu, C.-F.; Rao, G.-W. Monoclonal antibodies, small molecule inhibitors and antibody-drug conjugates as HER2 inhibitors. Curr. Med. Chem. 2021, 28, 3339–3360.
- Vuong, H.G.; Ho, A.T.N.; Tran, T.T.K.; Capdevila, J.; Benekli, M.; Nakazawa, T.; Katoh, R.; Kondo, T. Efficacy and toxicity of sorafenib in the treatment of advanced medullary thyroid carcinoma: A systematic review and meta-analysis. Head Neck 2019, 41, 2823–2829.
- Alavi, S.E.; Cabot, P.J.; Moyle, P.M. Glucagon-Like Peptide-1 receptor agonists and strategies to improve their efficiency. Mol. Pharm. 2019, 16, 2278–2295.
- Hackenberger, C.P.R.; Dawson, P.E.; Chen, Y.-X.; Hojo, H. Modern peptide and protein chemistry: Reaching new heights. J. Org. Chem. 2020, 85, 1328–1330.
- Joseph, P.; Leong, D.; McKee, M.; Anand, S.S.; Schwalm, J.-D.; Teo, K.; Mente, A.; Yusuf, S. Reducing the global burden of cardiovascular disease, part 1: The epidemiology and risk factors. Circ. Res. 2017, 121, 677–694.
- Montone, C.M.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Piovesana, S.; Zenezini Chiozzi, R.; Laganà, A. peptidomic strategy for purification and identification of potential ACE-inhibitory and antioxidant peptides in tetradesmus obliquus microalgae. Anal. Bioanal. Chem. 2018, 410, 3573–3586.
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; de Bold, M.K.; de Bold, A.J. Cardiac natriuretic peptides. Nat. Rev. Cardiol. 2020, 17, 698–717.
- Grieco, P.; Gomez-Monterrey, I. Natural and synthetic peptides in the cardiovascular diseases: An update on diagnostic and therapeutic potentials. Arch. Biochem. Biophys. 2019, 662, 15–32.
- Recio, C.; Maione, F.; Iqbal, A.J.; Mascolo, N.; De Feo, V. The Potential Therapeutic Application of Peptides and Peptidomimetics in Cardiovascular Disease. Front. Pharmacol. 2017, 7, 526.
- Huang, J.; Li, X.; Shi, X.; Zhu, M.; Wang, J.; Huang, S.; Huang, X.; Wang, H.; Li, L.; Deng, H.; et al. Platelet integrin AIIbβ3: Signal transduction, regulation, and its therapeutic targeting. J. Hematol. Oncol. 2019, 12, 26.
- Ahn, J.-M.; Kassees, K.; Lee, T.-K.; Manandhar, B.; Yousif, A.M. Strategy and Tactics for Designing Analogs: Biochemical Characterization of the Large Molecules. In Comprehensive Medicinal Chemistry III; Elsevier: Amsterdam, The Netherlands, 2017; pp. 66–115. ISBN 978-0-12-803201-5.
- Mohamed Abd El-Aziz, T.; Soares, A.G.; Stockand, J.D. Snake venoms in drug discovery: Valuable therapeutic tools for life saving. Toxins 2019, 11, 564.
- Eptifibatide. DB00063. Available online: https://go.drugbank.com/drugs/DB00063 (accessed on 1 March 2023).
- Cannon, C.P. Oral glycoprotein IIb/IIIa inhibition—Great idea, but it didn’t work. Am. J. Med. 2002, 112, 673–675.
- Newby, L.K.; Califf, R.M.; White, H.D.; Harrington, R.A.; Van de Werf, F.; Granger, C.B.; Simes, R.J.; Hasselblad, V.; Armstrong, P.W. The failure of orally administered glycoprotein IIb/IIIa inhibitors to prevent recurrent cardiac events. Am. J. Med. 2002, 112, 647–658.
- Cannon, C.P.; McCabe, C.H.; Wilcox, R.G.; Langer, A.; Caspi, A.; Berink, P.; Lopez-Sendon, J.; Toman, J.; Charlesworth, A.; Anders, R.J.; et al. Oral glycoprotein IIb/IIIa inhibition with orbofiban in patients with unstable coronary syndromes (OPUS-TIMI 16) trial. Circulation 2000, 102, 149–156.
- O’Neill, W.W.; Serruys, P.; Knudtson, M.; van Es, G.A.; Timmis, G.C.; van der Zwaan, C.; Kleiman, J.; Gong, J.; Roecker, E.B.; Dreiling, R.; et al. Long-Term Treatment with a platelet glycoprotein-receptor antagonist after percutaneous coronary revascularization. Excite trial investigators. Evaluation of oral xemilofiban in controlling thrombotic events. N. Engl. J. Med. 2000, 342, 1316–1324.
- Second SYMPHONY Investigators. Randomized trial of aspirin, sibrafiban, or both for secondary prevention after acute coronary syndromes. Circulation 2001, 103, 1727–1733.
- Topol, E.J.; Easton, J.D.; Amarenco, P.; Califf, R.; Harrington, R.; Graffagnino, C.; Davis, S.; Diener, H.C.; Ferguson, J.; Fitzgerald, D.; et al. Design of the blockade of the glycoprotein IIb/IIIa receptor to avoid vascular occlusion (BRAVO) trial. Am. Heart J. 2000, 139, 927–933.
- Mousa, S.A.; Khurana, S.; Forsythe, M.S. Comparative in vitro efficacy of different platelet glycoprotein IIb/IIIa antagonists on platelet-mediated clot strength induced by tissue factor with use of thromboelastography: Differentiation among glycoprotein IIb/IIIa antagonists. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1162–1167.
- Chan, Y.S.; Cheung, R.C.F.; Xia, L.; Wong, J.H.; Ng, T.B.; Chan, W.Y. Snake venom toxins: Toxicity and medicinal applications. Appl. Microbiol. Biotechnol. 2016, 100, 6165–6181.
- Lazarovici, P.; Marcinkiewicz, C.; Lelkes, P.I. From snake venom’s disintegrins and C-Type lectins to anti-platelet drugs. Toxins 2019, 11, 303.
- Hawgood, B.J. Abbé Felice Fontana (1730–1805): Founder of modern toxinology. Toxicon 1995, 33, 591–601.
- Phillips, D.R.; Scarborough, R.M. Clinical pharmacology of eptifibatide. Am. J. Cardiol. 1997, 80, 11B–20B.
- Bank, R.P.D. RCSB PDB—7THO: Integrin AlaphIIBbeta3 Complex with Eptifibatide. Available online: https://www.rcsb.org/structure/7THO (accessed on 1 March 2023).
- Bank, R.P.D. RCSB PDB—2VDN: Re-Refinement of Integrin AlphaIIbBeta3 Headpiece Bound to Antagonist Eptifibatide. Available online: https://www.rcsb.org/structure/2VDN (accessed on 1 March 2023).
- Bank, R.P.D. RCSB PDB—7U60: Integrin AlaphIIBbeta3 Complex with CRGDfV. Available online: https://www.rcsb.org/structure/7U60 (accessed on 1 March 2023).
- PubChem Eptifibatide. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/123610 (accessed on 9 January 2023).
- Disintegrin Barbourin—Sistrurus Miliarius Barbouri (Dusky Pigmy Rattlesnake) | UniProtKB | UniProt. Available online: https://www.uniprot.org/uniprotkb/P22827/entry (accessed on 1 March 2023).
- Scarborough, R.M.; Naughton, M.A.; Teng, W.; Rose, J.W.; Phillips, D.R.; Nannizzi, L.; Arfsten, A.; Campbell, A.M.; Charo, I.F. Design of potent and specific integrin antagonists. peptide antagonists with high specificity for glycoprotein IIb-IIIa. J. Biol. Chem. 1993, 268, 1066–1073.
- Liu, L.; Ding, Y.; Jiao, Z.; Wu, M.; Li, C.; Liu, J.; Liu, C.; Hu, Y.; Li, Q.; Zhang, H. Clinical evaluation of the tolerability, pharmacokinetics, and inhibition of platelet aggregation of eptifibatide in healthy chinese subjects. Clin. Pharmacol. Drug Dev. 2020, 9, 267–276.
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the european society of cardiology (ESC). Eur. Heart J. 2021, 42, 1289–1367.
- Pancioli, A.M.; Broderick, J.; Brott, T.; Tomsick, T.; Khoury, J.; Bean, J.; del Zoppo, G.; Kleindorfer, D.; Woo, D.; Khatri, P.; et al. The combined approach to lysis utilizing eptifibatide and Rt-PA in acute ischemic stroke: The clear stroke trial. Stroke 2008, 39, 3268–3276.
- Adeoye, O.; Sucharew, H.; Khoury, J.; Vagal, A.; Schmit, P.A.; Ewing, I.; Levine, S.R.; Demel, S.; Eckerle, B.; Katz, B.; et al. Combined approach to lysis utilizing eptifibatide and recombinant tissue-type plasminogen activator in acute ischemic stroke-full dose regimen stroke trial. Stroke 2015, 46, 2529–2533.
- Zhu, X.; Cao, G. Safety of glycoprotein IIb-IIIa inhibitors used in stroke-related treatment: A systematic review and meta-analysis. Clin. Appl. Thromb./Hemost. 2020, 26, 1076029620942594.
- Luo, L.; Lin, J.; Deng, Y.; Li, Z.; Yuan, Y.; Zhang, W. Treatment of progressive ischemic stroke with low-dose eptifibatide: A retrospective case-control study. Exp. Ther. Med. 2022, 25, 22.
- Berthelsen, R.E.; Ostrowski, S.R.; Bestle, M.H.; Johansson, P.I. Co-administration of iloprost and eptifibatide in septic shock (CO-ILEPSS)-a randomised, controlled, double-blind investigator-initiated trial investigating safety and efficacy. Crit. Care 2019, 23, 301.
- Cheddad El Aouni, M.; Magro, E.; Abdelrady, M.; Nonent, M.; Gentric, J.C.; Ognard, J. Safety and Efficacy of Cangrelor Among Three antiplatelet regimens during stent-assisted endovascular treatment of unruptured intracranial aneurysm: A single-center retrospective study. Front. Neurol. 2022, 13, 727026.
- Bhatt, D.L.; Lopes, R.D.; Harrington, R.A. Diagnosis and treatment of acute coronary syndromes: A review. JAMA 2022, 327, 662–675.
- Singh, A.; Museedi, A.S.; Grossman, S.A. Acute Coronary Syndrome. In StatPearls; StatPearls: Treasure Island, FL, USA, 2022.
- Bergmark, B.A.; Mathenge, N.; Merlini, P.A.; Lawrence-Wright, M.B.; Giugliano, R.P. Acute coronary syndromes. Lancet 2022, 399, 1347–1358.
- PURSUIT Trial Investigators. Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) Trial Investigators Inhibition of Platelet Glycoprotein IIb/IIIa with Eptifibatide in Patients with Acute Coronary Syndromes. N. Engl. J. Med. 1998, 339, 436–443.
- Karathanos, A.; Lin, Y.; Dannenberg, L.; Parco, C.; Schulze, V.; Brockmeyer, M.; Jung, C.; Heinen, Y.; Perings, S.; Zeymer, U.; et al. Routine glycoprotein IIb/IIIa inhibitor therapy in st-segment elevation myocardial infarction: A meta-analysis. Can. J. Cardiol. 2019, 35, 1576–1588.
- Gibson, C.M.; Kirtane, A.J.; Murphy, S.A.; Rohrbeck, S.; Menon, V.; Lins, J.; Kazziha, S.; Rokos, I.; Shammas, N.W.; Palabrica, T.M.; et al. Early initiation of eptifibatide in the emergency department before primary percutaneous coronary intervention for st-segment elevation myocardial infarction: Results of the time to integrilin therapy in acute myocardial infarction (TITAN)-TIMI 34 trial. Am. Heart J. 2006, 152, 668–675.
- Ellis, S.G.; Tendera, M.; de Belder, M.A.; van Boven, A.J.; Widimsky, P.; Janssens, L.; Andersen, H.R.; Betriu, A.; Savonitto, S.; Adamus, J.; et al. Facilitated PCI in patients with ST-elevation myocardial infarction. N. Engl. J. Med. 2008, 358, 2205–2217.
- ten Berg, J.M.; van ’t Hof, A.W.J.; Dill, T.; Heestermans, T.; van Werkum, J.W.; Mosterd, A.; van Houwelingen, G.; Koopmans, P.C.; Stella, P.R.; Boersma, E.; et al. Effect of early, pre-hospital initiation of high bolus dose tirofiban in patients with ST-segment elevation myocardial infarction on short- and long-term clinical outcome. J. Am. Coll. Cardiol. 2010, 55, 2446–2455.
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the european society of cardiology (ESC). Eur. Heart J. 2018, 39, 119–177.
- Saleiro, C.; Teixeira, R.; De Campos, D.; Lopes, J.; Oliveiros, B.; Costa, M.; Gonçalves, L. Glycoprotein IIb/IIIa Inhibitors for Cardiogenic Shock Complicating Acute Myocardial Infarction: A Systematic Review, Meta-Analysis, and Meta-Regression. J. Intensive Care 2020, 8, 85.
- Kanic, V.; Kompara, G.; Suran, D. GP IIb/IIIa receptor inhibitors in mechanically ventilated patients with cardiogenic shock due to myocardial infarction in the era of potent P2Y12 receptor antagonists. J. Clin. Med. 2022, 11, 7426.
- Myrda, K.; Gąsior, M.; Dudek, D.; Nawrotek, B.; Niedziela, J.; Wojakowski, W.; Gierlotka, M.; Grygier, M.; Stępińska, J.; Witkowski, A.; et al. One-year outcome of glycoprotein IIb/IIIa inhibitor therapy in patients with myocardial infarction-related cardiogenic shock. J. Clin. Med. 2021, 10, 5059.
- Hoole, S.P.; Bambrough, P. Recent advances in percutaneous coronary intervention. Heart 2020, 106, 1380–1386.
- Ahmad, M.; Mehta, P.; Reddivari, A.K.R.; Mungee, S. Percutaneous coronary intervention. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E.; Ganiats, T.G.; Holmes, D.R.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: Executive summary. Circulation 2014, 130, 2354–2394.
- Impact-II Investigators. Randomised placebo-controlled trial of effect of eptifibatide on complications of percutaneous coronary intervention: IMPACT-II. Lancet 1997, 349, 1422–1428.
- Giugliano, R.P.; White, J.A.; Bode, C.; Armstrong, P.W.; Montalescot, G.; Lewis, B.S.; van ’t Hof, A.; Berdan, L.G.; Lee, K.L.; Strony, J.T.; et al. Early versus delayed, provisional eptifibatide in acute coronary syndromes. N. Engl. J. Med. 2009, 360, 2176–2190.
- Stone, G.W.; McLaurin, B.T.; Cox, D.A.; Bertrand, M.E.; Lincoff, A.M.; Moses, J.W.; White, H.D.; Pocock, S.J.; Ware, J.H.; Feit, F.; et al. Bivalirudin for patients with acute coronary syndromes. N. Engl. J. Med. 2006, 355, 2203–2216.
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165.
- Rubboli, A.; Patti, G. What is the role for glycoprotein IIb/IIIa inhibitor use in the catheterization laboratory in the current era? Curr. Vasc. Pharmacol. 2018, 16, 451–458.
- Friedland, S.; Eisenberg, M.J.; Shimony, A. Meta-analysis of randomized controlled trials of intracoronary versus intravenous administration of glycoprotein IIb/IIIa inhibitors during percutaneous coronary intervention for acute coronary syndrome. Am. J. Cardiol. 2011, 108, 1244–1251.
- Ghazal, A.; Shemirani, H.; Amirpour, A.; Kermani-Alghoraishi, M. The effect of intracoronary versus intralesional injection of eptifibatide on myocardial perfusion outcomes during primary percutaneous coronary intervention in acute ST-segment elevation myocardial infarction; a randomized clinical trial study. ARYA Atheroscler. 2019, 15, 67–73.
- Van Tuyl, J.S.; Newsome, A.S.; Hollis, I.B. Perioperative bridging with glycoprotein IIb/IIIa Inhibitors versus cangrelor: Balancing efficacy and safety. Ann. Pharmacother. 2019, 53, 726–737.
- Wu, F.; Ma, K.; Xiang, R.; Han, B.; Chang, J.; Zuo, Z.; Luo, Y.; Mao, M. Efficacy and safety of a bridging strategy that uses intravenous platelet glycoprotein receptor inhibitors for patients undergoing surgery after coronary stent implantation: A meta-analysis. BMC Cardiovasc. Disord. 2022, 22, 125.
- Dargham, B.B.; Baskar, A.; Tejani, I.; Cui, Z.; Chauhan, S.; Sum-Ping, J.; Weideman, R.A.; Banerjee, S. Intravenous antiplatelet therapy bridging in patients undergoing cardiac or non-cardiac surgery following percutaneous coronary intervention. Cardiovasc. Revascularization Med. 2019, 20, 805–811.
- Yun, A.N.; Toyoda, A.Y.; Solomon, E.J.; Roberts, R.J.; Ji, C.S. Safety and Efficacy of Periprocedural Bridging With Cangrelor Versus Eptifibatide. J. Cardiovasc. Pharm. 2022, 79, 383–389.
- Liu, J.; Yang, Y.; Liu, H. Efficacy outcomes and safety measures of intravenous tirofiban or eptifibatide for patients with acute ischemic stroke: A systematic review and meta-analysis of prospective studies. J. Thromb. Thrombolysis 2022, 53, 898–910.
- Rana, A.; Yu, S.; Reid-Herrera, S.; Kamen, S.; Hunter, K.; Shaikh, H.; Jovin, T.; Thon, O.R.; Patel, P.; Siegler, J.E.; et al. Eptifibatide use in ischemic stroke patients undergoing endovascular thrombectomy: A matched cohort analysis. Front. Neurol 2022, 13, 939215.
- Ma, G.; Sun, X.; Cheng, H.; Burgin, W.S.; Luo, W.; Jia, W.; Liu, Y.; He, W.; Geng, X.; Zhu, L.; et al. Combined approach to eptifibatide and thrombectomy in acute ischemic stroke because of large vessel occlusion: A matched-control analysis. Stroke 2022, 53, 1580–1588.
- Osteraas, N.D.; Crowley, R.W.; Panos, N.; Dafer, R.M. Eptifibatide use following emergent carotid stenting in acute anterior circulation ischemic stroke with tandem occlusion. J. Stroke Cerebrovasc. Dis. 2020, 29, 105021.
- Horev, A.; Zlotnik, Y.; Borodetsky, V.; Biederko, R.; Star, M.; Zvenigorodsky, V.; Shelef, I.; Ifergane, G. Adjunctive treatment with low dose intra-arterial eptifibatide and intravenous aspirin during carotid stenting: A case series. J. Clin. Neurosci. 2021, 84, 29–32.
- Jacobi, J. The pathophysiology of sepsis-2021 update: Part 1, immunology and coagulopathy leading to endothelial injury. Am. J. Health-Syst. Pharm. 2022, 79, 329–337.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.5K
Entry Collection:
Peptides for Health Benefits
Revisions:
2 times
(View History)
Update Date:
11 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




