Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Isabella Reccia | -- | 1392 | 2023-04-04 11:13:14 | | | |
| 2 | Conner Chen | Meta information modification | 1392 | 2023-04-06 09:41:31 | | | | |
| 3 | Conner Chen | + 3 word(s) | 1395 | 2023-04-06 09:42:13 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Reccia, I.; Pai, M.; Kumar, J.; Spalding, D.; Frilling, A. Tumour Heterogeneity in Gastroenteropancreatic-Neoplasms. Encyclopedia. Available online: https://encyclopedia.pub/entry/42774 (accessed on 02 March 2026).
Reccia I, Pai M, Kumar J, Spalding D, Frilling A. Tumour Heterogeneity in Gastroenteropancreatic-Neoplasms. Encyclopedia. Available at: https://encyclopedia.pub/entry/42774. Accessed March 02, 2026.
Reccia, Isabella, Madhava Pai, Jayant Kumar, Duncan Spalding, Andrea Frilling. "Tumour Heterogeneity in Gastroenteropancreatic-Neoplasms" Encyclopedia, https://encyclopedia.pub/entry/42774 (accessed March 02, 2026).
Reccia, I., Pai, M., Kumar, J., Spalding, D., & Frilling, A. (2023, April 04). Tumour Heterogeneity in Gastroenteropancreatic-Neoplasms. In Encyclopedia. https://encyclopedia.pub/entry/42774
Reccia, Isabella, et al. "Tumour Heterogeneity in Gastroenteropancreatic-Neoplasms." Encyclopedia. Web. 04 April, 2023.
Copy Citation
Tumour heterogeneity is a common phenomenon in gastroenteropancreatic (GEP)-neoplasms (NENs) and has a negative impact on treatment success and prognosis as it produces cell clones that do not express treatment targets (i.e., SSTR, mammalian target of rapamycin–mTOR- signalling pathway, Ki-67).
neuroendocrine tumour
neuroendocrine neoplasms
gastroenteropancreatic
1. Introduction
Tumour heterogeneity refers to spatial and temporal variations that may occur within the tumour environment (intra-tumour) or within individual tumour foci, and also between tumour sites (inter-tumour) [1]. Such heterogeneity may encompass genetic and epigenetic variations, or differences in the tumour microenvironment [2][3][4][5][6]. Tumour heterogeneity can also evolve over time due to selective pressures, such as those imposed by treatment, leading to selection and clonal expansion of subpopulations [1][7][8][9]. Tumour heterogeneity is common in human tumours and its occurrence is essential to understand and predict tumour progression and response to specific treatment [10]. Higher intra-tumour and/or inter-tumour heterogeneity can be associated with negative outcomes [7].
Neuroendocrine tumours (NETs), better defined as neoplasms (NENs), are a heterogeneous group of neoplasms that range from well-differentiated tumours to more aggressive carcinomas (Table 1) [11].
Table 1. Classification for gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs).
| Terminology | Differentiation | Grade | Mitotic Rate (Mitoses/2 mm2) * | Ki-67 Index % ** |
|---|---|---|---|---|
| NET, G1 | Well differentiated | Low | <2 | <3% |
| NET, G2 | Intermediate | 2–20 | 3–20% | |
| NET, G3 | High | >20 | >20% | |
| NEC, small-cell type (SCNEC) | Poorly differentiated | High | >20 | >20% |
| NEC, large-cell type (LCNEC) | >20 | >20% | ||
| MiNEN | Well or poorly differentiated | Variable | Variable | Variable |
* mitotic rates are determined by counting in 50 fields of 0.2 mm2; ** Ki-67 values are expressed as the percentage of positive cells. Abbreviations: LCNEC, large-cell neuroendocrine carcinoma; MiNEN, mixed neuroendocrine–non-neuroendocrine neoplasm; NEC, neuroendocrine carcinoma; NET, neuroendocrine tumour; SCNEC, small-cell neuroendocrine carcinoma.
The hallmark of NENs is their expression of somatostatin receptors (SSTRs), as somatostatin inhibits cell growth and hormone secretion in normal and cancerous neuroendocrine cells [12]. Somatostatin receptors are G-protein-coupled receptors with a typical transmembrane domain that includes five distinct subtypes named 1 to 5, with the gene encoding for the SSTR2 also producing two splice variants, SSTR2 isoform A and B. While the natural ligands of SSTRs (i.e., somatostatin-14, somatostatin-28 and cortistatin) all bind to the receptors with high affinity, somatostatin analogues (SSAs)-octreotide, vapreotide and lanreotide-bind only to SSTR2 and with a lower affinity to SSTR3 and 5 [13][14][15]. Neuroendocrine neoplasms express all SSTRs at different concentrations, with SSTR2 being the predominant receptor found across NENs of different origins, followed by SSTR3 in gastroenteropancreatic (GEP)-NENs and SSTR1 and SSTR5 in midgut NENs [16][17][18].
The GEP tract is the most common site for NENs, with the small intestine (SI) and the pancreas being the most prevalent sites of origin for more advanced neoplasms. For these neoplasms, treatment strategies are based on information on SSTRs expression, tumour stage and grade (including differentiation) and the expression of neuroendocrine biomarkers [19].
The definitive diagnosis of an NEN is made by histopathological examination of tumour tissue, obtained either via a biopsy or following surgery. Morphologic imaging, however, is essential as a baseline evaluation for staging, in particular for identifying the presence of metastases, while functional imaging is important to assess the functional and metabolic status of the tumour. Combining morphological (e.g., computer tomography-CT) and functional imaging techniques is fundamental in the decision-making process of the therapeutic approach to patients with GEP-NENs [20]. Gallium68 (68Ga)-DOTA-peptide positron emission tomography (PET)/CT, i.e., 68Ga-DOTATATE or 68Ga-DOTATOC, remains the gold standard for assessing the eligibility and response to peptide receptor radionuclide therapy (PRRT), especially for well-differentiated grade 1 and grade 2 GEP-NETs [21][22]. However, NENs often show heterogeneous expression of SSTR, which could lead to inferior outcomes following targeted treatment and subsequently influence relapse and progression of the disease [21][22][23][24][25]. High-grade lesions and metastases can have a lower expression of SSTRs which may not be fully assessed on receptor-based imaging alone.
Spatial and temporal heterogeneity should be taken into account in the assessment of NENs, as it is not uncommon for GEP primary and metastatic sites to show intra-tumour and inter-tumour heterogeneity in their Ki-67 index, as well as in their SSTR expression and cell signalling pathways, leading to incomplete understanding of their tumour biology and behaviour [26][27][28][29][30][31].
2. Tumour Heterogeneity in GEP-NENs
Tumour heterogeneity is a common phenomenon in GEP-NENs (Figure 1) and has a negative impact on treatment success and prognosis as it produces cell clones that do not express treatment targets (i.e., SSTR, mammalian target of rapamycin–mTOR- signalling pathway, Ki-67) [32].
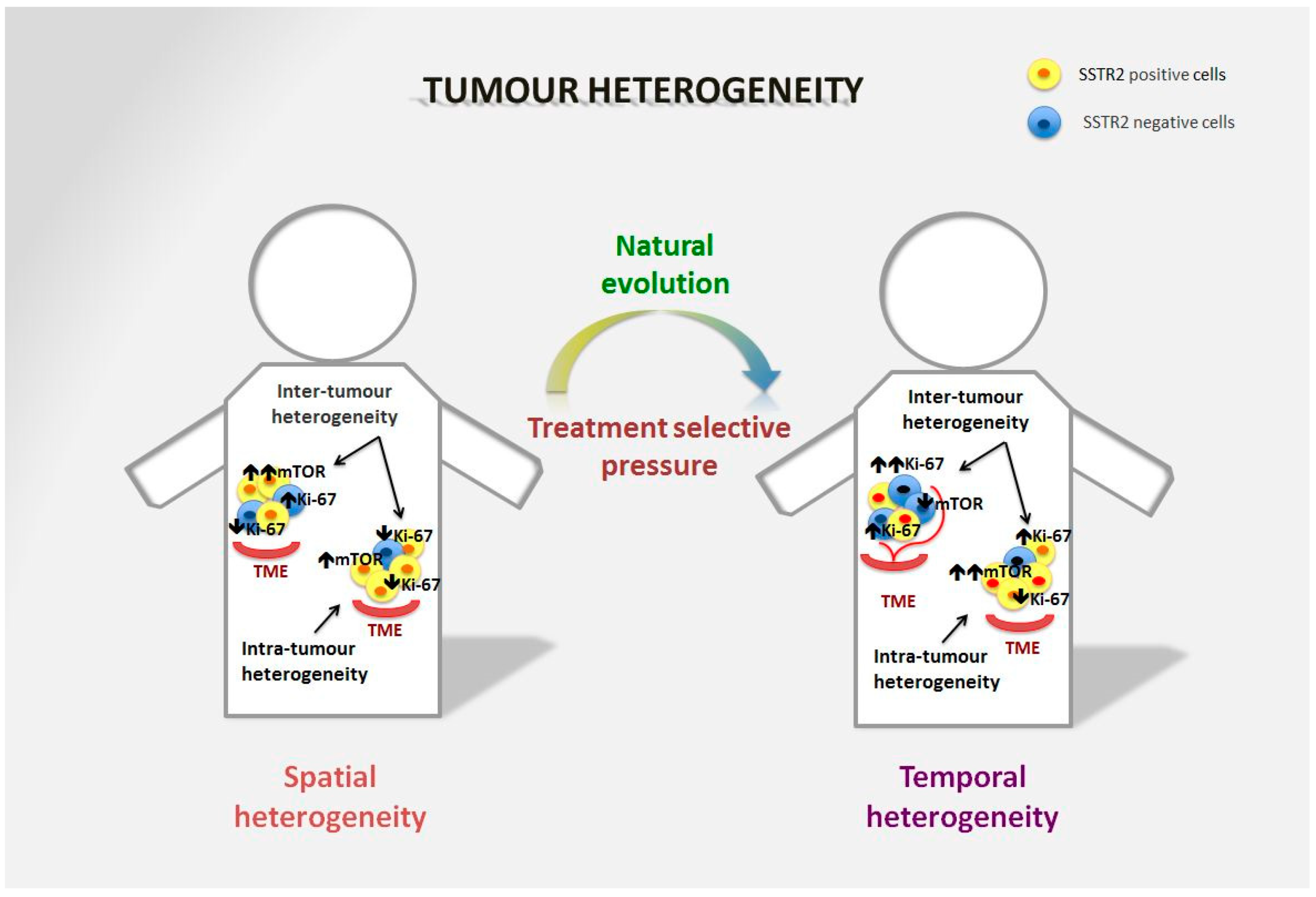
Figure 1. Spatial and temporal heterogeneity in NENs. Neuroendocrine neoplasms generally express SSTR2 on the tumour surface, and are well-differentiated tumours in the majority of cases. However, spatial heterogeneity within the primary tumour may lead to the presence of areas with lower expression of SSTR2 and/or a different Ki-67 index. This heterogeneity is also frequent in metastatic sites and can differ significantly from the primary lesion. The mTOR pathway is also commonly involved in the onset of the disease and is particularly relevant in distant metastases, although over time alternative pathways may be involved in tumour survival. Moreover, temporal heterogeneity that can be linked to treatment selective pressure may lead to significant changes in tumour biology that affect prognosis and survival.
Pancreatic NENs can show a progressive increase in their Ki-67 index or progression to a more aggressive disease, events that are linked to poorer prognosis [33][34]. Changes in the intra-tumoural distribution of Ki-67 in GEP-NENs can lead to significant downgrading of tumours as a consequence of sample bias, especially when small samples are collected that include areas of non-neoplastic tissue [31][35][36]. The Ki-67 is one of the prognostic markers for NENs; however, evaluation of the Ki-67 depends on the site and size of the tumour biopsy and assessment by the pathologist, therefore it may be not representative of tumour behaviour in heterogeneous lesions and especially in intermediate grade 2 lesions [26].
Small-intestine NENs are generally considered to have a relatively low somatic mutation rate, but a more florid epigenetic derangement. It has been shown, however, that there is a high degree of genetic variability between the primary site and liver metastases [37]. Although they are generally well-differentiated tumours with low proliferation rates, distant metastases, in particular hepatic, are a common event and an important cause of poor prognosis [38][39]. The rate of mutations is high, especially in liver metastases, with the mutations often being different to the mutations seen within the primary tumour, thus demonstrating a unique pattern of metastatic spread of SI-NENs [37][40][41]. A large molecular profiling study on SI neuroendocrine liver metastases showed that the expression of several cancer-related pathways that promote tumour development, progression and angiogenesis, including phosphoinositide 3 kinase (PI3K), epidermal growth factor receptor (ErbB1), platelet-derived growth factor receptor beta (PDGFRβ) and mTOR, is upregulated in neuroendocrine liver metastases in comparison to their primary site, and that neuroendocrine liver metastases harbour progressive genomic aberrations that occur mostly during the metastatic progression of the tumour [42]. It has also been shown that the pattern of metastatic growth within the liver may be the expression of the different biological behaviour of the disease, as less-differentiated NENs more often showed an aggressive pattern of growth (disseminated metastatic spread) linked to higher Ki-67 and more advanced disease [43]. Most metastases of GEP-NENs show a higher Ki-67 proliferation index than the primary tumour site, meaning that metastatic spread is potentially unrelated to its initial phenotype or genotype [26][44][45]. SI-NENs are usually believed to display an even expression of SSTR2 isoform A [24]. However, SI neuroendocrine liver metastases often show heterogeneous SSTR2 isoform A expression between lesions in the same patient, this seems to be unrelated to the tumour proliferation index or the tumour size, confirming that expression in metastatic lesions is not always similar to that in the primary tumour or between lesions in the same patient [24][31]. Moreover, no correlation has been shown with the SSTR2 isoform A expression of the primary tumours [24]. Liver metastases from ileal NENs have also been found to show a higher expression of SSTR5, which is potentially linked to tumour aggressiveness [46]. Somatostatin receptor type 5 expression seems to correlate with the presence of metastases and angioinvasion in NENs [47]. Although imaging, in particular 68Ga-DOTATATE PET/CT scans, seems to detect most liver metastases even when SSTRs expression is weak, response to radioligand therapy (RLT) may be lower and different between lesions in the same patient [24][48]. Intra-tumour, and especially inter-tumour, heterogeneity should therefore be taken into account in the diagnosis and management of GEP-NENs, as it represents a major challenge for the efficacy of targeted therapies. A better understanding of tumour biology will help in maximizing treatment outcomes.
References
- Liu, J.; Dang, H.; Wang, X.W. The significance of intertumor and intratumor heterogeneity in liver cancer. Exp. Mol. Med. 2018, 50, e416.
- Fisher, R.; Pusztai, L.; Swanton, C. Cancer heterogeneity: Implications for targeted therapeutics. Br. J. Cancer 2013, 108, 479–485.
- Lovly, C.M.; Salama, A.K.S.; Salgia, R. Tumor Heterogeneity and Therapeutic Resistance. Am. Soc. Clin. Oncol. Educ. Book 2016, 36, e585–e593.
- Prasetyanti, P.R.; Medema, J.P. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol. Cancer 2017, 16, 41.
- Vito, A.; El-Sayes, N.; Mossman, K.L. Hypoxia-Driven Immune Escape in the Tumor Microenvironment. Cells 2020, 9, 992.
- Davies, A.E.; Albeck, J.G. Microenvironmental Signals and Biochemical Information Processing: Cooperative Determinants of Intratumoral Plasticity and Heterogeneity. Front. Cell Dev. Biol. 2018, 6, 44.
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94.
- Brodt, P. Role of the Microenvironment in Liver Metastasis: From Pre- to Prometastatic Niches. Clin. Cancer Res. 2016, 22, 5971–5982.
- Zhu, L.; Jiang, M.; Wang, H.; Sun, H.; Zhu, J.; Zhao, W.; Fang, Q.; Yu, J.; Chen, P.; Wu, S.; et al. A narrative review of tumor heterogeneity and challenges to tumor drug therapy. Ann. Transl. Med. 2021, 9, 1351.
- Diaz-Cano, S.J. Tumor heterogeneity: Mechanisms and bases for a reliable application of molecular marker design. Int. J. Mol. Sci. 2012, 13, 1951–2011.
- Klimstra, D.; Klöppel, G.; La Rosa, S. Classification of neuroendocrine neoplasms of the digestive system. In WHO Classification of Tumours of Digestive System, 5th ed.; IARC Publications: Lyon, France, 2019; pp. 16–19.
- Rogoza, O.; Megnis, K.; Kudrjavceva, M.; Gerina-Berzina, A.; Rovite, V. Role of Somatostatin Signalling in Neuroendocrine Tumours. Int. J. Mol. Sci. 2022, 23, 1447.
- Briest, F.; Grabowski, P. PI3K-AKT-mTOR-signaling and beyond: The complex network in gastroenteropancreatic neuroendocrine neoplasms. Theranostics 2014, 4, 336–365.
- Patel, Y.C. Somatostatin and Its Receptor Family. Front. Neuroendocrinol. 1999, 20, 157–198.
- Veenstra, M.J.; de Herder, W.W.; Feelders, R.A.; Hofland, L.J. Targeting the somatostatin receptor in pituitary and neuroendocrine tumors. Expert Opin. Ther. Targets 2013, 17, 1329–1343.
- Kaemmerer, D.; Peter, L.; Lupp, A.; Schulz, S.; Sänger, J.; Baum, R.P.; Prasad, V.; Hommann, M. Comparing of IRS and Her2 as immunohistochemical scoring schemes in gastroenteropancreatic neuroendocrine tumors. Int. J. Clin. Exp. Pathol. 2012, 5, 187–194.
- Reubi, J.C.; Waser, B. Concomitant expression of several peptide receptors in neuroendocrine tumours: Molecular basis for in vivo multireceptor tumour targeting. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 781–793.
- Papotti, M.; Bongiovanni, M.; Volante, M.; Allìa, E.; Landolfi, S.; Helboe, L.; Schindler, M.; Cole, S.; Bussolati, G. Expression of somatostatin receptor types 1–5 in 81 cases of gastrointestinal and pancreatic endocrine tumors. Virchows Arch. 2002, 440, 461–475.
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 844–860.
- Sahani, D.V.; Bonaffini, P.A.; Castillo, C.F.D.; Blake, M.A. Gastroenteropancreatic Neuroendocrine Tumors: Role of Imaging in Diagnosis and Management. Radiology 2013, 266, 38–61.
- Graf, J.; Pape, U.-F.; Jann, H.; Denecke, T.; Arsenic, R.; Brenner, W.; Pavel, M.; Prasad, V. Prognostic Significance of Somatostatin Receptor Heterogeneity in Progressive Neuroendocrine Tumor Treated with Lu-177 DOTATOC or Lu-177 DOTATATE. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 881–894.
- Ortega, C.; Wong, R.K.S.; Schaefferkoetter, J.; Veit-Haibach, P.; Myrehaug, S.; Juergens, R.; Laidley, D.; Anconina, R.; Liu, A.; Metser, U. Quantitative 68Ga-DOTATATE PET/CT Parameters for the Prediction of Therapy Response in Patients with Progressive Metastatic Neuroendocrine Tumors Treated with 177Lu-DOTATATE. J. Nucl. Med. 2021, 62, 1406–1414.
- Shi, C.; Morse, M.A. Mechanisms of Resistance in Gastroenteropancreatic Neuroendocrine Tumors. Cancers 2022, 14, 6114.
- Charoenpitakchai, M.; Liu, E.; Zhao, Z.; Koyama, T.; Huh, W.J.; Berlin, J.; Hande, K.; Walker, R.; Shi, C. In liver metastases from small intestinal neuroendocrine tumors, SSTR2A expression is heterogeneous. Virchows Arch. 2017, 470, 545–552.
- Cives, M.; Strosberg, J. The Expanding Role of Somatostatin Analogs in Gastroenteropancreatic and Lung Neuroendocrine Tumors. Drugs 2015, 75, 847–858.
- Yang, Z.; Tang, L.H.; Klimstra, D.S. Effect of Tumor Heterogeneity on the Assessment of Ki67 Labeling Index in Well-differentiated Neuroendocrine Tumors Metastatic to the Liver: Implications for Prognostic Stratification. Am. J. Surg. Pathol. 2011, 35, 853–860.
- Nuñez-Valdovinos, B.; Carmona-Bayonas, A.; Jimenez-Fonseca, P.; Capdevila, J.; Castaño-Pascual, Á.; Benavent, M.; Pi Barrio, J.J.; Teule, A.; Alonso, V.; Custodio, A.; et al. Neuroendocrine Tumor Heterogeneity Adds Uncertainty to the World Health Organization 2010 Classification: Real-World Data from the Spanish Tumor Registry (R-GETNE). Oncologist 2018, 23, 422–432.
- Shi, H.; Jiang, C.; Zhang, Q.; Qi, C.; Yao, H.; Lin, R. Clinicopathological heterogeneity between primary and metastatic sites of gastroenteropancreatic neuroendocrine neoplasm. Diagn. Pathol. 2020, 15, 108.
- Zhang, W.H.; Gao, H.L.; Liu, W.S.; Qin, Y.; Ye, Z.; Lou, X.; Wang, F.; Zhang, Y.; Chen, X.M.; Chen, J.; et al. A real-life treatment cohort of pancreatic neuroendocrine tumors: High-grade increase in metastases confers poor survival. Front. Endocrinol. 2022, 13, 941210.
- Furukawa, T.; Ozaka, M.; Takamatsu, M.; Takazawa, Y.; Inamura, K.; Inoue, Y.; Mie, T.; Takeda, T.; Kanata, R.; Kasuga, A.; et al. Ki-67 Labeling Index Variability Between Surgically Resected Primary and Metastatic Hepatic Lesions of Gastroenteropancreatic Neuroendocrine Neoplasms. Int. J. Surg. Pathol. 2021, 29, 475–481.
- Shi, C.; Gonzalez, R.S.; Zhao, Z.; Koyama, T.; Cornish, T.C.; Hande, K.R.; Walker, R.; Sandler, M.; Berlin, J.; Liu, E.H. Liver metastases of small intestine neuroendocrine tumors: Ki-67 heterogeneity and World Health Organization grade discordance with primary tumors. Am. J. Clin. Pathol. 2015, 143, 398–404.
- El-Sayes, N.; Vito, A.; Mossman, K. Tumor Heterogeneity: A Great Barrier in the Age of Cancer Immunotherapy. Cancers 2021, 13, 806.
- Alexandraki, K.I.; Kaltsatou, M.; Kyriakopoulos, G.; Mavroeidi, V.; Kostopoulou, A.; Atlan, K.; Theocharis, S.; Rindi, G.; Grossman, A.B.; Grozinsky-Glasberg, S.; et al. Distinctive features of pancreatic neuroendocrine neoplasms exhibiting an increment in proliferative activity during the course of the disease. Endocrine 2021, 72, 279–286.
- Botling, J.; Lamarca, A.; Bajic, D.; Norlén, O.; Lönngren, V.; Kjaer, J.; Eriksson, B.; Welin, S.; Hellman, P.; Rindi, G.; et al. High-Grade Progression Confers Poor Survival in Pancreatic Neuroendocrine Tumors. Neuroendocrinology 2020, 110, 891–898.
- Grillo, F.; Valle, L.; Ferone, D.; Albertelli, M.; Brisigotti, M.P.; Cittadini, G.; Vanoli, A.; Fiocca, R.; Mastracci, L. KI-67 heterogeneity in well differentiated gastro-entero-pancreatic neuroendocrine tumors: When is biopsy reliable for grade assessment? Endocrine 2017, 57, 494–502.
- Trikalinos, N.A.; Chatterjee, D.; Lee, J.; Liu, J.; Williams, G.; Hawkins, W.; Hammill, C. Accuracy of Grading in Pancreatic Neuroendocrine Neoplasms and Effect on Survival Estimates: An Institutional Experience. Ann. Surg. Oncol 2020, 27, 3542–3550.
- Walter, D.; Harter, P.N.; Battke, F.; Winkelmann, R.; Schneider, M.; Holzer, K.; Koch, C.; Bojunga, J.; Zeuzem, S.; Hansmann, M.L.; et al. Genetic heterogeneity of primary lesion and metastasis in small intestine neuroendocrine tumors. Sci. Rep. 2018, 8, 3811.
- Ahmed, A.; Turner, G.; King, B.; Jones, L.; Culliford, D.; McCance, D.; Ardill, J.; Johnston, B.T.; Poston, G.; Rees, M.; et al. Midgut neuroendocrine tumours with liver metastases: Results of the UKINETS study. Endocr. Relat. Cancer 2009, 16, 885–894.
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.-N.; Rashid, A.; et al. One Hundred Years After “Carcinoid”: Epidemiology of and Prognostic Factors for Neuroendocrine Tumors in 35,825 Cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072.
- Karpathakis, A.; Dibra, H.; Pipinikas, C.; Feber, A.; Morris, T.; Francis, J.; Oukrif, D.; Mandair, D.; Pericleous, M.; Mohmaduvesh, M.; et al. Prognostic Impact of Novel Molecular Subtypes of Small Intestinal Neuroendocrine Tumor. Clin. Cancer Res. 2016, 22, 250–258.
- Francis, J.M.; Kiezun, A.; Ramos, A.H.; Serra, S.; Pedamallu, C.S.; Qian, Z.R.; Banck, M.S.; Kanwar, R.; Kulkarni, A.A.; Karpathakis, A.; et al. Somatic mutation of CDKN1B in small intestine neuroendocrine tumors. Nat. Genet. 2013, 45, 1483–1486.
- Karpathakis, A.; Dibra, H.; Pipinikas, C.; Feber, A.; Morris, T.; Francis, J.; Oukrif, D.; Mandair, D.; Pericleous, M.; Mohmaduvesh, M.; et al. Progressive epigenetic dysregulation in neuroendocrine tumour liver metastases. Endocr. Relat. Cancer 2017, 24, L21–L25.
- Frilling, A.; Li, J.; Malamutmann, E.; Schmid, K.-W.; Bockisch, A.; Broelsch, C.E. Treatment of liver metastases from neuroendocrine tumours in relation to the extent of hepatic disease. BJS 2009, 96, 175–184.
- Miller, H.C.; Drymousis, P.; Flora, R.; Goldin, R.; Spalding, D.; Frilling, A. Role of Ki-67 Proliferation Index in the Assessment of Patients with Neuroendocrine Neoplasias Regarding the Stage of Disease. World J. Surg. 2014, 38, 1353–1361.
- Alexandraki, K.I.; Spyroglou, A.; Kykalos, S.; Daskalakis, K.; Kyriakopoulos, G.; Sotiropoulos, G.C.; Kaltsas, G.A.; Grossman, A.B. Changing biological behaviour of NETs during the evolution of the disease: Progress on progression. Endocr. Relat. Cancer 2021, 28, R121–R140.
- Borga, C.; Dal Pozzo, C.A.; Trevellin, E.; Bergamo, F.; Murgioni, S.; Milanetto, A.C.; Pasquali, C.; Cillo, U.; Munari, G.; Martini, C.; et al. mTOR pathway and somatostatin receptors expression intratumor-heterogeneity in ileal NETs. Endocr. Relat. Cancer 2021, 28, 449–456.
- Schmid, H.A.; Lambertini, C.; van Vugt, H.H.; Barzaghi-Rinaudo, P.; Schäfer, J.; Hillenbrand, R.; Sailer, A.W.; Kaufmann, M.; Nuciforo, P. Monoclonal Antibodies against the Human Somatostatin Receptor Subtypes 1–5: Development and Immunohistochemical Application in Neuroendocrine Tumors. Neuroendocrinology 2012, 95, 232–247.
- Feijtel, D.; Doeswijk, G.N.; Verkaik, N.S.; Haeck, J.C.; Chicco, D.; Angotti, C.; Konijnenberg, M.W.; de Jong, M.; Nonnekens, J. Inter and intra-tumor somatostatin receptor 2 heterogeneity influences peptide receptor radionuclide therapy response. Theranostics 2021, 11, 491–505.
More
Information
Subjects:
Oncology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
629
Revisions:
3 times
(View History)
Update Date:
06 Apr 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





