1. Introduction
Plants and their derivatives have been used since ancient times, and have, through empirical knowledge, acquired great importance for humanity. The observations obtained from the behavior of animals that consumed plants created a kind of library in which humans began to relate the effects arising from the use of some plants
[1]. Plants are called medicinal when they play an important role in curing and treating disease. In some parts of the world, these plants symbolize the only way to treat specific pathologies
[2]. Historical references related to medicinal plants date from the first written documents that mention clay tablets, currently preserved in the British Museum, 3000 years before the Christian era. The well-known code of Hammurabi also described
Papaver somniferum L. (opium),
Ferula galbaniflua Boiss and Buhse (galbanum),
Ferula asa-foetida L. (asafetida), and
Hyoscyamus niger L. (henbane)
[3], among many other vegetables that are still used in the treatment of diseases caused by microorganisms, such as flu
[4] and bacterial infections
[5]. Most molecules with healing properties present in plants are secondary metabolites involved in different processes, such as defense against pathogens and pollination
[6]. Among the main substances with pharmacological action found in plants, one can highlight alkaloids, flavonoids, tannins, saponins, and terpenes
[7].
Terpenes are volatile compounds, insoluble in water, and are often involved in the odors released by plants, protection, and defense against abiotic and biotic stresses
[8][9]. Different pharmacological properties are associated with them, such as limonene present in citrus fruits, which has antitumor, antiparasitic, and neuroprotective activity
[10][11]. The diterpene taxol was initially found in the
Taxus brevifolia plant and is widely used in the treatment of various tumors
[12], whereas menthol has analgesic, antifungal, antibacterial, and anti-inflammatory potential
[13]. Alkaloids have a variety of molecules with many biological properties, such as morphine and codeine already used as analgesics; caffeine as stimulating agent; and quinones used to treat malaria
[14][15]. Flavonoids are abundant and diverse and are related to numerous benefits and applications in the treatment of heart disease, inflammatory diseases, diabetes, tumors, neuronal diseases, and even the fight against aging
[16][17]. Tannins are mainly associated with their astringent, antioxidant, and antimicrobial role
[18][19].
The use of medicinal plants is directly related to widespread knowledge, broadly disseminated on empirical grounds and without scientific validation. This practice aims to prevent and treat diseases, use cheaper alternatives, and be less dependent on the traditional health system
[20]. Despite this, such traditional knowledge of each region tends to be passed on over years of use and thus should be considered and investigated further
[21].
There are different approaches to preparing and administering plant material, such as infusions, popularly known as teas, leaf and fruit macerates, or, more commonly, the preparation of plant extracts that can be obtained from any plant organ
[22]. Each preparation methodology is indicated for extracting a specific group of molecules, which is necessary to evaluate their physicochemical parameters, such as the thermostability and solubility of the compounds to be extracted
[22].
The synergy between nanotechnology and biotechnology, and bionanotechnology, emerges as an efficient alternative for various applications in a sustainable approach to the environment
[23]. Nature, through the resources of medicinal plants, helps nanotechnology through biological functions that favor the interaction between the nanostructure and the medium in which it will be dispersed. Bionanotechnology presents itself as a clean and eco-friendly option, capable of solving significant difficulties. From the development of potential antimicrobial agents, with the interaction between the natural dispersive medium and the nanostructure, it operates in the food sector in search of preservatives with zero cytotoxicity and within the environmental sector with recovery techniques and environmental remediation
[24][25][26].
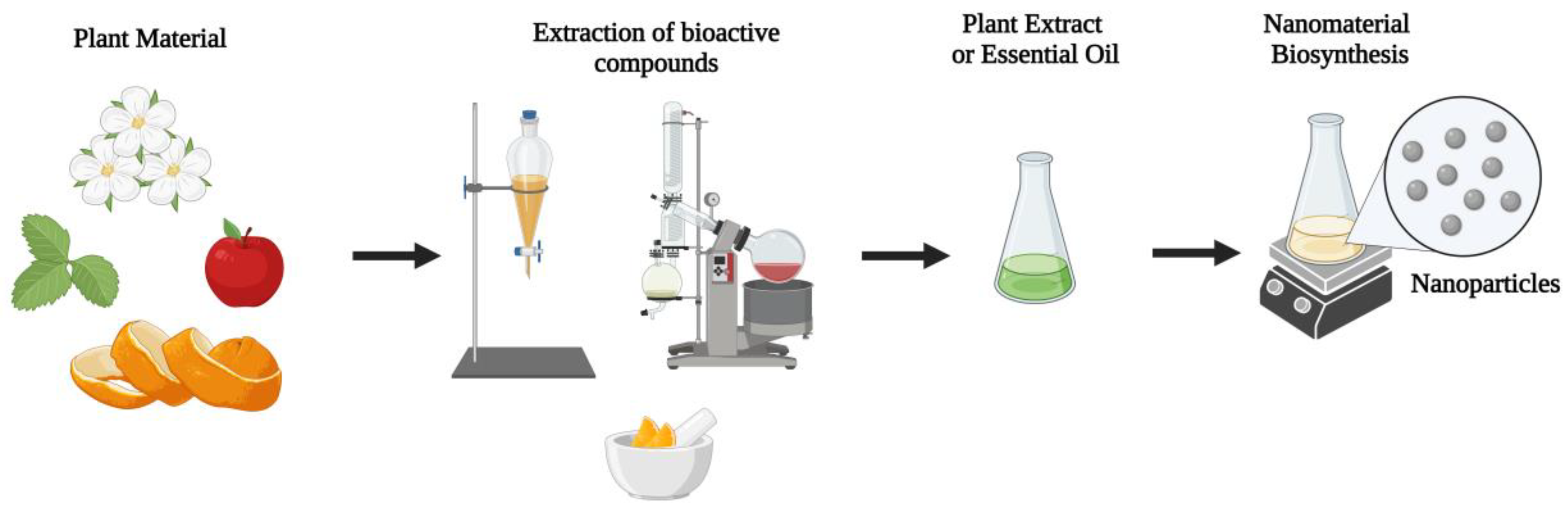
Bionanotechnology efficiently integrates the benefits of plant extracts, such as those obtained by the aforementioned methodologies, with the high power of nanostructures. In this regard, an extract with medicinal power and high antioxidant efficiency promotes a perfect dispersive medium for stabilizing nanoparticles through its bioactivity, which simultaneously acts with its properties, thereby enhancing actions on its targets (
Figure 1). This means that, for example, a plant extract has antimicrobial potential. When nanoparticles with this potential are combined, the synergistic effect between these components enhances the action and minimizes cytotoxicity and disposal impacts. This occurs because the use of precursors is reduced, as what will sustain the nanosystem is the combined actions of the nanostructure with the plant extract that they are associated with
[27].
Figure 1. Various compounds can be extracted from different plant sources and applied to different areas, including the biosynthesis of nanomaterials. Different plant parts, such as flowers, fruits, and leaves, can be used to prepare extracts or oils. Various techniques can be employed, such as maceration and extraction using a rotary evaporator, affecting which composts will be efficiently extracted from the plant material. The plant extracts or essential oils produced can be used to synthesize nanomaterials through different protocols that may or may not involve heating.
2. Plant-Based Antibacterial Green Nanomaterials
Nanotechnology is a multidisciplinary science that allows for the manipulating of matter on an atomic and molecular scale to develop new applications. This science impacts different sectors of society, ranging from agriculture to medicine. Furthermore, nanotechnology is expected to mobilize the economy, with more than 125 billion dollars in 2024, as this market is increasingly growing in various areas
[23]. Among the vastness of products and applications of nanotechnology, nanomaterials have a clinical potential in medicine due to their nanoscale. This causes nanomaterials to present physicochemical characteristics, such as size, morphology, charge, luminescence, and magnetism, in addition to electronic and optical characteristics different from materials with larger scales
[28]. Nanomaterials are a set of atoms bonded together, about 10–10
5 atoms, with at least one dimension from 1 to 100 nm
[29].
The synthesis of the nanomaterial is crucial for its cytotoxicity, physicochemical characteristics, and biological properties. Traditional syntheses of nanomaterials use compounds and solvents that are toxic and harmful to human and animal health, and the environment. The adversities surrounding traditional syntheses with toxic solvents involve problems from production to disposal, since all the toxic input produced will follow a course to the fauna, flora, oceans, and terrestrial layers
[30]. However, the green synthesis of nanomaterials consists of organic compounds, such as plant extracts and solvents of zero or low toxicity, through an eco-friendly approach to the entire life cycle of the nanomaterial
[31][32]. To carry out the synthesis, it is necessary to have a precursor source that can have an inorganic, organic, or metallic composition. Next to the precursor, it is necessary to have organic compounds, such as biomolecules, that are capable of reducing and stabilizing the ionic precursors to atoms. The first stage of green synthesis is called bioreduction. The bioactive (reducing agents) present in plant extracts, such as flavonoids, carotenoids, and polysaccharides, can reduce precursor ions to atoms. This step is fundamental for the biological property and cytotoxicity of the nanomaterial. Ions are more reactive than atoms due to the greater possibility of interaction. After bioreduction, the second step is nucleation. In this step, the atoms are joined together in synergy with the shielding of bioactivity, forming several nanoparticles. These nanoparticles form a nanosystem with bioactivity that stabilizes the synthesis process. During the preparation of the green synthesis, several physical–chemical factors (luminosity, thermal and electrical energy, pH, among others) can influence the stability of the nanoparticles and the properties that the nanomaterials will obtain
[33][34][35][36][37][38][39].
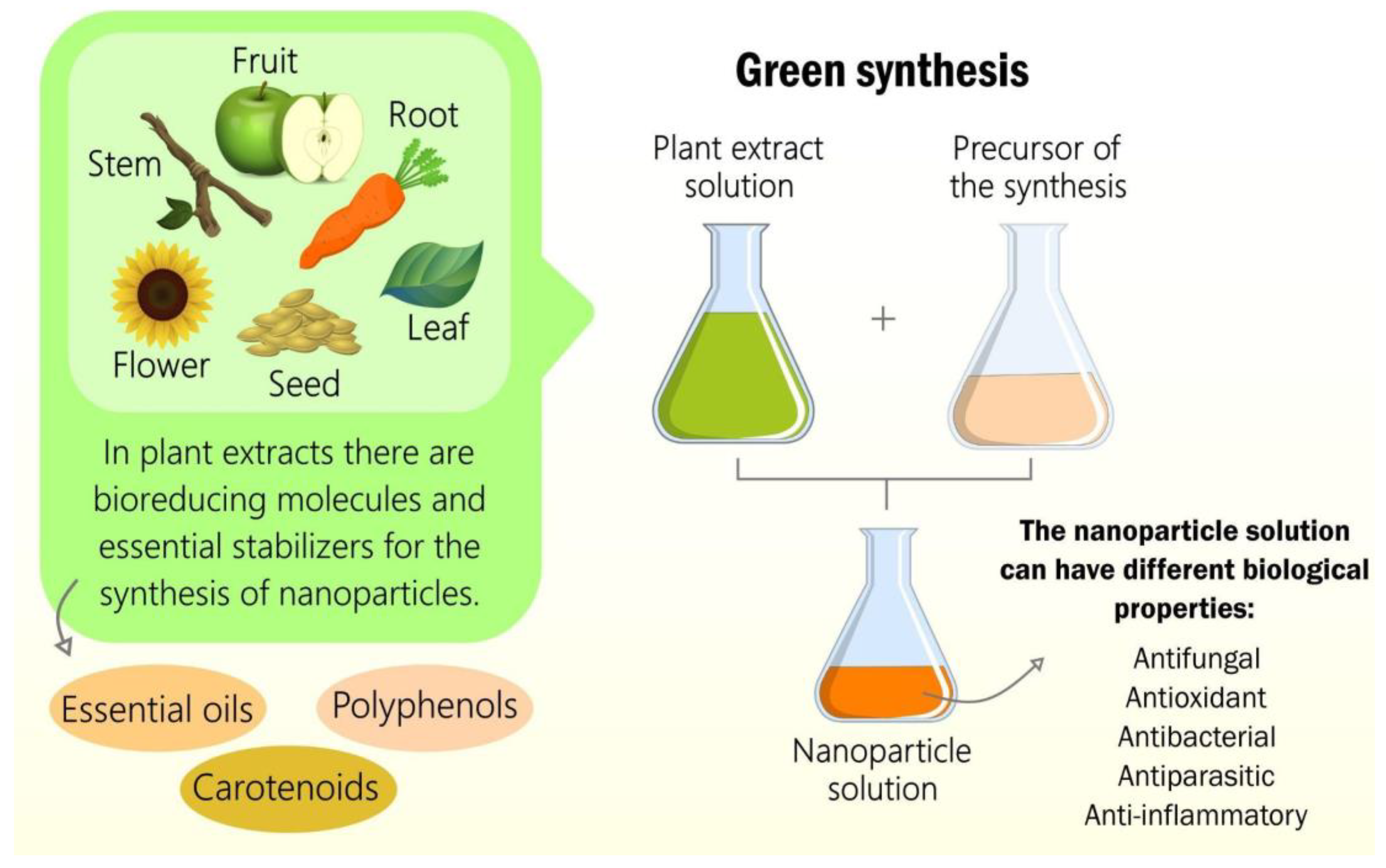
In this sense, natural extracts are responsible for the reduction and stabilization of atoms through their biomolecules to form nanomaterials. These extracts can be of animal origin, for example, bee products, but they can also come from by-products produced by microorganisms and algae
[40]. However, due to the dynamic and economical synthesis, the green synthesis of nanomaterials from plant extracts is used more frequently. These extracts can be made from a plant organ or synergy between several organs, such as leaves, fruits, seeds, roots, stems, and flowers. All these plant organs have different fundamental structures in the green synthesis process, such as essential oils, carotenoids, and phenolics responsible for the nanosystem’s antioxidant potential, as illustrated in
Figure 2 [40].
Figure 2. The green synthesis of nanomaterials from plant extracts with different biological properties.
It is noteworthy that seasonality, agrochemicals (fertilizers and pesticides), and environmental pollution are factors that can affect the plant extracts used to synthesize nanoparticles
[39]. In environmental pollution, such as acid rain, the plant can suffer damage to the leaves that alters wettability, pubescence, and morphophysiological characteristics, such as reducing glandular trichomes
[39][41]. Likewise, seasonal variations and climate changes can alter the size, availability, and development of leaves, flowers, fruits, and roots, among other plant organs. The secondary metabolism of plants is related to phenology, growing conditions, and seasonality. Therefore, the availability of bioactives may vary according to climate, light, temperature, soil nutrients, and agricultural treatments, such as agrochemicals
[42][43][44][45]. Therefore, the characteristics of the plant can be altered by different factors, causing modification, loss, or excess of bioactive elements necessary for the green synthesis of nanoparticles.
Many plants have efficient antimicrobial activities, depending on the bioactive extraction protocol. In this sense, the interaction with plant extracts and nanostructures can be an essential factor in activating potentialities associated with antibacterial agents. Furthermore, using only plant extracts has proven efficiency in the literature
[46][47][48][49][50], but the synergistic effects between plant-based nanostructures by green synthesis have also been proven. As an example, researchers can mention the study of the green synthesis of gold nanoparticles (AuNPs) made from the extract (leaves and fruits) of
Pistacia atlantica. AuNPs with a size between 40 nm and 60 nm and circular morphology showed antibacterial properties in Gram-negative bacteria
Escherichia coli and
Pseudomonas aeruginosa and Gram-positive bacteria
Staphylococcus aureus and
Bacillus subtilis. Notably, these are prevalent bacteria in hospital environments and are associated with cases of multidrug resistance. This nanosystem showed no cytotoxicity in Hela NCBI-C115 cells (Human cervix carcinoma) and obtained an antioxidant potential from assays that demonstrated the scavenging of free radicals such as 1,1-diphenyl-2-picryl-hydrazil (DPPH)
[51][52]. The action of magnesium oxide nanoparticles (MgONPs) made from Saussurea costus root extract for different biological properties was also analyzed. The synthesis showed no cytotoxicity for mammalian cells, indicating that it is possible to synthesize biocompatible MgONPs. The nanoparticles, with cubic morphology and a size between 30 nm and 34 nm, showed antibacterial activity against
Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, and
Staphylococcus aureus, in addition to fungal activity against strains
Candida tropicalis and
Candida glabrata. The same synthesis route exhibited anticancer activity against MCF-7 cancer cells
[53]. In another study, bioactive compounds from the aqueous extract of
Medicago sativa were investigated to synthesize nickel nanoparticles (NiNPs). It was observed that in bioactive compounds such as flavonoids, reducing sugars, proteins, and polysaccharides decreased after the green synthesis of NiNPs. This indicates that these bioactivities may be related to the bioreduction of nickel to NiNPs during green synthesis
[54]. The same plant species has been used in ZnO nanoparticles (ZnONPs) and has been shown to have antimicrobial activity in strains of
Lactococcus lactis, Lactobacillus casei, and
Staphylococcus epidermidis and against the fungus
Candida albicans [55].
Table 1 lists different plant-based nanomaterials by green synthesis. It is possible to highlight many essential and recent studies that act efficiently for Gram-positive and Gram-negative bacteria.
Table 1. Examples of plant-based green synthesis of nanoparticles with antibacterial properties.
| Nanomaterials |
Plant-Based Green Synthesis of Nanoparticles |
Antibacterial Properties |
References |
| AgNPs |
Acacia lignin |
Antibacterial activity against Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis Staphylococcus aureus, Bacillus circulam, and Ralstonia eutropha. |
[56] |
| AgNPs |
Dodonaea viscosa |
Antibacterial activity against Streptococcus pyogene. |
[57] |
| AgNPs |
Euterpe oleracea |
Antibacterial dressings against Staphylococcus aureus and Escherichia coli. |
[58] |
| AgNPs |
Pedalium murex |
Antibacterial activity against Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Micrococcus flavus, Pseudomonas aeruginosa, Klebsiella pheumoniae, and Bacillus pumilus. |
[59] |
| AgNPs |
Beta vulgaris |
Antibacterial textiles against Staphylococcus aureus, Staphylococcus epidermidis, and Escherichia coli. |
[60] |
| AuNPs |
Pistacia atlantica |
Antibacterial activity against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Pseudomonas aeruginosa. |
[52] |
| AuNPs |
Amorphophallus paeoniifolius |
Antibacterial activity against Escherichia coli, Citrobacter freundii, Bacillus subtilis, Pseudomonas aeruginosa, Salmonella typhimurium, and Staphylococcus aureus. |
[61] |
| AuNPs |
Jatropha integerrima Jacq. |
Antibacterial activity against Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and klebsiella pneumoniae. |
[62] |
| AuNPs |
Citrus maxima |
Antibacterial activity against Staphylococcus aureus, and Escherichia coli. |
[63] |
| Al2O3NPs |
Prunus xyedonesis |
Antibacterial activity against Pseudomonas aeruginosa. |
[64] |
| Al2O3NPs |
Cymbopogan citratus |
Antibacterial activity against Pseudomonas aeruginosa. |
[65] |
| MgONPs |
Sargassum wightii |
Antibacterial activity against Streptococcus pneumonia, Escherichia coli, Pseudomonas aeruginosa, and Aeromonas baumannii. |
[66] |
| MgONPs |
Annona squamosa |
Antibacterial activity against Pactobacterium carotovorume. |
[67] |
| MgONPs |
Rhododendron arboreum |
Antibacterial activity against Escherichia coli, Spectrococous mutans, and Proteus vulgaris. |
[68] |
| MgONPs |
Saussurea costus |
Antibacterial activity against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, and Pseudomonas aeruginosa. |
[53] |
| ZnONPs |
Pongamia pinnata |
Antibacterial activity against Pseudomonas aeruginosa. |
[69] |
| ZnONPs |
Ailanthus altissima |
Antibacterial activity against Staphylococcus aureus and Escherichia coli. |
[70] |
| ZnONPs |
Medicago sativa L. |
Antibacterial activity against Staphylococcus epidermidis, Lactococcus lactis, and Lactobacillus casei. |
[55] |
| TiO2NPs |
Psidium guajava |
Antibacterial activity against Staphylococcus aureus and Escherichia coli. |
[71] |
| TiO2NPs |
Mentha arvensis |
Antibacterial activity against Escherichia coli, Proteus vulgaris, and Staphylococcus aureus. |
[72] |
| TiO2NPs |
Trigonella foenum-graecum |
Antibacterial activity against Staphylococcus aureus, Enterococcus faecalis, Klebsiella pneumoniae, Streptococcus faecalis, Pseudomonas aeruginosa, Escherichia coli, Proteus vulgaris, Bacillus subtilis, and Yersinia enterocolitica. |
[73] |
| TiO2NPs |
Azadirachta indica |
Antibacterial activity against Salmonella typhi and Escherichia coli. |
[74] |
| TiO2NPs |
Hypsizygus ulmarius |
Antibacterial activity against Escherichia coli, Staphylococcus aureus, klebsiella pneumoniae, and Bacillus cereus. |
[75] |
| TiO2NPs |
Pristine pomegranate peel extract |
Bacterial disinfection against Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa. |
[76] |
Nanoparticles of various materials have different mechanisms of action through their synergistic or additive properties to natural extracts used as dispersive means and stabilizers of the nanosystem
[78]. For example, NiO nanoparticles ranging in size from 2 to 16 nm were biosynthesized using
Stevia rebaudiana leaf extract and demonstrated antibacterial activity against
Streptococcus mutans [79]. On the other hand, the green synthesis of silver nanoparticles, with a size of 15 to 20 nm with a cubic crystalline structure, from the aqueous extract of
Coptis chinensis rhizome and the modification of the surface of the nanomaterial with a chitosan biopolymer, led this antibacterial nanosystem against
E. coli and
Bacillus subtilis through cell wall damage and generation of reactive oxygen species, causing damage and release of biomolecules and bacterial intracellular structures
[80]. The same effect can be observed in AgNPs biosynthesized by
Ocimum gratissimum leaf extract against
E. coli and
S. aureus bacteria, having the same mechanisms of action
[81]. Copper oxide nanoparticles (CuONPs) also have several green synthesis routes from plant extracts and diverse mechanisms of action, such as oxidative stress, protein, and genetic damage to bacteria
[82]. As a last example, researchers can mention the ZnO nanoparticles synthesized using the extract of
Dysphania ambrosioides, with a size of 5 to 30 nm and an almost spherical shape and hexagonal crystalline structure, and with antibacterial properties similar to the commercial ZnONPs used as a reference in the study. The
Prevotella intermedia bacteria were the most sensitive to ZnONPs, with the most common mechanisms of action being the disruption of the cell wall and vital molecules of the cell, such as enzymes, DNA, and other proteins
[83].
In this context, the synergistic effect between an antimicrobial plant extract and metallic nanoparticles synthesized in this dispersive medium potentiates the bactericidal and bacteriostatic activities of the technologies and studies developed, extending the synergistic effect with antibiotics, including against multi-antibiotic-resistant bacterial strains
[84][85]. In this way, using nanotechnology synthesized from plant extracts presents itself as a potential alternative to the increasing occurrence of nosocomial infections multiresistant to antibiotics and as a key to combating bacterial dissemination through different technological applications made possible in several studies developed over the last few decades. However, it is noteworthy that studying the mechanisms of action from the interaction between nanosystems and bacteria still lacks more details for developing nanotechnologies addressed to mechanisms and actions in specific pathogenic strains.