Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Andrea G. Capodaglio | -- | 6143 | 2023-04-07 10:26:24 | | | |
| 2 | Camila Xu | Meta information modification | 6143 | 2023-04-10 02:53:45 | | | | |
| 3 | Camila Xu | Meta information modification | 6143 | 2023-04-10 08:15:55 | | | | |
| 4 | Camila Xu | + 4 word(s) | 6147 | 2023-04-10 08:48:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Capodaglio, A.G. Biorefinery of Sewage Sludge. Encyclopedia. Available online: https://encyclopedia.pub/entry/42865 (accessed on 07 February 2026).
Capodaglio AG. Biorefinery of Sewage Sludge. Encyclopedia. Available at: https://encyclopedia.pub/entry/42865. Accessed February 07, 2026.
Capodaglio, Andrea G.. "Biorefinery of Sewage Sludge" Encyclopedia, https://encyclopedia.pub/entry/42865 (accessed February 07, 2026).
Capodaglio, A.G. (2023, April 07). Biorefinery of Sewage Sludge. In Encyclopedia. https://encyclopedia.pub/entry/42865
Capodaglio, Andrea G.. "Biorefinery of Sewage Sludge." Encyclopedia. Web. 07 April, 2023.
Copy Citation
The disposal of excess biological sewage sludge from wastewater treatment is a growing environmental issue due to the vast quantities generated worldwide. Due to its composition, sewage sludge could be potentially exploited as a renewable carbon source, rather than being considered an inevitable “nuisance” linked to the main task of wastewater treatment. Biorefinery encompasses any technology used to process excess biological sewage sludge (EBSS) for biofuel and/or resource recovery: proper process integration can contribute to generating multiple possible final products (energy and/or materials).
sewage sludge
biorefinery
energy
biopolymers
1. Introduction
Excess biological sewage sludge (EBSS) and its disposal are growing environmental issues worldwide: production is estimated at more than 13 million t/y (dry solids, DS) in Europe [1], approximately as much in the USA. [2], more than 30 million t/y in China [3], and at over 43 million t/y in India, where a large part is dumped into surface waters or land without treatment [4]. At the urban level, sewage sludge constitutes an abundant organic waste stream with per capita production estimated at 20–25 kg DS/person-y in the EU [5], second only to food waste (production estimated at 1.3 billion t/y) [6]. Due to its composition, sewage sludge could be potentially exploited as a renewable carbon source, rather than being considered an inevitable “nuisance” linked to the main task of wastewater treatment. The need for environmentally safer and more sustainable EBSS disposal routes is becoming acute due to increasing quantities and concerns related to its emerging possible pollutants content, which discourage or rule out traditional practices. EBSS, in fact, often contains pathogenic microorganisms, toxic compounds, including heavy metals, polycyclic aromatic hydrocarbons, dioxins and furans, per- and polyfluoroalkyl substances (PFASs), pharmaceutical compounds, and others [7], which remain embedded in biomass after their incomplete degradation during biological wastewater processes [8].
EBSS processing and disposal have a significant effect on the final energy and economic performance of wastewater treatment plants (WWTP), accounting for over 50% of their related burden, and contributing to almost 40% of their energy consumption and greenhouse gas emissions [9]. Sludge treatment and disposal costs vary depending on adopted methods and local conditions and have been estimated at between 160 and 310 €/t (DS) in EU countries [10]. Disposal in agriculture has represented the most widespread reuse application since early history [11], and is still an option in some countries due to low investment costs and nutrient recycling benefits; however, increasingly stringent regulations related to pollutants transfer into soils are being introduced [12]. An alternative to soil application was traditionally represented by incineration but this route implies loss of energy and materials and potentially negative environmental impacts. Sludge requires some preliminary dewatering/drying and transportation onto fields or combustion facilities, with significant energy inputs in either case [13]. In addition to soil and crop pollutants accumulation, spreading can create environmental nuisances through the propagation of odours, insects, and pests. Incineration, on the other hand, requires stringent flue gas emission control and may find strong opposition from environmental activists; combustion residuals (incinerated sewage sludge ash (ISSA)) concentrate metals contained in the sludge and are generally disposed of in secure landfills at considerable cost, with the risk of inducing further environmental contamination. The prevalent current challenge in WWTP waste management, in addition to the reduction in these impacts, has been identified in the recovery of energy and materials, by implementing EBSS-centred circular economy schemes that could provide greater added value than traditional disposal methods.
According to current legislation (European Directives 91/271/EEC and 86/278/EEC), EBSS, whether treated or untreated, is defined as a residual product, hence as “waste”, classified in the European Waste Catalogue (EWC) with code number 190,805 [14]. The Waste Framework Directive (2008/98/EC) specifically introduces the “end-of-waste” concept, whereby end products are no longer considered waste upon the fulfilment of four conditions: their use for specific purposes; the existence of a market for the product; the existence of technical requirements and standards for products; and the proof of no adverse environmental/health effects induced by their use. Upon fulfilment of these prerequisites, sludge could then become a valuable secondary raw material on its own. Compliance with these conditions can be ascertained on a case-by-case basis for some specific final uses.
In 2021, the European Commission closed its public consultations on the planned Sewage Sludge Directive (SSD): in circulated drafts, it is stated that “the reuse of sewage sludge should contribute to the exploitation of the value in water, unlocking the full potential of opening up markets for reused sewage sludge opportunities and raw materials embedded in it” [15]. This would create a pivotal paradigmatic shift in the status of sewage sludge since it would permanently move from its current “waste status” to the condition of a valuable by-product of the wastewater treatment cycle. This concept is closely related to the new paradigm that is being currently introduced in WWTP planning and design, moving from the traditional concept of ‘treatment’ to the new function of the water resource recovery facility (WRRF). The new definition clearly states the dual purpose of these facilities to a) protect the environment from pollution and, at the same time, b) recover water [16], nutrients [17], and energy [18] to reduce the environmental impact and enhance the sustainability of the urban water cycle.
Several existing or potential options for EBSS-embedded resource recovery have been investigated, ranging from biological process innovation, new thermochemical technologies, the integration of bioelectrochemical processing, biorefinery, and others. Traditional recoverable resources, such as biogas from sludge fermentation [19], are now complemented by liquid and solid energy end products (e.g., biodiesel and biochar) [20], and nutrients [21]. The possibility of recovering other high-value-added products, such as polyhydroxyalkanoates (PHAs), extracellular polymeric substances (EPS), and others, is also intensively investigated [22][23]. All of these process technologies can be grouped under the definition of “biorefinery”.
2. Sewage Sludge Characteristics
Raw sewage sludge could be defined as a multiphase medium containing organic and inorganic particles, microbial biomass aggregates, and extracellular polymeric substances (EPSs), all suspended within a predominant volume of water. Originally dissolved wastewater components, such as organics (i.e., chemical energy) and nutrients, are concentrated in biomass, which, therefore, contains (in terms of dry matter) an equivalent energy comparable to that of lignite, about 11–22 MJ/kg [24], and stoichiometric or superstoichoimetric amounts of P and N. Sewage sludge is qualitatively characterized by three main classes of parameters: physical, relating to its structure and suitability to mechanical processing; chemical, relating to the presence of complex organics, nutrients, and toxic/dangerous compounds; biological, relating to the microbial composition, mainly concerning the presence of pathogens. The composition and type of raw wastewater and the type of biological treatment processes determine the final properties of EBSS; therefore, the variability in sludge characteristics according to location and time (even for the same location) can be quite high.
In addition to the “conventional” parameters reported in the table, more specific chemical characterization is needed for each sludge disposal option according to applicable regulations, which may exist at the supernational (e.g., European Union), national, and local (regional, county, etc.) levels [25][26][27]. Many pollutants that are originally present in wastewater could go undegraded or only partly degraded in WWTPs and may end up entrapped or adsorbed in sludge cells or flocs. These include inorganic compounds (silicates, aluminates, and calcium and magnesium compounds), toxic pollutants, such as heavy metals (Zn, Pb, Cu, Cr, Ni, Cd, Hg, and As) [28], dioxins, pesticides, nanomaterials, microplastics [29][30], and a long list of over 70 emerging pollutants including personal care and pharmaceutical products (PPCP), hormones and endocrine disrupters [31], per- and polyfluoroalkyl substances (PFASs), linear alkylbenzene sulfonate (LAS), alkylphenols (APs), phthalates (PAEs), polycyclic aromatic hydrocarbons (PAHs), and polychlorobiphenyls (PCBs) [32] with concentrations ranging from ˂1 mg to several g per kg [33]. Pathogens and other microbiological pollutants are also present in sludge [34].
Sludge mass consists of approximately 60% (DS) biodegradable, nontoxic organic C compounds; TKN and P fractions follow in lower percentages. Volatile solid content is usually in the range of 65–77 g/L; dry matter may vary between 7–12 g/L. The sludge organic fraction is mainly composed of EPS, negatively charged polymers with high molecular weight originating from microbial cell secretions and lysis. EPS contains most of the sludge proteins, nucleic acids, humic substances, polysaccharides, and lipids; these comprise about 20% of the total EPS mass [35]. EPS forms a complex structure with biological cells, interlocking high amounts of humidity in flocs, and protecting them against dewatering. The presence of EPS manifests itself in the accumulation of large amounts of positively charged ions within flocs. This generates an osmotic gradient that affects the effectiveness of dewatering processes, and the sludge’s rheologic behaviour [36]. In particular, granular sludge (GS) formation (either from aerobic or anaerobic processes, i.e., activated granular sludge, anaerobic sludge blanket, or anammox) is strongly affected by the presence of EPS. EPS is found in much higher amounts in granulated sludge than in any other biomass aggregation (e.g., activated sludge flocs) [37]: depending on the nature of GS granules (aerobic, anaerobic, and anammox) EPS content may vary between approximately 30 and 600 mg/g [38]. Due to its constituents, EPS is one of the most attractive and studied sludge components at the moment, given the high possibility for the sustainable recovery of novel raw materials through its biorefinery processing.
3. Principles of Biorefinery
Biorefinery is not an entirely novel concept of this century: traditional bioconversion processes, i.e., in the sugar, starch, pulp, paper, and vegetable oil industries that have been around for a long time can also be classified as belonging to the biorefinery concept [39]. Nowadays, the common definition of biorefinery refers more specifically to a process or a chain of processes capable of producing a broad spectrum of value-added products, such as biochemicals or biofuels, from a single feedstock or combinations thereof. Hence, biorefinery converts biomass (animal, crop, or waste) to chemicals, energy, and materials, replacing nonrenewable energy or chemical products of fossil origin. Biomass is, by definition, renewable, since plants naturally synthesize organic matter from environmentally available CO2, H2O, and solar energy. Organic waste streams from anthropic activity also contain embedded energy and materials that can be similarly recovered and exploited. The sustainable harvesting and processing of natural and waste biomasses does not negatively affect the environment, and could be carbon neutral in most cases. Therefore, biorefinery plays a key role in ensuring the closure of the cycle of biomass production and consumption, while satisfying society’s need for energy and chemicals.
4. Sewage Sludge Biorefinery
According to the previous definition, biorefinery encompasses any technology used to process EBSS for biofuel and/or resource recovery: proper process integration can contribute to generating multiple possible final products (energy and/or materials), as schematically shown in Figure 1. Process integration could ensure flexibility and operational optimization in view of possible changes in sewage sludge quantities, composition, and characteristics in time. Ideally, treatment processes or operational adjustments could be introduced in WWTPs themselves, in order to improve EBSS characteristics to enhance their recovery options. Since the chemical composition and energy content of sludge vary according to sludge stream and type (e.g., primary, secondary, or mixed sludge, conventional activated sludge, MBR or EBPR sludge, or GS), these streams could be separately processed, aiming at specific final products. For example, as mentioned earlier, GS presents a higher EPS content than CAS, and primary sludge has a higher content of free fatty acids than secondary.
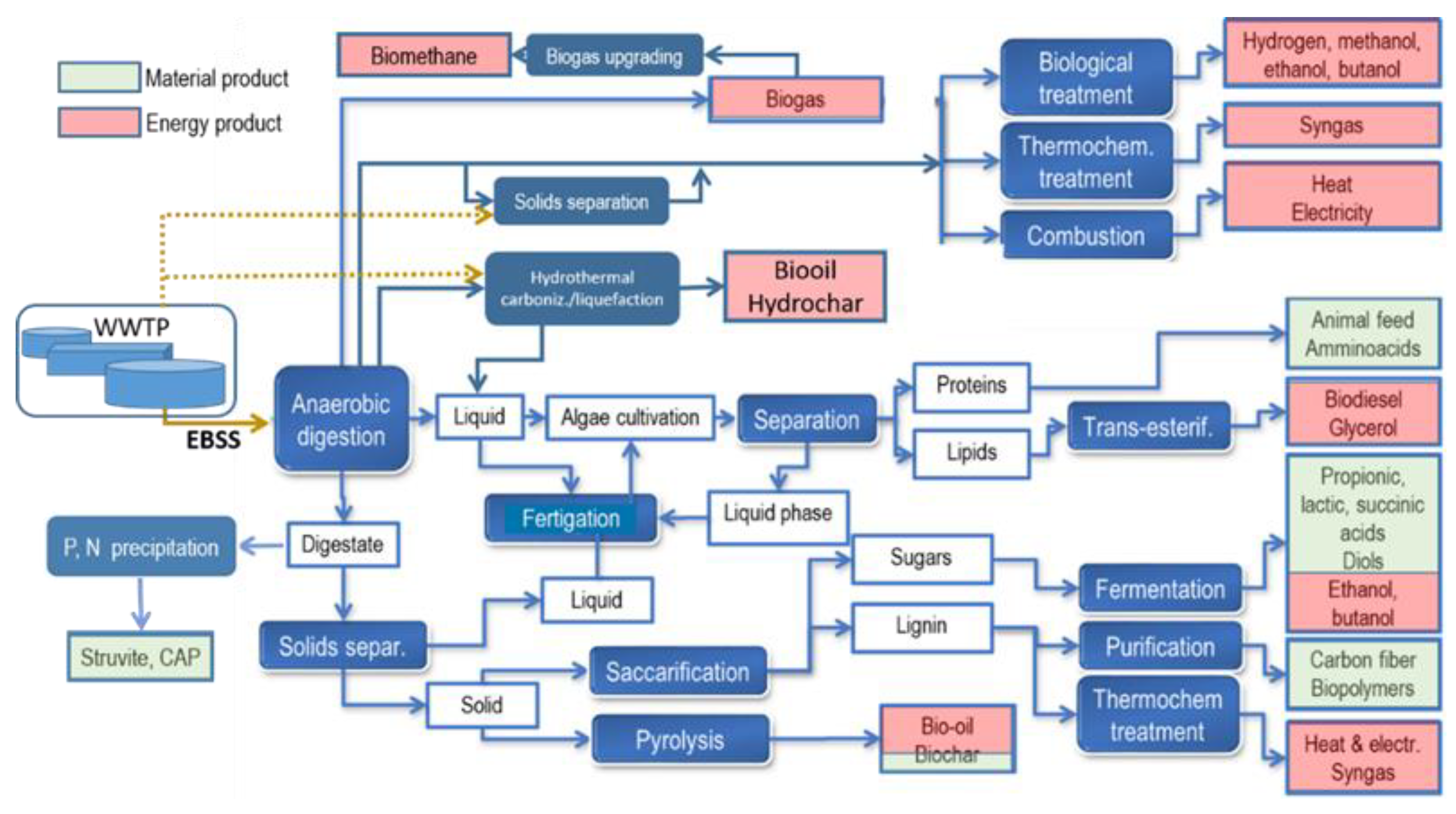
Figure 1. Possible biorefinery full-cycle implementation from EBSS feedstock.
Some of the processes shown in Figure 1 have been traditionally adopted as sludge management practices for many years, and have been amply described in the literature. For example, the anaerobic digestion (AD) of excess sludge was introduced in its earliest form in 1885, at the Exeter (UK) WWTP, and has been constantly improved since. AD for the production of methane-rich biogas is an early example and current staple process combining resource recovery and organic matter stabilization. AD is not limited to feedstocks such as sewage sludge and manure but can include the co-digestion of organic fraction of municipal solid waste (OFMSW), food waste, energy crops, and crop biomasses residuals, as well as agroindustrial and chemical industry wastewaters. While biogas can contribute to the energy requirements of WWTPs, its economic added value is limited compared to that of other higher-value end products that could be alternatively obtained. While the sequence of biorefinery processes can be optimized to exploit the most valuable or desirable end products, AD could still remain an important step within many biorefinery schemes.
Even if thermochemical combustion processes could be considered the final steps of sludge biorefinery, they will not be discussed specifically in this entry. These are traditionally well-known and have been reviewed recently by several authors [40][41][42][43]; pyrolysis and gasification have also been amply reviewed [44][45][46]. This entry of sludge biorefinery techniques focuses on recent approaches aimed at the direct recovery of fermentation products, such as organic acids, alcohols, and hydrogen, or at their postprocessing for the recovery of industrially valuable molecules, such as PHAs and VFAs, or bioproducts.
4.1. Raw Chemicals Recovery from EBSS
Many studies have been published on the physical–chemical characterization of sewage sludge with regard to its final disposal: a Google Scholar search (conducted on 25 August 2022) yielded around 28,000 hits with the keywords “sewage sludge characterization for thermochemical processes” (over 6000 of which were published before the year 2000) and almost 99,000 with the keywords “sewage sludge characterization for land application”. The literature on EBSS characterization for biorefinery purposes is more recent: a search with the keyword “sewage sludge characterization for biorefinery” returned just 90 results prior to the year 2000 and just over 20,000 until the present. This indicates that biorefinery has received a lot of interest in the scientific and industrial communities in the past 20 years.
Thermochemical processes (incineration, pyrolysis, and gasification) can recover heat, oil, gas, or char from EBSS, but they also destroy valuable organic macromolecules, polymers, or enzymes contained therein: three major biochemical families (carbohydrates, proteins, and lipids) represent approximately 80% of the organic matter in sludge, mostly in the form of extracellular polymeric substances (EPS). Biorefinery can address the recovery of those components, following a “pyramidal approach” in which the extraction of the most valuable constituents has the greatest priority.
4.1.1. Central Role of Anaerobic Fermentation in EBSS Exploitation
AD (fermentation) is currently considered one of the most efficient solutions for managing large amounts of organic substrates, including EBSS, food, and agricultural biomass wastes. Most process chains aimed at producing high-value end products from low or negative-value feedstocks are based on this initial step. The process reduces their volume and allows the production of valuable methane (or possibly hydrogen); these renewable fuels (from sludge and other sources) are expected to contribute to the EU renewable energy portfolio in the coming years [47]. The first intermediates of the AD process are short-chain organic acids and alcohols: short-chain fatty acids (SCVFAs) are volatile fatty acids (VFAs and carboxylic acids) with 2–5 C atoms; acetate, propionate, butyrate, and lactate are usually referred to as carboxylates. These industrial raw materials are normally produced from fossil sources but can be generated through the fermentation of organic matter following the inhibition of the conversion step of organic acids to CH4 and CO2 and the minimization of molecular hydrogen production (Figure 2).
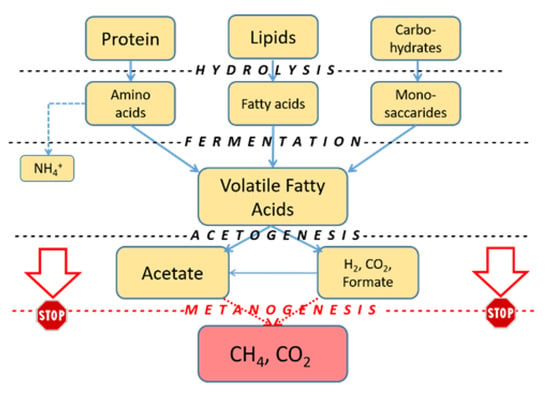
Figure 2. Anaerobic fermentation steps. By suppressing methanogenesis, VFAs and hydrogen could be recovered from the process.
Ample literature on feedstock pretreatment for biogas production enhancement in AD exists; however, there is little specifically concerning VFA production potential. SCVFA production rates from the AD of EBSS are often limited by slow hydrolysis and poor substrate availability. Hydrolysis enhancement can increase production rates: Li et al. [48] observed that SCVFA production increased 3.7 times by 1.8 mg/L of HNO2 sludge treatment prior to AD; Zhang et al. [49] observed that pretreating EBSS with 176.5 mg/L of NH3 increased VFA concentrations to 267.2 mgCOD/gVSS. Liu et al. [50] applied heat–CaO2 thermal hydrolysis pretreatment (67.4 °C and 0.12 g/gVSS) to sludge, increasing the fraction of biodegradable organic matter and achieving maximum VFA concentrations of 336.5 mgCOD/gVSS. The methanation rate is significantly reduced by acid accumulation in the digester [51]; therefore, it is evident that proper fermentation control is essential for successful VFA recovery from AD processes.
Due to their characteristics of high flexibility, low stiffness and brittleness, high tensile strength and toughness, VFAs are highly valuable in the bioplastics and biotextiles production processes; acetate and butyrate are starting blocks for polyhydroxybutyrate (PHB) production, propionate for polyhydroxyvalerate (PHV) production [52]. VFAs, as such, are unsuitable as fuels due to their high O:C ratio (in the range 1:1–2:5) and low energy density; however, they can serve as raw materials for liquid biofuels synthesis: acetate, for example, is a precursor of fuel alcohol that is blended with gasoline [53]. The benefits of AD’s VFA production should be carefully evaluated compared to those from biogas recovery [54]. Apart from the high commercial value of the produced VFAs, these also have reuse potential within the same WWTP as an internal carbon source in biological nutrient removal, instead of external sources such as acetate and methanol [55].
Fermentation within the VFA platform originates a mixture of short-chain carboxylates that, after concentration by nanofiltration membranes, liquid extraction, or anion exchange [56], undergoes secondary bioconversion to yield medium-chain fatty acids (MCFAs, i.e., carboxylic acids with C atoms in the range of 6 to 12). Due to their properties, MCFAs are considered more valuable chemicals than SCVFAs. Compared to SCVFAs, characterized by a high O:C ratio, MCFAs (with lower O:C ratios, between 1:3–1:6) feature higher energy densities (approximately 3500–4800 KJ/mol) and serve as biofuel precursors or raw materials for various commercial products, such as fragrances, food additives, antimicrobial agents, and others [57].
Formate (COOH−) can be consumed as the sole carbon source by formatotrophic microbes: it can be thus converted into an ample array of final products, including fuels, solvents, plastic monomers, pigments, and even protein meal for food or animal feed, creating the basis of an entire formate-centred Circular Bioeconomy [58]. Due to their value, the optimization of acetic and formic acid production is a research hotspot in the AD field: formate generation via VFA degradation by acetogenic microbes is limited by thermodynamics, while it is easier for acidogens to directly metabolize organic matter into these forms. Factors such as pH, ORP, and initial C/N ratio are known to affect the production of acetic and formic acids. Optimal conditions occur at pH 7.0 and low ORP (−450 mV): Wang et al. [59] indicated that neutral pH could provide a suitable niche for Petrimonas, Trichococcus, and other diverse acetic/formic acid-producing bacteria.
Biogas, the most common final AD product, is a valuable energy product, consisting of approximately 40–75% CH4 and 25–60% CO2, plus other minor components (H2S, NH3, siloxanes, etc.) depending on the feedstock. Biogas is suitable for upgrade strategies (biomethanation) to generate a fuel product compatible with natural gas standards [47]; however, biogas bioconversion into biopolymers such as polyhydroxyalkanoates (PHA), protein, or ectoine has emerged as a promising alternative approach [60]. Biogas was tested for the industrial production of polyhydroxybutyrate (PHB), a specific short-chain, biodegradable, and biocompatible PHA thermoplastic synthesized by methanotrophs (methane oxidising bacteria (MOBs)) from C1 carbon sources (CO2 and CH4), under growth limiting conditions [61].
The major constraints associated with CH4/biogas bioconversion technologies are represented by the limited mass transfer rates of O2 and CH4 (1.3 × 10−3 and 1.4 × 10−3 M/atm, respectively) [62]. This requires the development of highly efficient turbulent contactors to support enhanced methane transformation even in the presence of unfavourable values of Henry’s law coefficients [63]. For this reason, notwithstanding this potential, long-term continuous operation of biogas-to-PHB production processes have not been yet reported [64].
4.1.2. Biopolymers
Polyhydroxyalkanoates are biodegradable polymers internally accumulated by microorganisms as energy and carbon storage reserves, generally under nutrient imbalance conditions during biological wastewater treatment. PHAs made up of monomeric units of (R)-3-hydroxyalkanoic acids accumulate as granules inside microbial cells in variable proportions, from 1 to 90% of cell dry weight. More than 150 monomeric units of PHA have been identified so far: poly-beta-hydroxybutyric acid and its co-polymer poly (3-hydroxybutyrate-co-hydroxyvalerate [P(3HB-co-HV)]) are among the most common. These molecules exhibit properties comparable to those of petroleum-based plastics, hence their use as a biodegradable and biocompatible conventional plastic substitute could reduce environmental and human health problems related to nondegradable petrochemically derived materials. PHAs may also serve as feedstock for biofuel (3- hydroxybutyrate methyl ester (HAME)) production, or for the production of specific chemicals such as acrylate and propene [65][66].
PHA has been extensively studied and characterized in terms of properties and possible industrial applications; however, their cost is still higher (up to 6 fold) than fossil-derived plastics [67], since industrial processes for their production are mostly based on pure microbial cultures and expensive substrates such as glucose, fructose, and propionic acid, hindering their commercial appeal so far. More cost-effective production technologies, using wastes and/or industrial by-product streams as raw materials for PHA production could decrease overall production costs by up to 50% [68]. Low-value substrates, such as EBSS, and the use of microbial mixed cultures could also allow energy (no sterilization required) and fermentation (less expensive reactor construction) cost reduction and control equipment minimization, with the possibility of efficiently integrating this technology in traditional WWTPs [69]. Preferred substrates for PHA synthesis are butyrate, lactate, and, to a lesser extent, acetate—all major products of primary fermentation within the VFA platform [70].
As pointed out by Valentino et al. [71], PHA extraction could also be advantageous for final sludge disposal optimization: separately fermented primary sludge could be minimized by the conversion of organics into VFAs, which would favour subsequent internal PHA accumulation in EBSS since PHA-storing bacteria feed on VFAs from the liquid phase and accumulate them intracellularly as biopolymers. In enhanced biological phosphorus removal (EBPR) systems, for example, substrate VFA production is encouraged, so that phosphate-accumulating organisms (PAOs) can synthesise and intracellularly accumulate PHAs under anaerobic conditions, allowing them to perform alternate P accumulation and release. Glycogen-accumulating organisms (GAOs) are a second group of bacteria responsible for PHA accumulation under anaerobic conditions. Microbiological PHA production, therefore, implies favouring the selective growth of PHA-storing bacteria (PAOs or GAOs) by exerting dynamic substrate feeding conditions (“feast and famine” cycles, i.e., alternating conditions of C availability and unavailability) [72][73]. During the “feast” phase, ammonia and carbon are assimilated by microorganisms, which synthesise PHA. After substrate exhaustion (“famine”), stored PHAs are consumed along with external ammonia. Process optimization leads to the development of mixed bacterial cultures with significant PHA storage capacity, up to 65% of cell content [74]; however, insufficient process stability was reported [75]. PHA accumulation has been observed in the range of 0.30 to 22.7 mg polymer/g sludge, depending on process conditions [76][77]. While many approaches based on mixed-culture processes have been proposed, none have been implemented at the industrial scale, so far. The most successful approaches for PHA extraction from biomass so far are based on alkaline fermentation [6][78].
In contrast to intracellular PHA needing a specific extraction process, high-value EPS macromolecular cellular components are released by cell lysis or excreted across cell membranes into solution, and typically remain trapped in the intracellular spaces of microbial aggregates. Unless recovered, EPS are discarded with the effluent or returned to the biological units after sludge processing. EPS recovery could further shift treatment processes to zero-waste discharge, minimising the final organic load output of WWTPs by recovering materials in production processes.
Granular sludge-derived EPS demonstrated hydrating properties and gel-forming capacities similar to alginates; therefore, several biotechnical uses of EPS exist in the food, paints, oil drilling ‘muds’, cosmetics, and pharmaceutical industries. EPS may have potential uses as biosurfactants in tertiary oil production, biological glues, and as a component of biofilm systems; due to its viscous properties, it is used to increase the viscosity of technical materials and food preparations, with another still unexplored biotechnological potential, including medical applications [79]. EPS coatings improve the self-extinguishing properties of fabrics due to their content of phosphates which improves flame retardation [80]. Pollutant adsorption by EPS has been studied. The presence of functional groups such as carboxyl, phosphoric, sulfhydryl, phenolic, and hydroxyl groups in EPS can complex heavy metals, hence EPSs have been proposed for metals removals: for example, EPS from Bacillus megaterium was tested for Cu removal, while other bacterial EPS types exhibited high affinity for Cd [81][82]. EPS also adsorbs organic pollutants: Spath et al. [83] reported adsorption of solute BTX greater than 60%; the adsorptions of phenanthrene [84], humic acids [85], and dyes [86] were also reported. In fact, only a small fraction of pollutants in biological mixed liquor is actually adsorbed within bacterial cells themselves: since EPS is always negatively charged, it binds with positively charged organic pollutants by strong electrostatic interaction. Furthermore, soluble EPS contains a higher protein fraction than bound EPS; since proteins have higher binding capacities than humic substances, soluble EPS captures most of the pollutants [85].
EPS is involved in most biofouling occurrences in WWTPs, allowing the formation and persistence of microbial aggregates on exposed surfaces such as heat exchangers, piping, and membrane media. Biological sludge EPS accounts for 10–40% of sludge dry weight, with 75–89% of its extracellular organic carbon consisting of proteins and saccharides; hence, it could serve as a C or energy source in conditions of substrate shortage [87]. The quantity of EPS generated varies according to the biological process: it was estimated that a UASB facility of 100,000 P.E. capacity could yield 150 kg-EPS/d [88], while an anammox unit of the same capacity could generate up to 185 kg-EPS/d [89].
Techniques for EPS extraction include physical, chemical, and biological approaches: physical treatments include centrifugation [50], sonication [90], electroporation [91][92], blending [93], and heating [94]. Chemical extraction involves the use of cation exchange resins [95], or of alkaline, acid, or aldehydic reagents and organic solvents (ethylenediamine tetraacetic acid and formaldehyde plus NaOH) [50][96][97]; finally, the biological method includes enzymatic treatment [98]. Bound and soluble EPS fractions can be separated from sludge cells by centrifugation: the former remains attached to solids while the latter is recovered with the centrate. Physical (ultrasonication and heating) and chemical methods have proven effective in recovering bound EPS from most types of sludge. EPS final composition largely depends on adopted extraction methods, which can result in significant yield and quality (properties and functional groups) variations in the extracted exopolymers, affecting their exploitation. However, common extraction and recovery methods have rarely been evaluated in detail so far in terms of the suitability of recovered EPS for specific practical applications. Discussion is still ongoing between researchers recommending cell lysis minimization techniques, and those advocating heavy cell integrity destruction for the extraction of different, novel biopolymers [94][96]. These aspects need further research to optimize EPS recovery and reuse.
4.1.3. Proteins
Proteins are essential components of animal feed, and their recovery from EBSS could offer various benefits over traditional sources: the high fraction of proteins (∼50% dry weight) in sludge makes it a highly suitable source for their recovery; therefore, this is a well-studied waste-to-resource approach. Currently, two patented technologies for protein recovery from sludge are applied in practice [99][100]. Recovered sludge-derived proteins find industrial uses as fertilizers, adhesives [101], fire-extinguishing foam [102], or animal feedstuff [103]. Efficient protein recovery and reuse methods depend on sludge type, nature of pretreatment, and separation and purification methods since all of these factors can modify protein properties (e.g., molecular weight, conformation, type, etc.) [104].
The dissolution of bacterial cell walls and release of bound EPS, and the disruption of intracellular materials (cytoplasm) are critical prerequisites in order to release soluble proteins into the bulk liquid. Physical, thermal, chemical, and biological processes and combinations thereof have been proposed: these include ultrasonication, microwaves, electric pulse, deflaking, and thermal decomposition. Oxidative cell destruction by hydroxyl radical generation has been reported as effective for protein solubilisation, but high oxidant dosages may lead to their undesired mineralisation. Ozonation was indicated as an effective and environmentally-friendly chemical technique for protein solubilisation [104]. Biological pretreatment by the addition of enzymes, or specific enzyme-producing microbes, is considered attractive compared to chemical or physical methods for the solubilisation of sludge proteins since it is considered more environmental-friendly, does not introduce external chemicals in the process, and does not require special equipment. Studies, however, reported that enzyme (e.g., protease and lipase) addition could cause protein degradation into polypeptides and amino acids [105].
Intracellular protein recovery was achieved by sludge disintegration using alkali treatment coupled with ultra-sonication [103] or combined enzymatic methods consisting of disintegration by enzyme, acid, and base hydrolyzation processes followed by isoelectric precipitation, centrifugation, and freeze-drying [106]. Protein extraction yields up to 80%, with a composition comparable to commercial protein feeds, have been achieved, with most of the metals initially present in the sludge being removed from the final products. A coupling of chemical and biological methods was shown to increase the solute concentration of protein: the former (with a dosage of ethylenediamine tetraacetic acid (EDTA), SDS, formaldehyde, and NaOH) aids the release of trapped EPS and the disintegration of cell walls; the latter degrades released organics enhancing protein accumulation [107].
A proper comparison of all possible methods available for protein recovery in view of their circular economy application is virtually impossible due to limited information available on costs, sludge properties, and suitability of the recovered materials for specific applications. For example, protein recovered with alkaline pretreatment may not be suitable for applications in the food industry; pathogens and micropollutant contamination should be investigated with respect to possible final uses. In general, physical methods are more easily controllable; however, they may require considerable energy input, e.g., a thermal method will effectively remove odours and pathogens, but usually demand higher energy and capital costs. Chemical methods are effective in the disruption sludge flocs and are easy to operate, but the added chemical costs and possible corrosion issues due to acid or alkaline reagents should be evaluated. Protein loss by mineralization is a possible risk if aggressive oxidants are used. Biological methods, despite their low adverse impacts and effectiveness, are typically limited by slow start-up and process scale-up issues [107]. Since the destruction of microbial cells by any means induces increased dewaterability, sludge processed for protein recovery will result in reduced ultimate disposal needs.
4.1.4. Added-Value Products from Biological Processing of EBSS
In addition to the recoverable biotechnological products just discussed, others such as biopesticides, bioherbicides, and enzymes can be recovered from the EBSS biorefinery. Bacillus thuringiensis (BT) is considered the most effective biopesticide in use in agriculture, forestry, and the health sector at present, with specific action against organisms of the orders Lepidoptera (moths), Diptera (flies and mosquitoes), and Coleoptera (beetles). BT forms crystals of proteinaceous δ-endotoxin insecticidal that, due to its target specificity and little or no harmful effects on wildlife, most pollinators, beneficial insects, and humans, is regarded as environmentally friendly. The conventional fermentation medium for BT production involves a significant portion (40–60%) of its total industrial costs; recently, however, waste sewage sludge was found to be a highly nutritional and cost-effective medium for BT [108] and other biopesticides growth, such as endo- and enterotoxins, vegetative insecticidal proteins, hemolysins, enterotoxins, chitinases, proteases, phospholipases, etc. [109]. It has been estimated that the use of EBSS as a growing medium could reduce the industrial cost of BT by about 50% [110].
Enzymes (e.g., lipases, dehydrogenase, glycosidase, peroxidase, and aminopeptidases) are a class of proteins with biological catalyst properties, used in the pharmaceutical, food, and fine chemicals industries to increase chemical reaction rates and, thus, production yield. As such, they contribute to the synthesis of high-value-added products, without undergoing any biochemical transformation. Microorganisms are an important source of enzymes and, due to their fast growth, they produce them more quickly and efficiently than plants or animals; furthermore, since they can secrete abundant extracellular supplies directly into a fermentation broth, they simplify their downstream processing compared to those obtained from higher living organisms.
A large number of nontoxic and nonpathogenic microbes (bacteria, fungi, yeast, and actinomycetes) are known to produce enzymes considered appropriate for industrial applications. Conventional enzyme production occurs on synthetic substrates derived from soybean, fishmeal, glucose, yeast extracts, peptone and other trace elements, which can ultimately account for up to 40% of their industrial production cost. EBSS is an inexpensive and nutrient-rich substrate that could replace conventional substrates, improving the process cost/benefit ratio. Alkaline protease enzyme production via the submerged fermentation or solid-state fermentation of wastewater sludge substrate by Bacillus licheniformis was reported [111]. Additional literature confirms EBSS as a substrate from which different enzymes can be obtained, including protease, dehydrogenase, catalase, peroxidase, o-diphenol oxidase, esterase, α-amylase, glycosidases, and α-glucosidase [112][113].
These enzymes have application potential in detergents, leather processing, silver mining, medical, food processing, animal feeds, and various other chemical industries, as well as in waste processing [114]. Sludge-derived enzymes can promote diethylene glycol terephthalate (DTP) and polyethene terephthalate (PET) plastic fibre biodegradation. Naturally produced sludge-derived enzymes have been shown to enhance WWTP operations by improving solids degradation, the hydrolysis of organic matter, and the biodegradation of toxic pollutants [115]. Enzymes are attached to cell surfaces or embedded in EPS or cell structures; their release is improved by disrupting sludge cells [116][117][118], or by extraction from the solution with cation exchange resins, nonionic detergents, or EDTA [113][119].
4.1.5. Added-Value Products from the Chemical Processing of EBSS
Protein and lipids contained in sewage sludge can also yield bio-oils and chemical added-value products. Thermochemical processes, such as pyrolysis [120][121][122][123], gasification [124], hydrothermal carbonization [125], and hydrothermal liquefaction [126] have been tested at the laboratory and pilot scale for the production of bio-oils. Biodiesel, a cleaner mixture of fatty acid methyl esters (FAMEs) compared to bio-oil, can be industrially obtained by methanol transesterification of vegetable oils or animal fats in what is called a “first-generation” biofuel [127]. However, the potential of first-generation biodiesel is hindered by the high cost of the feedstocks, and by the ensuing competition between food and energy, which raises ethical concerns. Nevertheless, biodiesel is among the most promising biofuels that could be recovered from urban waste streams; EBSS, as widely available and nonedible lipid-rich feedstock, could, therefore, represent a viable alternative for its sustainable production. However, bio-oil derived from the sludge protein fraction is high in N and S content, precluding its direct use as fuel; since lipids are considered the major precursor of “good” biodiesel, separation of the protein fraction before pyrolysis can be performed to lower undesired contents in thermally-recovered bio-oil. Biodiesel can then be obtained by the direct esterification/transesterification (i.e., the process of exchanging the organic group of an ester with that of an alcohol) of lipids, with or without the addition of acid or basic catalysts, or enzymes (lipases). Since primary sludge is mainly constituted by soaps and free fatty acids (FFAs) [128], its FAME yield is much higher than from secondary sludge [126], mainly constituted by mono-, di- and triglycerides, phospholipids, and waxes.
Chemical solvent (chloroform, toluene, or methanol/chloroform/water) extraction methods are effective for lipid selective extraction from raw sewage sludge, producing low N and S content feedstocks. Since solvents are relatively volatile, they can be recovered with relative ease after lipid extraction [129]. Liquid–liquid extraction techniques contemplate the use of an organic phase (usually hexane, either pure or with added methanol or acetone) to extract lipids from sludge flocs. Acetone seems to help in the disruption of cell membranes. Liquid/liquid extraction is usually less efficient than with solvent since it is negatively affected by water presence in sludge, which also hinders the subsequent esterification/transesterification reactions. The presence of water residuals is one of the main challenges in the full-scale production of biodiesel from sludge; therefore, dewatering pretreatment is required [130]. Lipids can be extracted from dried sludge, using a hexane/dried sludge ratio of about 10:1, and biodiesel production can then be carried out by acid catalysis [131]. The residual biomass can be returned to the WWTP’s anaerobic digestion reactor for additional biogas production [132].
The limit of organic solvents, such as hexane, is their possible release into the environment during lipid extraction; such release could produce ozone or other photochemical oxidants, or generate process residuals that may contain mineral acids. Furthermore, in the case of residual biomass recirculation to AD, the presence of solvent traces could reduce biogas production capacity by up to tenfold, possibly modifying the process’s economic balance, and worsening its environmental impact [133]. The use of more environmentally-friendly solvents, such as ionic liquids and bio-derived ethyl esters of volatile fatty acids was studied: ethylbutyrate, a green, bio-derived solvent, demonstrated the recovery of more than 90% of saponifiable lipids in primary sewage sludge without the addition of acids, and with greater effectiveness than hexane [128]. Other organic-based, green solvents and ionic liquids (e.g., deep eutectic solvents) or CO2 (typically used in supercritical conditions) were also indicated as potential substitutes for fossil solvents in lipid extraction. Their excessively high costs and the lack of tested protocols and extracted product certification still constitute significant barriers to the industrial adoption of these substances as fossil solvent substitutes [134]. Other extraction alternatives that have been proposed include sludge sonication and mechanical disintegration, but with yet inconclusive results [135].
Innovative paradigms in wastewater treatment strategies with full implementation of the WRRF concept could favour the use of sewage sludge as feedstock for biodiesel production: new process technologies based on granular sludge can promote the increase in lipid fraction in sludge; in addition, the residual biomass after lipid extraction still contains organic compounds, consisting of about 30% proteins, which can, in turn, be exploited further. Hence, biodiesel recovery by biorefinery can contribute to the improvement of sludge management solutions by embracing both circular economy principles and EU policies concerning sustainable mobility [136].
References
- Sludgetreat. Eco-Friendly and Energy Efficient Sewage SLUDGE dewaTeRing through Novel Nanomaterials and Electro-Osmotic Process. Project Co-Funded by the European Commission within the FP7 (2007–2013) Marie Curie Actions—Industry-Academia Partnerships and Pathways (IAPP). 2014. Available online: https://sludgetreat.eu/wp-content/uploads/2016/11/D2.2-Preliminary-market-analysis-review1.pdf (accessed on 11 November 2022).
- University of Michigan. U.S. Wastewater Treatment—Factsheet Water. Center for Sustainable Systems. 2021. Available online: https://css.umich.edu/sites/default/files/Wastewater%20Treatment_CSS04-14_e2021.pdf (accessed on 11 November 2022).
- Wei, L.; Zhu, F.; Lia, Q.; Xue, C.; Xia, X.; Yu, H.; Zhao, Q.; Jiang, J.; Bai, B. Development, current state and future trends of sludge management in China: Based on exploratory data and CO2-equivaient emissions analysis. Environ. Int. 2020, 144, 106093.
- Dubey, M.; Mohapatra, S.; Tyagi, V.K.; Suthar, S.; Kazmi, A.A. Occurrence, fate, and persistence of emerging micropollutants in sewage sludge treatment. Environ. Pollut. 2021, 273, 116515.
- Sivaramakrishnan, S. 120,000 tonnes of Faecal Sludge: Why India Needs a Market for Human Waste. World Econ. Forum. 2019. Available online: https://www.weforum.org/agenda/2019/09/how-to-improve-sanitation-in-india/#:~:text=India’s%20urban%20areas%20produce%20120%2C000,connected%20to%20the%20sewer%20system (accessed on 9 December 2022).
- Battista, F.; Frison NPavan, P.; Cavinato, C.; Gottardo, M.; Fatone, F.; Eusebi, A.L.; Majone, M.; Zeppilli, M.; Valentino, F.; Fino, D.; et al. Food wastes and sewage sludge as feedstock for an urban biorefinery producing biofuels and added-value bioproducts. J. Chem. Technol. Biotechnol. 2020, 95, 328–338.
- Trojanowicz, M.; Bojanowska-Czajka, A.; Capodaglio, A.G. Can radiation chemistry supply a highly efficient AO(R)P process for organics removal from drinking and waste water? A review. Environ. Sci. Pollut. Res. 2017, 24, 20187–20208.
- EurEau. Waste Water Treatment—Sludge Management—Briefing Note 2021. Available online: https://www.eureau.org/resources/briefing-notes/5629-briefing-note-on-sludge-management/file (accessed on 11 December 2022).
- Capodaglio, A.G.; Olsson, G. Energy issues in sustainable urban wastewater management: Use, demand reduction and recovery in the urban water cycle. Sustainability 2020, 12, 266.
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorata, A.; Brattebob, H.; Almåsc, Å.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46.
- Angelakis, A.N.; Capodaglio, A.G.; Dialynas, E.G. Wastewater Management: From Ancient Greece to Modern Times and Future. Water 2023, 15, 43.
- Hudcová, H.; Vymazal, J.; Rozkošný, M. Present restrictions of sewage sludge application in agriculture within the European Union. Soil Water Res. 2019, 14, 104–120.
- Boguniewicz-Zablocka, J.; Klosok-Bazan, I.; Capodaglio, A.G. Sustainable management of biological solids in small treatment plants: Overview of strategies and reuse options for a solar drying facility in Poland. Environ. Sci. Pollut. Res. 2021, 28, 24680–24693.
- Eurostat. Guidance on Classification of Waste according to EWC-Stat Categories. Supplement to the Manual for the Implementation of the Regulation (EC) No 2150/2002 on Waste Statistic; Commission of The European Communities, Directorate E: Sectoral and regional statistics; Eurostat: Brussels, Belgium, 2010.
- Water Europe. Public Consultation on the “Sewage Sludge Use in Farming” Directive is Now Open. 2020. Available online: https://watereurope.eu/public-consultation-of-sewage-sludge-use-in-farming-directive-is-now-open/ (accessed on 27 October 2022).
- Capodaglio, A.G. Fit-for-purpose urban wastewater reuse: Analysis of issues and available technologies for sustainable multiple barrier approaches. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1619–1666.
- Daneshgar, S.; Buttafava, A.; Callegari, A.; Capodaglio, A.G. Economic and energetic assessment of different phosphorus recovery options from aerobic sludge. J. Clean. Prod. 2019, 223, 729–738.
- Cecconet, D.; Raček, J.; Callegari, A.; Hlavínek, P. Energy Recovery from Wastewater: A Study on Heating and Cooling of a Multipurpose Building with Sewage-Reclaimed Heat Energy. Sustainability 2020, 12, 116.
- Appels, L.; Baeyens, J.; Degreve, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. 2008, 34, 755–781.
- Callegari, A.; Hlavinek, P.; Capodaglio, A.G. Production of energy (biodiesel) and recovery of materials (biochar) from pyrolysis of urban waste sludge. Rev. Ambiente Água 2018, 13, e2128.
- Daneshgar, S.; Vanrolleghem, P.A.; Vaneeckhaute, C.; Buttafava, A.; Capodaglio, A.G. Optimization of P compounds recovery from aerobic sludge by chemical modeling and response surface methodology combination. Sci. Total Environ. 2019, 668, 668–677.
- Crutchik, D.; Franchi, O.; Caminos, L.; Jeison, D.; Belmonte, M.; Pedrouso, A.; Val del Rio, A.; Mosquera-Corral, A.; Campos, J.L. Polyhydroxyalkanoates (PHAs) Production: A Feasible Economic Option for the Treatment of Sewage Sludge in Municipal Wastewater Treatment Plants? Water 2020, 12, 1118.
- Li, J.; Hao, X.D.; Gan, W.; van Loosdrecht, M.C.M.; Wu, Y.Y. Recovery of extracellular biopolymers from conventional activated sludge: Potential, characteristics and limitation. Water Res. 2021, 205, 117706.
- Shizas, I.; Bagley, D.M. Experimental determination of energy content of unknown organics in municipal wastewater streams. J. Energy Eng. 2004, 130, 45–53.
- Spinosa, L. Standardized characterization procedures: A necessary support to regulations. Water Sci. Technol. 2016, 74, 220–228.
- National Biosolids Partnership. National Manual of Good Practice for Biosolids; Water Environment Research Foundation: Alexandria, VA, USA, 2011.
- EC, Sewage Sludge. European Commission Directorate-General for Environment. 2022. Available online: https://environment.ec.europa.eu/topics/waste-and-recycling/sewage-sludge_en#publications (accessed on 7 December 2022).
- Li, J.; Luo, G.; Xu, J. Fate and Ecological Risk Assessment of Nutrients and Metals in Sewage Sludge from Ten Wastewater Treatment Plants in Wuxi City, China. Bull. Environ. Contam. Toxicol. 2019, 102, 259–267.
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwang, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420.
- Hennebert, P.; Anderson, A.; Merdy, P. Mineral Nanoparticles in Waste: Potential Sources, Occurrence in Some Engineered Nanomaterials Leachates, Municipal Sewage Sludges and Municipal Landfill Sludges. J. Biotechnol. Biomater. 2017, 7, 261.
- Fijalkowski, K. Emerging contaminants in sludge (endocrine disruptors, pesticides, and pharmaceutical residues, including illicit drugs/controlled substances, etc.). In Industrial and Municipal Sludge Emerging Concerns and Scope for Resource Recovery; Butterworth: Oxford, UK, 2019; pp. 455–473.
- Rosinska, A. Traditional contaminants in sludge. In Industrial and Municipal Sludge Emerging Concerns and Scope for Resource Recovery; Butterworth: Oxford, UK, 2019; pp. 426–453.
- Mailler, R.; Gasperi, J.; Patureau, D.; Vulliet, E.; Delgenes, N.; Danel NDeshayesa, S.; Eudes, V.; Guerine, S.; Moilleron, R.; Chebbo, G.; et al. Fate of emerging and priority micropollutants during the sewage sludge treatment: Case study of Paris conurbation. Part 1: Contamination of the different types of sewage sludge. Waste Manag. 2017, 59, 379–393.
- Huang, K.; Mao, Y.; Zhao, F.; Zhang, X.-X.; Ju, F.; Ye, L.; Wang, Y.; Li, B.; Ren, H.; Zhang, T. Free-living bacteria and potential bacterial pathogens in sewage treatment plants. Appl. Microbiol. Biotechnol. 2018, 102, 2455–2464.
- Tyagi, V.K.; Lo, S.L. Sludge: A waste or renewable source for energy and resources recovery? Renew. Sustain. Energy Rev. 2013, 25, 708–728.
- Keiding, K.; Wybrandt, L.; Nielsen, P.H. Remember the water: A comment on EPS colligative properties. Water Sci. Technol. 2001, 43, 17–23.
- Ding, Z.; Bourven, I.; Guibaud, G.; van Hullebusch, E.D.; Panico, A.; Pirozzi, F.; Esposito, G. Role of extracellular polymeric substances (EPS) production in bioaggregation: Application to wastewater treatment. Appl. Microbiol. Biotechnol. 2015, 99, 9883–9905.
- Feng, C.; Lotti, T.; Canziani, R.; Lin, Y.; Tagliabue, C.; Malpei, F. Extracellular biopolymers recovered as raw biomaterials from waste granular sludge and potential applications: A critical review. Sci. Total Environ. 2021, 753, 142051.
- BIOPOL. 2009. Available online: http://www.biorenery.nl/leadmin/biopol/user/documents/PublicDeliverables/BIOPOL_D_7_6_-_Final_240609.pdf (accessed on 27 October 2022).
- Bora, R.R.; Richardson, R.E.; You, F. Resource recovery and waste-to-energy from wastewater sludge via thermochemical conversion technologies in support of circular economy: A comprehensive review. BMC Chem. Eng. 2020, 2, 8.
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical conversion of sewage sludge: A critical review. Prog. Energy Combust. Sci. 2020, 79, 100843.
- Hu, M.; Ye, Z.; Zhang, H.; Chen, B.; Pan, Z.; Wang, J. Thermochemical conversion of sewage sludge for energy and resource recovery: Technical challenges and prospects. Environ. Pollut. Bioavailab. 2021, 33, 145–163.
- Shatir, S.; Syed-Hassan, A.; Wang, Y.; Hua, S.; Su, S.; Xiang, J. Thermochemical processing of sewage sludge to energy and fuel: Fundamentals, challenges and considerations. Renew. Sustain. Energy Rev. 2017, 80, 888–913.
- Capodaglio, A.G.; Callegari, A.; Dondi, D. Microwave-Induced Pyrolysis for Production of Sustainable Biodiesel from Waste Sludges. Waste Biom. Valoriz 2016, 7, 703–709.
- Ledakowicz, S.; Stolarek, P.; Malinowski, A.; Lepez, O. Thermochemical treatment of sewage sludge by integration of drying and pyrolysis/autogasification. Renew. Sustain. Energy Rev. 2019, 104, 319–327.
- Wang, X.; Chi, Q.; Liu, X.; Wang, Y. Influence of pyrolysis temperature on characteristics and environmental risk of heavy metals in pyrolyzed biochar made from hydrothermally treated sewage sludge. Chemosphere 2019, 216, 698–706.
- Capodaglio, A.G.; Callegari, A.; Lopez, M.V. European Framework for the Diffusion of Biogas Uses: Emerging Technologies, Acceptance, Incentive Strategies, and Institutional-Regulatory Support. Sustainability 2016, 8, 298.
- Li, X.; Zhao, J.; Wang, D.; Yang, Q.; Zeng, G. An efficient and green pretreatment to stimulate short-chain fatty acids production from waste activated sludge anaerobic fermentation using free nitrous acid. Chemosphere 2016, 144, 160–167.
- Zhang, C.; Qin, Y.; Xu, Q.; Liu, X.; Liu, Y.; Ni, B.J.; Yang, Q.; Wang, D.; Li, X.; Wang, Q. Free ammonia-based pretreatment promotes short-chain fatty acid production from waste activated sludge. ACS Sustain. Chem. Eng. 2018, 6, 9120–9129.
- Liu, H.; Fang, H.H. Extraction of extracellular polymeric substances (EPS) of sludges. J. Biotechnol. 2002, 95, 249–256.
- Cord-Ruwisch, R. Thermodynamics of anaerobic digestion: Mechanism of suppression on biogas production during acidogenesis. INMATEH Agric. Eng. 2019, 57, 287–301.
- Shen, L.; Hu, H.; Ji, H.; Cai, J.; He, N.; Li, Q.; Wang, Y. Production of poly(hydroxybutyrate-hydroxyvalerate) from waste organics by the two-stage process: Focus on the intermediate volatile fatty acids. Biores Technol. 2014, 166, 194–200.
- Levy, P.F.; Sanderson, J.E.; Kispert, R.G.; Wise, D.L. Biorefining of biomass to liquid fuels and organic chemicals. Enzym. Microb. Technol. 1981, 3, 207–215.
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786.
- Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Production of volatile fatty acids through co-digestion of sewage sludge and external organic waste: Effect of substrate proportions and long-term operation. Waste Manag. 2020, 112, 30–39.
- Zacharof, M.P.; Lovitt, R.W. Complex effluent streams as a potential source of volatile fatty acids. Waste Biomass Valorization 2013, 4, 557–581.
- Wu, S.L.; Wei, W.; Sun, J.; Xu, Q.; Dai, X.; Ni, B.J. Medium-Chain fatty acids and long-chain alcohols production from waste activated sludge via two-stage anaerobic fermentation. Water Res. 2020, 186, 116381.
- Yishai, O.; Lindner, S.N.; de la Cruz, J.G.; Tenenboim, H.; Bar-Even, A. The formate bio-economy. Curr. Opin. Chem. Biol. 2016, 35, 1–9.
- Wang, R.; Lv, N.; Li, C.; Cai, G.; Pan, X.; Li, Y.; Zhu, G. Novel strategy for enhancing acetic and formic acids generation in acidogenesis of anaerobic digestion via targeted adjusting environmental niches. Water Res. 2021, 193, 116896.
- Mühlemeier, I.M.; Speight, R.; Strong, P.J. Biogas, bioreactors and bacterial methane oxidation. In Methane Biocatalysis: Paving the Way to Sustainability; Kalyuzhnaya, M.G., Xing, X.-H., Eds.; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2018; pp. 213–235.
- Zhang, T.; Zhou, J.; Wang, X.; Zhang, Y. Coupled effects of methane monooxygenase and nitrogen source on growth and poly-β-hydroxybutyrate (PHB) production of Methylosinus trichosporium OB3b. J. Environ. Sci. 2017, 52, 49–57.
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981.
- Cantera, S.; Estrada, J.M.; Lebrero, R.; García-Encina, P.A.; Muñoz, R. Comparative performance evaluation of conventional and two-phase hydrophobic stirred tank reactors for methane abatement: Mass transfer and biological considerations. Biotechnol. Bioeng. 2016, 113, 1203–1212.
- Rodríguez, Y.; Firmino, P.I.M.; Pérez, V.; Lebrero, R.; Muñoz, R. Biogas valorization via continuous polyhydroxybutyrate production by Methylocystis hirsuta in a bubble column bioreactor. Waste Manag. 2020, 113, 395–403.
- Gao, X.; Chen, J.C.; Wu, Q.; Chen, G.Q. Polyhydroxyalkanoates as a source of chemicals, polymers, and biofuels. Curr. Opin. Biotechnol. 2011, 22, 768–774.
- Spekreijse, J.; Le Nôtre, J.; van Haveren, J.; Scott, E.L.; Sanders, J.P.M. Simultaneous production of biobased styrene and acrylates using ethenolysis. Green Chem. 2014, 14, 2747–2751.
- Bluemink, E.D.; van Nieuwenhuijzen, A.F.; Wypkema, E.; Uijterlinde, C.A. Bio-plastic (poly-hydroxy-alkanoate) production from municipal sewage sludge in the Netherlands: A technology push or a demand driven process? Water Sci. Technol. 2016, 74, 353–358.
- Yadav, B.; Talan, A.; Tyagi, R.D.; Drogui, P. Concomitant production of value-added products with polyhydroxyalkanoate (PHA) synthesis: A review. Bioresour. Technol. 2021, 337, 125419.
- Morgan-Sagastume, F.; Hjort, M.; Cirne, D.; Gérardin, F.; Lacroix, S.; Gaval, G.; Karabegovic, L.; Alexandersson, T.; Johansson, P.; Karlsson, A.; et al. Integrated production of polyhydroxyalkanoates (PHAs) with municipal wastewater and sludge treatment at pilot scale. Bioresour. Technol. 2015, 181, 78–89.
- Agler, M.T.; Wrenn, B.A.; Zinder, S.H.; Angenent, L.T. Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends Biotechnol. 2011, 29, 70–78.
- Valentino, F.; Morgan-Sagastume, F.; Fraraccio, S.; Corsi, G.; Zanaroli, G.; Werker, A.; Majone, M. Sludge minimization in municipal wastewater treatment by polyhydroxyalkanoate (PHA) production. Environ. Sci. Pollut. Res. 2015, 22, 7281–7294.
- Frison, N.; Katsou, E.; Malamis, S.; Oehmen, A.; Fatone, F. Development of a novel process integrating the treatment of sludge reject water and the production of polyhydroxyalkanoates (PHAs). Environ.Sci. Technol. 2015, 49, 10877–10885.
- Serafim, L.S.; Lemos, P.C.; Albuquerque, M.G.E.; Reis, M.A.M. Strategies for PHA production by mixed cultures and renewable waste materials. Appl. Microbiol. Biotechnol. 2008, 81, 615–628.
- Serafim, L.S.; Lemos, P.C.; Oliveira, R.F.; Reis, M.A.M. Optimisation of polyhydroxybutyrate production by mixed cultures submitted to aerobic dynamic feeding conditions. Biotechnol. Bioeng. 2004, 87, 145–160.
- Takabatake, H.; Satoh, H.; Mino, T.; Matsuo, T. Recovery of biodegradable plastics from activated sludge process. Water Sci. Technol. 2000, 42, 351–356.
- Albuquerque, M.G.E.; Martino, V.; Pollet, E.; Avérous, L.; Reis, M.A.M. Mixed culture polyhydroxyalkanoate (PHA) production from volatile fatty acid (VFA)-rich streams: Effect of substrate composition and feeding regime on PHA productivity, composition and properties. J. Biotechnol. 2011, 151, 66–76.
- Baetens, D.; Aurola, A.M.; Foglia, A.; Dionisi, D.; van Loosdrecht, M.C.M. Gas chromatographic analysis of polyhydroxybutyrate in activated sludge: A round-robin test. Water Sci. Technol. 2002, 46, 357–361.
- Morgan-Sagastume, F.; Karlsson, A.; Johansson, P.; Pratt, S.; Boon, N.; Lant, P.; Werker, A. Production of polyhydroxyalkanoates in open, mixed cultures from a waste sludge stream containing high levels of soluble organics, nitrogen and phosphorus. Water Res. 2010, 44, 5196–5211.
- Flemming, H.C.; Wingender, J. Relevance of microbial extracellular polymeric substances (EPSs) Part II: Technical aspects. Water Sci. Technol. 2001, 43, 9–16.
- Kim, N.K.; Mao, N.; Lin, R.; Bhattacharyya, D.; van Loosdrecht, M.C.M.; Lin, Y. Flame retardant property of flax fabrics coated by extracellular polymeric substances recovered from both activated sludge and aerobic granular sludge. Water Res. 2020, 170, 115344.
- Schlekat, C.E.; Decho, A.W.; Chandler, G.T. Sorption of cadmium to bacterial extracellular polymeric sediment coatings under estuarine conditions. Environ. Toxicol. Chem. 1998, 17, 1867–1874.
- Mohapatra, S.P.; Siebel, M.A.; Alaerts, G.J. Effect of Bacillus megaterium on removal of copper from aqueous solutions by activated carbon. J. Environ. Sci. Health Part A Environ. Sci. Eng. Tox. Haz. Subst. Contr. 1993, 28, 615–629.
- Spath, R.; Flemming, H.C.; Wuertz, S. Sorption properties of biofilms. Water Sci. Technol. 1998, 37, 207–210.
- Liu, A.B.; Ahn, I.S.; Mansfield, C.; Lion, L.W.; Shuler, M.L.; Ghiorse, W.C. Phenanthrene desorption from soil in the presence of bacterial extracellular polymer: Observations and model predictions of dynamic behaviour. Water Res. 2000, 35, 835–843.
- Esparza-Soto, M.; Westerhoff, P. Biosorption of humic and fulvic acids to live activated sludge biomass. Water Res. 2003, 37, 2301–2310.
- Sheng, G.P.; Zhang, M.L.; Yu, H.Q. Characterization of adsorption properties of extracellular polymeric substances (EPS) extracted from sludge. Colloids Surf. B 2008, 62, 83–90.
- Zhang, X.Q.; Bishop, P.L. Biodegradability of biofilm extracellular polymeric substances. Chemosphere 2003, 50, 63–69.
- Andreoli, C.V.; Von Sperling, M.; Fernandes, F.; Ronteltap, M. Sludge Treatment and Disposal; IWA Publishing: London, UK, 2007.
- Lackner, S.; Gilbert, E.M.; Vlaeminck, S.E.; Joss, A.; Horn, H.; van Loosdrecht, M.C.M. Full-scale partial nitritation/anammox experiences–an application survey. Water Res. 2014, 55, 292–303.
- Dubé, C.D.; Guiot, S.R. Characterization of the protein fraction of the extracellular polymeric substances of three anaerobic granular sludges. AMB Express 2019, 9, 23.
- Seviour, T.; Donose, B.C.; Pijuan, M.; Yuan, Z. Purification and conformational analysis of a key exopolysaccharide component of mixed culture aerobic sludge granules. Environ. Sci. Technol. 2010, 44, 4729–4734.
- Capodaglio, A.G. Pulse electric field technology for wastewater and biomass residues’ improved valorization. Processes 2021, 9, 736.
- Gehr, R.; Henry, J. Removal of extracellular material techniques and pitfalls. Water Res. 1983, 17, 1743–1748.
- Felz, S.; Al-Zuhairy, S.; Aarstad, O.A.; van Loosdrecht, M.C.M.; Lin, Y.M. Extraction of structural extracellular polymeric substances from aerobic granular sludge. JOVE-J. Vis. Exp. 2016, 115, e54534.
- Frølund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996, 30, 1749–1758.
- Sheng, G.; Yu, H.; Li, X. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894.
- Boleij, M.; Seviour, T.; Wong, L.L.; van Loosdrecht, M.C.M.; Lin, Y. Solubilization and characterization of extracellular proteins from anammox granular sludge. Water Res. 2019, 164, 114952.
- Sesay, M.L.; Özcengiz, G.; Sanin, F.D. Enzymatic extraction of activated sludge extracellular polymers and implications on bioflocculation. Water Res. 2006, 40, 1359–1366.
- Markham, W.M.; Reid, J.H. Conversion of Biological Sludge and Primary Float Sludge to Animal Protein Supplement. U.S. Patent No 4,728,517, 1 March 1988.
- Rudolf, P.; Szabo, B.; Janko, F.; Neszmelyi, E.; Illes, J.; Takacs, I.; Havas, F.; Bende, G. Process and Apparatus for Extraction of Solid Matter Containing Fat And/or Protein from Sludge. US Patent 5200085A, 6 April 1993.
- Pervaiz, M.; Sain, M. Protein extraction from secondary sludge of paper mill wastewater and its utilization as a wood adhesive. Bioresources 2021, 6, 961–970.
- Zhu, H. Study on preparation of protein foam fire extinguishing agent. Special. Petrochem. 1994, 1, 1–7.
- Hwang, J.; Zhang, L.; Seo, S.; Lee, Y.W.; Jahng, D. Protein recovery from excess sludge for its use as animal feed. Bioresour. Technol. 2008, 99, 8949–8954.
- Xiao, K.; Zhou, Y. Protein recovery from sludge: A review. J. Clean. Prod. 2020, 249, 119373.
- Ayol, A. Enzymatic treatment effects on dewaterability of anaerobically digested biosolids-I: Performance evaluations. Process Biochem. 2005, 40, 2427–2434.
- Su, R.; Hussain, A.; Guo, J.; Guan, J.; He, Q.; Yan, X.; Li, D.; Guo, Z. Animal feeds extracted from excess sludge by enzyme, acid and base hydrolysis processes. ACS Sust. Chem. Eng. 2015, 3, 2084–2091.
- Kavitha, S.; Stella, P.C.; Kaliappan, S.; Yeom, I.T.; Banu, J.R. Enhancement of anaerobic degradation of sludge biomass through surfactant-assisted bacterial hydrolysis. Process Saf. Environ. Prot. 2016, 99, 207–215.
- Zhuang, L.; Zhou, S.; Wang, Y.; Liu, Z.; Xu, R. Cost-effective production of Bacillus thuringiensis biopesticides by solid-state fermentation using wastewater sludge: Effects of heavy metals. Bioresour. Technol. 2011, 102, 4820–4826.
- Brar, S.K.; Verma, M.; Tyagi, R.D.; Valéro, J.R.; Surampalli, R.Y. Biopesticides—Bacillus thuringiensis. In Sustainable Sludge Management: Production of Value Added Products; Tyagi, R.D., Surampalli, R.Y., Yan, S., Zhang, T.C., Kao, C.M., Lohani, B.N., Eds.; American Society of Civil Engineers: Reston, VA, USA, 2013; pp. 168–202.
- Montiel, M.T.; Tyagi, R.; Valero, J.; Surampalli, R. Production of biopesticides using wastewater sludge as a raw material-effect of process parameters. Water Sci. Technol. 2003, 48, 239–246.
- Drouin, M.; Lai, C.K.; Tyagi, R.D.; Surampalli, R.Y. Bacillus licheniformis proteases as high value added products from fermentation of wastewater sludge: Pre-treatment of sludge to increase the performance of the process. In Proceedings of the IWA Specialist Conference on Moving Forward Wastewater Biosolids Sustainability: Technical, Managerial, and Public Synergy, Moncton, NB, Canada, 24–27 June 2007; pp. 599–605.
- Balasubramanian, S.; Tyagi, R.D. Value-Added Bio-products From Sewage Sludge. In Current Developments in Biotechnology and Bioengineering; Wong, J.-C., Tyagi, R., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–42.
- Frølund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Enzymatic activity in the activated sludge floc matrix. Appl. Microbiol. Biotechnol. 1995, 43, 708–716.
- Subramanian, S.B.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Enzymes: Production and Extraction. In Sustainable Sludge Management: Production of Value Added Products; Tyagi, R.D., Surampalli, R.Y., Yan, S., Zhang, T.C., Kao, C.M., Lohani, B.N., Eds.; American Society of Civil Engineers: Reston, VA, USA, 2013; pp. 231–261.
- Feng, S.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Cheng, D.; Varjani SLei, Z.; Liu, Y. Roles and applications of enzymes for resistant pollutants removal in wastewater treatment. Bioresour. Technol. 2021, 335, 125278.
- Guanghui, Y.; Pinjing, H.; Liming, S.; Yishu, Z. Enzyme extraction by ultrasound from sludge flocs. J. Environ. Sci. 2009, 21, 204–210.
- Nabarlatz, D.; Vondrysova, J.; Jenicek, P.; Stüber, F.; Font, J.; Fortuny, A.; Fabregat, A.; Bengoa, C. Hydrolytic enzymes in activated sludge: Extraction of protease and lipase by stirring and ultrasonication. Ultrason. Sonochem. 2010, 17, 923–931.
- Jung, J.; Xing, X.H.; Matsumoto, K. Recoverability of protease released from disrupted excess sludge and its potential application to enhanced hydrolysis of proteins in wastewater. Biochem. Eng. J. 2002, 10, 67–72.
- Petersen, S.B.; Nielsen, P.H. Lipase and protease extraction from activated sludge. Water Res. 2003, 37, 3652–3657.
- Agrafioti, E.; Bouras, G.; Kalderis, D.; Diamadopoulos, E. Biochar production by sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 72–78.
- Capodaglio, A.G.; Callegari, A. Feedstock and process influence on biodiesel produced from waste sewage sludge. J. Environ. Manag. 2018, 216, 176–182.
- Djandja, O.S.; Wang, Z.C.; Wang, F.; Xu, Y.P.; Duan, P.G. Pyrolysis of Municipal Sewage Sludge for Biofuel Production: A Review. Ind. Eng. Chem. Res. 2020, 59, 16939–16956.
- Mushtaq, F.; Mat, R.; Ani, R.F. A review on microwave assisted pyrolysis of coal and biomass for fuel production. Renew. Sustain. Energy Rev. 2014, 39, 555–574.
- Quan, L.M.; Kamyab, H.; Yuzir, A.; Ashokkumar, V.; Hosseini, S.E.; Balasubramanian, B.; Kirpichnikova, I. Review of the application of gasification and combustion technology and waste-to-energy technologies in sewage sludge treatment. Fuel 2022, 316, 123199.
- Tasca, A.L.; Puccini, M.; Gori, R.; Corsi, I.; Galletti AM, R.; Vitolo, S. Hydrothermal carbonization of sewage sludge: A critical analysis of process severity, hydrochar properties and environmental implications. Waste Manag. 2019, 93, 1–13.
- Thomsen, L.B.S.; Carvalho, P.N.; Dos Passos, J.S.; Anastasakis, K.; Bester, K.; Biller, P. Hydrothermal liquefaction of sewage sludge; energy considerations and fate of micropollutants during pilot scale processing. Water Res. 2020, 183, 116101.
- Callegari, A.; Bolognesi, S.; Cecconet, D.; Capodaglio, A.G. Production technologies, current role, and future prospects of biofuels feedstocks: A state-of-the-art review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 384–436.
- Olkiewicz, M.; Caporgno, M.P.; Fortuny, P.; Stüber, F.; Fabregat, A.; Font, J.; Bengoa, C.M. Direct liquid-liquid extraction of lipid from municipal sewage sludge for biodiesel production. Fuel Process. Technol. 2014, 128, 331–338.
- Boocock, D.G.B.; Konar, S.K.; Leung, A.; Ly, L.D. Fuels and chemicals from sewage sludge: 1. The solvent extraction and composition of a lipid from a raw sewage sludge. Fuel 1992, 71, 1283–1289.
- Siddiquee, M.N.; Rohani, S. Lipid extraction and biodiesel production from municipal sewage sludges: A review. Renew. Sustain. Energy Rev. 2011, 15, 1067–1072.
- Patiño, Y.; Mantecón, L.G.; Polo, S.; Faba, L.; Díaz, E.; Ordóñez, S. Effect of sludge features and extraction-esterification technology on the synthesis of biodiesel from secondary wastewater treatment sludges. Bioresour. Technol. 2018, 247, 209–216.
- Sakaveli, F.; Petala, M.; Tsiridis, V.; Darakas, E. Enhanced mesophilic anaerobic digestion of primary sewage sludge. Water 2021, 13, 348.
- D’Ambrosio, V.; di Bitonto, L.; Angelini, A.; Gallipoli ABraguglia, C.M.; Pastore, C. Lipid extraction from sewage sludge using green biosolvent for sustainable biodiesel production. J. Clean. Prod. 2021, 329, 129643.
- Olkiewicz, M.; Fortuny, P.; Stüber, F.; Fabregat, A.; Font, J.; Bengoa, C. Effects of pre-treatments on the lipid extraction and biodiesel production from municipal WWTP sludge. Fuel 2015, 141, 250–257.
- de Jesus, S.S.; Maciel Filho, R. Recent advances in lipid extraction using green solvents. Renew. Sustain. Energy Rev. 2020, 133, 110289.
- Raboni, M.; Viotti, P.; Capodaglio, A.G. A comprehensive analysis of the current and future role of biofuels for transport in the European Union (EU). Rev. Ambiente Agua 2015, 10, 9–21.
More
Information
Subjects:
Green & Sustainable Science & Technology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Entry Collection:
Wastewater Treatment
Revisions:
4 times
(View History)
Update Date:
10 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




