Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Manuel Aureliano | -- | 3342 | 2023-03-30 23:38:25 | | | |
| 2 | Jessie Wu | + 1 word(s) | 3343 | 2023-03-31 05:43:51 | | | | |
| 3 | Jessie Wu | + 2 word(s) | 3345 | 2023-03-31 05:44:27 | | | | |
| 4 | Jessie Wu | Meta information modification | 3345 | 2023-03-31 05:45:34 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Aureliano, M.; Sousa-Coelho, A.L.D.; Dolan, C.C.; Roess, D.A.; Crans, D.C. Vanadium Effects on Lipid Peroxidation and Disease Processes. Encyclopedia. Available online: https://encyclopedia.pub/entry/42672 (accessed on 07 February 2026).
Aureliano M, Sousa-Coelho ALD, Dolan CC, Roess DA, Crans DC. Vanadium Effects on Lipid Peroxidation and Disease Processes. Encyclopedia. Available at: https://encyclopedia.pub/entry/42672. Accessed February 07, 2026.
Aureliano, Manuel, Ana Luísa De Sousa-Coelho, Connor C. Dolan, Deborah A. Roess, Debbie C. Crans. "Vanadium Effects on Lipid Peroxidation and Disease Processes" Encyclopedia, https://encyclopedia.pub/entry/42672 (accessed February 07, 2026).
Aureliano, M., Sousa-Coelho, A.L.D., Dolan, C.C., Roess, D.A., & Crans, D.C. (2023, March 30). Vanadium Effects on Lipid Peroxidation and Disease Processes. In Encyclopedia. https://encyclopedia.pub/entry/42672
Aureliano, Manuel, et al. "Vanadium Effects on Lipid Peroxidation and Disease Processes." Encyclopedia. Web. 30 March, 2023.
Copy Citation
Lipid peroxidation (LPO), a process that affects human health, can be induced by exposure to vanadium salts and compounds. LPO is often exacerbated by oxidation stress, with some forms of vanadium providing protective effects. The LPO reaction involves the oxidation of the alkene bonds, primarily in polyunsaturated fatty acids, in a chain reaction to form radical and reactive oxygen species (ROS). The important question is which radical starts the chain first. On the one hand, a radical is needed (oxidative stress environment) while on the other hand, LPO amplifies and contributes to changing the redox state towards oxidation (what was called oxidative stress).
lipid peroxidation
vanadium
oxidative stress
cancer
Parkinson
Alzheimer
Decavanadate
Polyoxometalates
Diabetes
vanadate
amyloid
1. Role of Vanadium in Lipid Peroxidation Related to Cancer
Despite somewhat controversial reports suggesting that vanadium may be an essential trace element for humans [1][2], pharmacological amounts of vanadium needed to observe efficacy may be 10 to 100 times the normal intake [3]. Under certain levels, some vanadium complexes/compounds have shown anticancer and/or antidiabetic activity in mammals [4][5][6][7][8], while higher levels can cause toxicity. Under some conditions, vanadium can act as a pro-oxidant molecule, which interacts with other oxidants and synergistically enhances oxidative stress and potentially lipid peroxidation (LPO) [9].
Reports from the early 1990s showed that V complexes/compounds induced LPO, which was associated with tissue toxicity and carcinogenicity [10][11][12]. Tissue-specific responses were shown for vanadate, which produced a cytotoxic response in the murine osteoblast-like MC3T3E1 nontransformed cell line [13]. This level of cytotoxicity was higher than that in vanadate-treated osteosarcoma cancer UMR106 cells with respect to both time- and concentration-dependent responses [13]. Osteoblastic cells were more sensitive to the vanadate-induced free radical and biomarker thiobarbituric acid (TBARS) formation, particularly at low concentrations. Nevertheless, higher basal TBARS was observed in untreated osteosarcoma cells [13]. Other vanadium compounds (VOSO4) and a complex of vanadyl with aspirin (VO/Aspi)) were found to be more potent than vanadate in inducing TBARS and inhibiting the cellular growth, in both cell lines tested [13] (Table 1). However, when an equivalent low concentration of VO/Aspi was released from a controlled delivery system (poly(β-propiolactone) (PβPL) film), less TBARS formation was observed [14] (Table 1), which reflects lower cytotoxicity compared to that previously reported for the metallodrug in solution [13].
The development and testing of vanadium derivatives with different ligands and with improved bioavailability and toxicity profiles continues. Both naproxen- and glucose-complexed vanadium compounds (NapVO and GluVO) had antiproliferative effects that were more pronounced in osteosarcoma UMR106 cells than in the normal MC3T3E1 osteoblasts [15]. This supported the observation that a low level of GluVO and NapVO increased TBARS production in tumoral cells but not in the nontransformed cells [15] (Table 1), suggesting LPO was involved in the antineoplastic action observed. Interestingly, neither the free vanadyl cation nor ligands induced an antimitogenic effect in cells at the concentrations tested [15]. At low concentrations, a large number of different complexes/compounds of vanadium were found to be therapeutically active [2][16]. Possible mechanisms for the anticancer activity of vanadium complexes/compounds included an increase in oxygen species (ROS) generation, hyperactivation of the Ras-Raf-MEK-ERK pathway and cell cycle arrest [17][18][19]. It is also possible that vanadium may confer protection against chemical-induced carcinogenesis or toxicity in normal tissues by normalization of increased pathogenic LPO and oxidative stress. While an increase in hepatic LPO was observed in a group of carcinogen-treated female Sprague Dawley rats, this increase was lowered towards normal values by vanadium co-administration [20] (Table 1) and was associated with a significantly lower percentage of rats with tumors after vanadium treatment. In these experimental groups, SOD activity in the liver paralleled LPO. By contrast, hepatic glutathione (GSH) and cytochrome P450 (CYP) enzyme content and glutathione S-transferase (GST) activity decreased with carcinogenic treatment compared to control rats and recovered with vanadium treatment [20]. Similarly, in a model of hepatocarcinogenesis induced in rats by chronic feeding of 2-acetylaminofluorene (2-AAF), continuous vanadium administration inhibited LPO and suppressed cell proliferation [21] (Table 1), suggesting vanadium was chemopreventive.
The chemoprotective role of vanadium against cancer chemotherapy-induced toxicity is also relevant. Many chemotherapeutic agents such as cyclophosphamide (CP) and cisplatin (CDDP) are toxic due to multifactorial mechanisms that include increased oxidative stress in normal tissues and organs, namely the liver and kidney. The co-administration of compounds with antioxidant potential may be beneficial to patients. For example, the simultaneous treatment of female Swiss albino mice with CP and either vanadium(III)-L-cysteine complex (VC-III) [22] or oxovanadium(IV)-L-cysteine methyl ester (VC-IV) [23] reduced ROS levels when compared to the increase in ROS observed in CP-treated group vs. control [22][23]. With respect to LPO, partial normalization of TBARS in CP/VC-III- or CP/VC-IV-treated mice was observed (Table 1) [22][23]. After treatment with CP, there was a decrease in GSH levels and in GST, glutathione peroxidase (GPx), superoxide dismutase (SOD) and catalase (CAT) activities, while a recovery was observed with vanadium treatment [22][23]. Similar protective effects were observed with concomitant treatment with cisplatin (CDDP) and VC-III (Table 1) [24]. These results suggest that vanadium may be beneficial as an adjunct therapy to protect against the toxicity of anticancer drugs.
Table 1. Effects of vanadium in lipid peroxidation related to cancer. Main outcomes of studies using different V compounds in various organs/tissues of animal models or cancer cells.
| Vanadium Compound | Combined/Complexed | Carcinogenic/Toxic Agent or Cell Lines | Tissue/Model | Main Results/Outcome | Ref. |
|---|---|---|---|---|---|
| V1V; VO | Aspirin; polymeric film |
Osteosarcoma UMR106 cells in culture | Bone | Cytotoxic effects | [13][14] |
| V1V derivatives | Naproxen (Nap-VO); Glucose (GluVO) | Apoptosis mediated by lipid peroxidation | [15] | ||
| Ammonium monovanadate (NH4VO3, +V oxidation state) (vanadium supplemented in drinking water) | 7,12-dimethylbenz(a)anthracene (DMBA)-induced mammary carcinogenesis in rats | Mammary gland | Prevention of mammary cancer | [20] | |
| Vanadium (in the form of ammonium vanadate) | 2-acetylaminofluorene (2-AAF)-induced hepatocarcinogenesis in rats | Liver | Vanadium was chemopreventive; inhibition of lipid peroxidation | [21] | |
| Oxovanadium(IV)-L-cysteine methyl ester (VC-IV) | Cyclophosphamide (CP)-induced hepatotoxicity in mice | Liver | Protective role of VC-IV against CP-induced toxicity | [23] | |
| Vanadium(III)-L-cysteine complex (VC-III) | Protective role of VC-III against CP- and CDDP-induced toxicity | [22] | |||
| Cisplatin (CDDP)-induced nephrotoxicity in mice | Kidney | [24] | |||
Even though vanadium participates in Fenton-type reactions [25] and the mechanisms proposed for vanadate action involve redox cycling and the production of ROS [26][27], some results show a depression in ROS and the rate of ROS formation [28]. Previous results show that in certain experimental conditions, for example in rats with induced hepatocarcinogenesis [29] and diabetes [20], vanadate may decrease oxidative stress. Strong evidence supports the observation that V10 alters the production of mitochondrial O2.− differently from V1 and suggests the possibility that different pathways are involved in the biological activity of different vanadium species. Of the proposed intracellular pathways for vanadate, several involve the production of O2.− mediated by oxidoreductases of NADPH in the respiratory chain [26][27]. Considering the proposed mechanisms of action and detoxification of vanadate, which include reducing vanadate to vanadyl with O2.− production, the available data support the interpretation that V10 may participate in bioprocessing and metabolism differently than V1.
2. Effect of Vanadium in Diabetes-Induced Lipid Peroxidation
Diabetes mellitus is a complex metabolic disease characterized by a chronic state of hyperglycemia [30]. Although the impaired function of the pancreatic islets might be relevant in its etiology, other tissues are affected and may present complications in uncontrolled disease [31]. Diabetes can be generally classified into different categories with distinct clinical features. Type 1 diabetes is an autoimmune disease in which beta cells in the pancreas are unable to produce the hormone insulin while in type 2 diabetes, the most common form of diabetes, the body is either resistant to insulin or incapable of producing sufficient amounts of insulin [30].
An imbalance between the production and removal of ROS and RNS may contribute to insulin resistance and pancreatic beta cell dysfunction, which ultimately leads to the development of type 2 diabetes [32][33]. Increased levels of TBARS, a biomarker of LPO, were more highly elevated in type 2 diabetes patients than in healthy control subjects [34]. In patients with type 2 diabetes, hyperglycemia was associated with increased oxidative stress and free radical-mediated LPO [35][36][37] both of which may affect the development of micro- and macrovascular complications related to the intensification of systemic inflammation in these patients [33][38][39]. Compounds that modulate LPO and oxidative stress and have an antioxidant potential may contribute to improving the metabolic health in patients with diabetes and be a valuable therapeutic approach.
In 1979, Tolman et al. showed that vanadium salts exhibited insulin-mimetic effects which led to an interest in vanadium chemistry for the treatment of diabetes [40]. Since then, a series of reports have been published describing the insulin-like effects of various vanadium compounds, mainly VIV and VV salt and coordination complexes. One coordination complex, an organic vanadium compound, bis(ethylmaltolato)oxovanadium(IV) (BEOV), exhibited excellent efficacy in streptozotocin (STZ)-diabetic rats [41] and entered Phase I and II clinical trials [42][43].
Using different animal models of diabetes and analyzing diverse tissues, many reports showed effects of vanadium compounds on the activity of antioxidant enzymes and the levels of LPO (Figure 1). Early studies from the 1990s showed that treatment of STZ-induced diabetic Sprague Dawley rats with sodium metavanadate (NaVO3), did not lead to changes in the antioxidant defense system [44]. However, the tissue level of vanadium positively correlated with the TBARS level [44]. By contrast, sodium orthovanadate (SOV) treatment of STZ-induced diabetic male Wistar rats led to the STZ-induced decrease in the hepatic activities of SOD, CAT and GPx being restored to normal levels, while the elevated plasma lipid peroxides (as measured by MDA) were decreased almost to basal values [45] (Figure 1). This same pattern was observed in the liver enzymes of alloxan-induced diabetes female Wistar rats (Figure 1), but not in all the tissues evaluated [29]. SOV treatment also almost normalized the chemical-induced increase in the levels of TBARS in the brain, along with the normalization of the activity of the brain GST, which was decreased in the diabetic rats [46] (Figure 1). Interestingly, subsequent studies have used SOV combined with Trigonella graecum seed powder (TSP) which makes it possible to use lower concentrations of vanadate. Most authors have shown a reversal of non-physiologic antioxidant levels and peroxidative stress in different tissues from diabetic animals [47][48][49][50][51] (Figure 1).
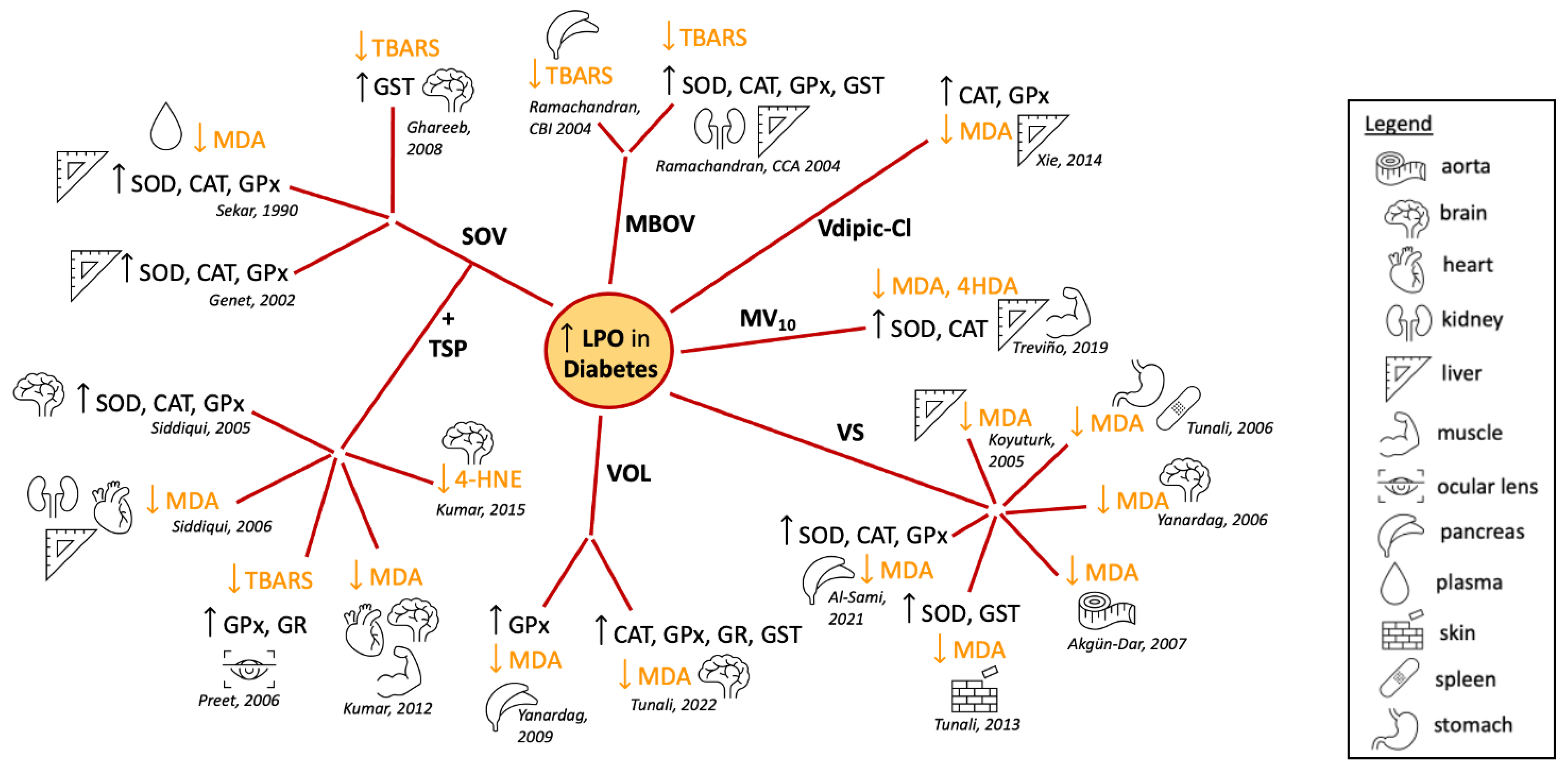
Figure 1. Summary of the reported effects of different vanadium compounds in lipid peroxidation and antioxidant enzyme activity, evaluated in different tissues of diabetes-induced animal models [29][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63]. Abbreviations: superoxide dismutase, SOD; catalase, CAT; glutathione peroxidase, GPx; glutathione reductase, GR; glutathione S-transferase, GST; malondialdehyde, MDA; 4-hydroxy-2-nonenal, 4-HNE; thiobarbituric acid reactivity, TBARS; 4-hydroxyalkenals, 4HDA; sodium orthovanadate, SOV; Trigonella graecum seed powder, TSP; macrocyclic binuclear oxovanadium complex, MBOV; N(1)-2,4-dihydroxybenzylidene-N(4)-2-hydroxybenzylidene-S-methyl-thiosemicarbazidato-oxovanadium (IV), VOL; vanadyl sulphate, VSO4; metformin-decavanadate, MV10 [29][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63].
The vanadium salt in oxidation state IV, vanadyl sulfate (VOSO4), has also been studied extensively. An early study showed that TBARS levels were elevated in vanadyl-treated animals, although cataract development was suppressed in STZ-diabetic Wistar rats [64]. By contrast, many reports showed in different tissues that the treatment with VOSO4 reversed the increased levels of LPO in response to diabetes induction [54][55][56][57][60][61] (Figure 1). These results were recently expanded to show similar normalization of the oxidative state in cardiac, lung, skeletal muscle and eye lens tissue [65]. Similar antioxidant effects were observed in the pancreas, liver and kidneys of diabetic rats treated with a macrocyclic binuclear oxovanadium complex (MBOV) [62][63] and in the pancreas and brain of diabetic rats treated with the N(1)-2,4-dihydroxybenzylidene-N(4)-2-hydroxybenzylidene-S-methyl-thiosemicarbazidato-oxovanadium (IV) (VOL) compound [58][59] (Figure 1).
Oxidative stress in the liver and muscle tissues of alloxan-induced diabetic rats was addressed after treatment with (H2Metf)3[V10O28] (metformin-decavanadate, MV10) [53]. After 60 days, decreased activity levels of SOD and CAT induced by alloxan were restored to normal levels (Figure 1). Furthermore, the increased levels of LPO markers in the diabetic animals were normalized after Metf-V10 treatment. This was observed for both MDA and 4-hydroxyalkenal (4HDA) levels in a similar fashion to treatment with insulin, while metformin alone had very limited effects [53] (Figure 1). Decavanadate was previously reported to increase the glucose uptake in rat adipocytes, in the presence or in the absence of insulin [66]. Together, these findings suggest that vanadium compounds are not only insulin mimetics but may also enhance the activity of insulin [4][67][68].
Changing the oxidation state of the vanadium compound changes the redox properties of the complex alters the formation of LPO products as described above for vanadate (VV) and VOSO4 (VIV). One study compared the effects of vanadium in oxidation states III, IV and V in a series of coordination complexes with the same ligand, chloro-substituted dipicolinic acid [52]. VIVdipic-Cl and VVdipic-Cl complexes in liver tissues produced improved blood glucose levels, while there were lesser effects of VIIIdipic-Cl [52] (Figure 1). This demonstrated that even high-oxidation-state vanadium compounds are beneficial in changing MDA levels toward normal and reducing ROS levels presumably through redox cycling. For complexes with the dipic-Cl ligand, it was surprising that the VV complex showed a trend towards being slightly better at normalizing the redox state of diabetic cells, though VV complexes would need to undergo Fenton chemistry first [52].
It is important to note that the animal models of diabetes using diabetogenic chemicals cause the destruction of β-cells resulting in type 1 diabetes, so it is unclear whether such effects would be observed in type 2 diabetes animal models or patients. Moreover, in some cases, the diabetic animals did not show a reduction in the activity of enzymes involved in antioxidant defense in all tissues analyzed. As an example, an increase in enzyme activity was observed in diabetic heart tissues when compared to normal animals [29][50], although even in such cases, treatment with vanadium compounds restored levels close to normal (non-diabetic) values [29][47][50][55].
Taken together, these data suggest treatment with vanadium compounds may contribute to alleviating oxidative stress in patients with diabetes and contribute to an overall improvement in metabolic function. However, more evidence of vanadium antioxidant beneficial effects and safety is still required.
3. Vanadium Lipid Peroxidation and Neurodegenerative Diseases
Vanadium is known to have neurotoxic effects and contribute to a number of neurodegenerative diseases presumably through the introduction of oxidative stress and LPO production. The brain contains high amounts of PUFAs, making it a prime target for LPO, which can cause the destruction of the myelin sheath, loss of neurons via cell death, disruption of the cell membrane potential, depletion of dopamine, and inactivation of phosphatase enzymes. Neurons are surrounded by a myelin sheath which is important for the development of the electric potential and the ability to transmit electrical impulses in the form of action potentials quickly. Vanadium exposure has been reported to cause damage to the myelin sheath [69] and, as a result of LPO, neuronal death. LPO in the mitochondria also leads to cell death through effects on mitochondrial membranes. Vanadium accumulates in the brain after exposure [70], indicating that the toxic effects of vanadium relating to membrane destruction may play a role in the reported neurodegenerative diseases such as Parkinson’s and Alzheimer’s. The metal content and transporters in the rat brain have been reported to be sensitive to the presence of other metals, including Mn, chromium, zinc, cobalt, aluminum, molybdenum and vanadium [71].
3.1. Parkinson’s Disease
Parkinson’s disease (PD) is a neurodegenerative disease that has been associated with several failures in brain function. A decrease in the neurotransmitter dopamine has been correlated with the onset of Parkinson’s which, with disease progression, leads to a failure in the dopaminergic system. Some basis of knowledge around metals, specifically manganese (Mn), and the onset of Parkinson’s or the onset of similar symptoms called Parkinsonism exists [72]. The latter is a condition that results in loss of motor and neurological function similar to that of Parkinson’s but does not exhibit the symptoms of Parkinson’s disease. Symptoms produced by Mn are called manganism [73]. Mn, like vanadium, undergoes redox cycling and is known to have many neurotoxic effects. The ability of Mn to produce ROS has been well characterized [74] and shown to cause effects on mitochondrial function similar to those observed with vanadium treatment, including the loss of the mitochondrial membrane potential and the release of Cyt C [75]. Additionally, LPO products have been observed in mitochondria and the endoplasmic reticulum system and are similar to the oxidative stress in response to manganese exposure. With high doses of Mn, symptoms of Parkinson’s disease are seen and correlated with the onset of Parkinson’s [76]. Given the similarities between vanadium and manganese, the effects of vanadium on the onset of Parkinson’s are likely to be similar. Ngwa and coworkers have reported a link between vanadium neurotoxicity and its effect on the dopaminergic system due to its effect on protein kinase C-delta and its function in cell signaling mechanisms [77]. Ohiomokhare and coworkers (2020) found that vanadium increased ROS and decreased motor function in Melanogaster drosophila, both wild-type and PD models, and that these effects were alleviated with chelators or the administration of L-DOPA [78].
3.2. Alzheimer’s Disease
Alzheimer’s disease (AD’s) is a neurodegenerative disease characterized by loss of memory. Although no single cause of AD’s has been discovered, there is evidence that metals, lipid peroxidation and oxidative stress can play a role in disease progression. The disease is associated with the accumulation of β-amyloid plaques in the brain that have the capability of interacting with redox-active metals, such as copper, zinc and iron [79]. These metal ions induce the disease because of their ability to generate ROS and damage the brain through DNA damage and oxidation of lipids and proteins. Studies have shown that 4-HNE, a product of LPO, is present in the brains of AD’s patients [80]. Mitochondrial ROS production and mitochondrial dysfunction have been associated with AD [81]. All of these are known products of vanadium-based oxidative stress and offer a basis for vanadium having a role in AD.
As is the case for Parkinson’s, there are only a limited number of studies characterizing vanadium effects on the development of AD’s disease or its progression. However, due to vanadium’s redox properties and ability to generate ROS, it has the potential to induce at least some similar effects. Montiel-Flores and coworkers found that the inhalation of vanadium pentoxide caused AD-like neuronal cell death in rats [82].
There is also a growing body of work investigating the use of vanadium in treating AD’s disease. Although vanadium has toxic effects, studies reported some potential of vanadium-based therapeutics for AD’s disease [83]. Vanadyl acetylacetonate was found to promote glucose and energy metabolism in neuronal cells but did not reduce β-amyloid plaque production [84]. Two peroxovanadium complexes were reported to inhibit β-amyloid fibril formation. He et al. (2015) showed that two complexes were able to inhibit the aggregation of amyloids using PrP106–126 and Aβ1–42, where PrP is from the prion disease and refers to protein-prion protein. Inhibition was more effective in PrP than in Aβ, but there was not much difference in its effects on cell toxicity. Peroxovanadium complexes increased cell viability perhaps due to the ability of peroxovanadium complexes to reduce methionine residues [85]. This group also found that BEOV was able to ameliorate AD symptoms through a number of mechanisms including inhibition of Aβ aggregate formation [86]. These results should encourage studies on the use of vanadium in the treatment of neurodegenerative diseases.
4. The Potential for Lipid Peroxidation as a Future Target for Therapeutic Treatments
The ability of vanadium compounds to impact oxidative stress and the formation of LPO products is well documented [87]. Since vanadium remains a comparatively underexplored metal [88], new compounds are being assayed to determine their potential for alleviating oxidative stress [89][90]. Novel compounds are being designed which affect LPO but lack cellular toxicity. New pathways are discovered by investigating organisms not traditionally investigated [91]. New approaches are being developed based on combatting oxidative stress in disease processes. For example, a 2D vanadium carbide synthetic enzyme referred to as V2C MXenzyme has been reported to alleviate ROS-mediated inflammation [74]. Specifically, the 2D V2C MXenzyme can replace SOD, CAT, POD, TPx, GPx and HPO, thus mimicking the intracellular antioxidant defense system against ROS-mediated oxidative damage including protein carbonylation, lipid peroxidation and DNA damage. In vitro and in vivo experiments demonstrated that V2C MXenzyme was biocompatible and exhibited ROS-scavenging capability, protecting cellular components against oxidative stress. Future investigations are likely to involve the characterization of novel biological systems, new compounds and agents such as the V2C MXenzyme and related systems [74] designed to combat the effects on oxidative stress and LPO.
References
- French, R.J.; Jones, P.J.H. Role of vanadium in nutrition: Metabolism, essentiality and dietary considerations. Life Sci. 1993, 52, 339–346.
- Ścibior, A.; Pietrzyk, Ł.; Plewa, Z.; Skiba, A. Vanadium: Risks and possible benefits in the light of a comprehensive overview of its pharmacotoxicological mechanisms and multi-applications with a summary of further research trends. J. Trace Elem. Med. Biol. 2020, 61, 126508.
- Harland, B.F.; Harden-Williams, B.A. Is vanadium of human nutritional importance yet? J. Am. Diet. Assoc. 1994, 94, 891–894.
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; Rompel, A.; Crans, D.C. Polyoxovanadates with emerging biomedical activities. Coord. Chem. Rev. 2021, 447, 214143.
- Crans, D.C.; Yang, L.; Allison Haase, X.Y. Health Benefits of Vanadium and Its Potential as an Anticancer Agent. Met. Ions Life Sci. 2018, 18, 251–279.
- Crans, D.C.; Henry, L.; Gabriel Cardiff, B.I.P. Developing Vanadium as an Antidiabetic or Anticancer Drug: A Clinical and Historical Perspective. Met Ions Life Sci. 2019, 19, 203–230.
- Willsky, G.R.; Chi, L.-H.; Godzala, M.; Kostyniak, P.J.; Smee, J.J.; Trujillo, A.M.; Alfano, J.A.; Ding, W.; Hu, Z.; Crans, D.C. Anti-diabetic effects of a series of vanadium dipicolinate complexes in rats with streptozotocin-induced diabetes. Coord. Chem. Rev. 2011, 255, 2258–2269.
- Sharfalddin, A.A.; Al-Younis, I.M.; Mohammed, H.A.; Dhahri, M.; Mouffouk, F.; Abu Ali, H.; Anwar, M.J.; Qureshi, K.A.; Hussien, M.A.; Alghrably, M.; et al. Therapeutic Properties of Vanadium Complexes. Inorganics 2022, 10, 244.
- Ścibior, A.; Kurus, J. Vanadium and Oxidative Stress Markers—In Vivo Model: A Review. Curr. Med. Chem. 2019, 26, 5456–5500.
- Byczkowski, J.Z.; Kulkarni, A.P. Lipid peroxidation and benzo(a)pyrene derivative co-oxygenation by environmental pollutants. Bull. Environ. Contam. Toxicol. 1990, 45, 633–640.
- Byczkowski, J.Z.; Kulkarni, A.P. Vanadium redox cycling, lipid peroxidation and co-oxygenation of benzo(a)pyrene-7,8-dihydrodiol. Biochim. Biophys. Acta Lipids Lipid Metab. 1992, 1125, 134–141.
- Younes, M.; Strubelt, O. Vanadate-induced toxicity towards isolated perfused rat livers: The role of lipid peroxidation. Toxicology 1991, 66, 63–74.
- Cortizo, A.M.; Bruzzone, L.; Molinuevo, S.; Etcheverry, S.B. A possible role of oxidative stress in the vanadium-induced cytotoxicity in the MC3T3E1 osteoblast and UMR106 osteosarcoma cell lines. Toxicology 2000, 147, 89–99.
- Cortizo, M.S.; Alessandrini, J.L.; Etcheverry, S.B.; Cortizo, A.M. A vanadium/aspirin complex controlled release using a poly(β-propiolactone) film. Effects on osteosarcoma cells. J. Biomater. Sci. Polym. Ed. 2001, 12, 945–959.
- Molinuevo, M.S.; Barrio, D.A.; Cortizo, A.M.; Etcheverry, S.B. Antitumoral properties of two new vanadyl(IV) complexes in osteoblasts in culture: Role of apoptosis and oxidative stress. Cancer Chemother. Pharmacol. 2004, 53, 163–172.
- Crans, D.C.; Bunch, R.L.; Theisen, L.A. Interaction of trace levels of vanadium(IV) and vanadium(V) in biological systems. J. Am. Chem. Soc. 1989, 111, 7597–7607.
- Amante, C.; De Sousa-Coelho, A.L.; Aureliano, M. Vanadium and Melanoma: A Systematic Review. Metals 2021, 11, 828.
- De Sousa-Coelho, A.L.; Aureliano, M.; Fraqueza, G.; Serrão, G.; Gonçalves, J.; Sánchez-Lombardo, I.; Link, W.; Ferreira, B.I. Decavanadate and metformin-decavanadate effects in human melanoma cells. J. Inorg. Biochem. 2022, 235, 111915.
- Kowalski, S.; Wyrzykowski, D.; Hac, S.; Rychlowski, M.; Radomski, M.W.; Inkielewicz-Stepniak, I. New Oxidovanadium(IV) Coordination Complex Containing 2-Methylnitrilotriacetate Ligands Induces Cell Cycle Arrest and Autophagy in Human Pancreatic Ductal Adenocarcinoma Cell Lines. Int. J. Mol. Sci. 2019, 20, 261.
- Bishayee, A.; Oinam, S.; Basu, M.; Chatterjee, M. Vanadium chemoprevention of 7,12-dimethylbenz(a)anthracene-induced rat mammary carcinogenesis: Probable involvement of representative hepatic phase I and II xenobiotic metabolizing enzymes. Breast Cancer Res. Treat. 2000, 63, 133–145.
- Chakraborty, T.; Chatterjee, A.; Rana, A.; Rana, B.; Palanisamy, A.; Madhappan, R.; Chatterjee, M. Suppression of Early Stages of Neoplastic Transformation in a Two-Stage Chemical Hepatocarcinogenesis Model: Supplementation of Vanadium, a Dietary Micronutrient, Limits Cell Proliferation and Inhibits the Formations of 8-Hydroxy-2′-deoxyguanosines and DN. Nutr. Cancer 2007, 59, 228–247.
- Basu, A.; Bhattacharjee, A.; Samanta, A.; Bhattacharya, S. Prevention of cyclophosphamide-induced hepatotoxicity and genotoxicity: Effect of an l-cysteine based oxovanadium(IV) complex on oxidative stress and DNA damage. Environ. Toxicol. Pharmacol. 2015, 40, 747–757.
- Basu, A.; Bhattacharjee, A.; Roy, S.S.; Ghosh, P.; Chakraborty, P.; Das, I.; Bhattacharya, S. Vanadium as a chemoprotectant: Effect of vanadium(III)-l-cysteine complex against cyclophosphamide-induced hepatotoxicity and genotoxicity in Swiss albino mice. J. Biol. Inorg. Chem. 2014, 19, 981–996.
- Basu, A.; Singha Roy, S.; Bhattacharjee, A.; Bhuniya, A.; Baral, R.; Biswas, J.; Bhattacharya, S. Vanadium(III)-L-cysteine protects cisplatin-induced nephropathy through activation of Nrf2/HO-1 pathway. Free Radic. Res. 2016, 50, 39–55.
- Stohs, S. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995, 18, 321–336.
- Zhang, Z.; Leonard, S.S.; Huang, C.; Vallyathan, V.; Castranova, V.; Shi, X. Role of reactive oxygen species and MAPKs in vanadate-induced G2/M phase arrest. Free Radic. Biol. Med. 2003, 34, 1333–1342.
- Capella, L.S.; Gefé, M.R.; Silva, E.F.; Affonso-Mitidieri, O.; Lopes, A.G.; Rumjanek, V.M.; Capella, M.A. Mechanisms of vanadate-induced cellular toxicity: Role of cellular glutathione and NADPH. Arch. Biochem. Biophys. 2002, 406, 65–72.
- Soares, S.S.; Gutiérrez-Merino, C.; Aureliano, M. Decavanadate Toxicity Effects Following In Vivo Administration; Aureliano, M., Ed.; Research Signpost: Kerala, India, 2007; ISBN 978-81-308-0184-1.
- Genet, S.; Kale, R.K.; Baquer, N.Z. Alterations in antioxidant enzymes and oxidative damage in experimental diabetic rat tissues: Effect of vanadate and fenugreek (Trigonellafoenum graecum). Mol. Cell. Biochem. 2002, 236, 7–12.
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46 (Suppl. 1), S19–S40.
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Gibbons, C.H.; Giurini, J.M.; Hilliard, M.E.; et al. 12. Retinopathy, Neuropathy, and Foot Care: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46 (Suppl. 1), S203–S215.
- Wright, E.; Scism-Bacon, J.L.; Glass, L.C. Oxidative stress in type 2 diabetes: The role of fasting and postprandial glycaemia. Int. J. Clin. Pract. 2006, 60, 308–314.
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070.
- Turk, H.M.; Sevinc, A.; Camci, C.; Cigli, A.; Buyukberber, S.; Savli, H.; Bayraktar, N. Plasma lipid peroxidation products and antioxidant enzyme activities in patients with type 2 diabetes mellitus. Acta Diabetol. 2002, 39, 117–122.
- Ceriello, A.; Quagliaro, L.; Catone, B.; Pascon, R.; Piazzola, M.; Bais, B.; Marra, G.; Tonutti, L.; Taboga, C.; Motz, E. Role of Hyperglycemia in Nitrotyrosine Postprandial Generation. Diabetes Care 2002, 25, 1439–1443.
- Likidlilid, A.; Patchanans, N.; Peerapatdit, T.; Sriratanasathavorn, C. Lipid peroxidation and antioxidant enzyme activities in erythrocytes of type 2 diabetic patients. J. Med. Assoc. Thai. 2010, 93, 682–693.
- Davì, G.; Falco, A.; Patrono, C. Lipid Peroxidation in Diabetes Mellitus. Antioxid. Redox Signal. 2005, 7, 256–268.
- de Souza Bastos, A.; Graves, D.T.; de Melo Loureiro, A.P.; Júnior, C.R.; Corbi, S.C.T.; Frizzera, F.; Scarel-Caminaga, R.M.; Câmara, N.O.; Andriankaja, O.M.; Hiyane, M.I.; et al. Diabetes and increased lipid peroxidation are associated with systemic inflammation even in well-controlled patients. J. Diabetes Complicat. 2016, 30, 1593–1599.
- Rendra, E.; Riabov, V.; Mossel, D.M.; Sevastyanova, T.; Harmsen, M.C.; Kzhyshkowska, J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology 2019, 224, 242–253.
- Tolman, E.L.; Barris, E.; Burns, M.; Pansini, A.; Partridge, R. Effects of vanadium on glucose metabolism. Life Sci. 1979, 25, 1159–1164.
- McNeill, J.H.; Yuen, V.G.; Hoveyda, H.R.; Orvig, C. Bis(maltolato)oxovanadium(IV) is a potent insulin mimic. J. Med. Chem. 1992, 35, 1489–1491.
- Thompson, K.H.; Orvig, C. Vanadium in diabetes: 100 years from Phase 0 to Phase I. J. Inorg. Biochem. 2006, 100, 1925–1935.
- Thompson, K.H.; Lichter, J.; LeBel, C.; Scaife, M.C.; McNeill, J.H.; Orvig, C. Vanadium treatment of type 2 diabetes: A view to the future. J. Inorg. Biochem. 2009, 103, 554–558.
- Oster, M.H.; Llobet, J.M.; Domingo, J.L.; Bruce German, J.; Keen, C.L. Vanadium treatment of diabetic Sprague-Dawley rats results in tissue vanadium accumulation and pro-oxidant effects. Toxicology 1993, 83, 115–130.
- Sekar, N.; Kanthasamy, A.; William, S.; Balasubramaniyan, N.; Govindasamy, S. Antioxidant effect of vanadate on experimental diabetic rats. Acta Diabetol. Lat. 1990, 27, 285–293.
- Ghareeb, D.A.; Hussen, H.M. Vanadium improves brain acetylcholinesterase activity on early stage alloxan-diabetic rats. Neurosci. Lett. 2008, 436, 44–47.
- Siddiqui, M.R.; Taha, A.; Moorthy, K.; Hussain, M.E.; Basir, S.F.; Baquer, N.Z. Amelioration of altered antioxidant status and membrane linked functions by vanadium andTrigonella in alloxan diabetic rat brains. J. Biosci. 2005, 30, 483–490.
- Siddiqui, M.R.; Moorthy, K.; Taha, A.; Hussain, M.E.; Baquer, N.Z. Low doses of vanadate and Trigonella synergistically regulate Na+/K+-ATPase activity and GLUT4 translocation in alloxan-diabetic rats. Mol. Cell. Biochem. 2006, 285, 17–27.
- Preet, A.; Siddiqui, M.R.; Taha, A.; Badhai, J.; Hussain, M.E.; Yadava, P.K.; Baquer, N.Z. Long-term effect of Trigonella foenum graecum and its combination with sodium orthovanadate in preventing histopathological and biochemical abnormalities in diabetic rat ocular tissues. Mol. Cell. Biochem. 2006, 289, 137–147.
- Kumar, P.; Taha, A.; Kale, R.K.; McLean, P.; Baquer, N.Z. Beneficial effects of Trigonella foenum graecum and sodium orthovanadate on metabolic parameters in experimental diabetes. Cell Biochem. Funct. 2012, 30, 464–473.
- Kumar, P.; Taha, A.; Kumar, N.; Kumar, V.; Baquer, N.Z. Sodium Orthovanadate and Trigonella Foenum Graecum Prevents Neuronal Parameters Decline and Impaired Glucose Homeostasis in Alloxan Diabetic Rats. Prague Med. Rep. 2015, 116, 122–138.
- Tunali, S.; Yanardag, R. Effect of vanadyl sulfate on the status of lipid parameters and on stomach and spleen tissues of streptozotocin-induced diabetic rats. Pharmacol. Res. 2006, 53, 271–277.
- Yanardag, R.; Tunali, S. Vanadyl Sulfate Administration Protects the Streptozotocin-Induced Oxidative Damage to Brain Tissue in Rats. Mol. Cell. Biochem. 2006, 286, 153–159.
- Akgün-Dar, K.; Bolkent, S.; Yanardag, R.; Tunalı, S. Vanadyl sulfate protects against streptozotocin-induced morphological and biochemical changes in rat aorta. Cell Biochem. Funct. 2007, 25, 603–609.
- Tunali, S.; Yanardag, R. Protective effect of vanadyl sulfate on skin injury in streptozotocin-induced diabetic rats. Hum. Exp. Toxicol. 2013, 32, 1206–1212.
- Al-Salmi, F.A.; Hamza, R.Z. Efficacy of Vanadyl Sulfate and Selenium Tetrachloride as Anti-Diabetic Agents against Hyperglycemia and Oxidative Stress Induced by Diabetes Mellitus in Male Rats. Curr. Issues Mol. Biol. 2021, 44, 94–104.
- Tunalı, S.; Yanardağ, R. The effects of vanadyl sulfate on glutathione, lipid peroxidation and nonenzymatic glycosylation levels in various tissues in experimental diabetes. İstanbul J. Pharm. 2021, 51, 73–78.
- Ramachandran, B.; Ravi, K.; Narayanan, V.; Kandaswamy, M.; Subramanian, S. Protective effect of macrocyclic binuclear oxovanadium complex on oxidative stress in pancreas of streptozotocin induced diabetic rats. Chem. Biol. Interact. 2004, 149, 9–21.
- Ramachandran, B.; Ravi, K.; Narayanan, V.; Kandaswamy, M.; Subramanian, S. Effect of macrocyclic binuclear oxovanadium complex on tissue defense system in streptozotocin-induced diabetic rats. Clin. Chim. Acta 2004, 345, 141–150.
- Yanardag, R.; Demirci, T.B.; Ülküseven, B.; Bolkent, S.; Tunali, S.; Bolkent, S. Synthesis, characterization and antidiabetic properties of N1-2,4-dihydroxybenzylidene-N4-2-hydroxybenzylidene-S-methyl-thiosemicarbazidato-oxovanadium(IV). Eur. J. Med. Chem. 2009, 44, 818–826.
- Tunali, S.; Bal-Demirci, T.; Ulkuseven, B.; Yanardag, R. Protective effects of N(1)-2,4-dihydroxybenzylidene-N(4)-2-hydroxybenzylidene-S-methyl-thiosemicarbazidato-oxovanadium (IV) on oxidative brain injury in streptozotocin-induced diabetic rats. J. Biochem. Mol. Toxicol. 2022, 36, e22991.
- Thompson, K.H.; McNeill, J.H. Effect of vanadyl sulfate feeding on susceptibility to peroxidative change in diabetic rats. Res. Commun. Chem. Pathol. Pharmacol. 1993, 80, 187–200.
- Koyuturk, M.; Tunali, S.; Bolkent, S.; Yanardag, R. Effects of Vanadyl Sulfate on Liver of Streptozotocin-Induced Diabetic Rats. Biol. Trace Elem. Res. 2005, 104, 233–248.
- Treviño, S.; González-Vergara, E. Metformin-decavanadate treatment ameliorates hyperglycemia and redox balance of the liver and muscle in a rat model of alloxan-induced diabetes. New J. Chem. 2019, 43, 17850–17862.
- Pereira, M.J.; Carvalho, E.; Eriksson, J.W.; Crans, D.C.; Aureliano, M. Effects of decavanadate and insulin enhancing vanadium compounds on glucose uptake in isolated rat adipocytes. J. Inorg. Biochem. 2009, 103, 1687–1692.
- Aureliano, M. Recent perspectives into biochemistry of decavanadate. World J. Biol. Chem. 2011, 2, 215.
- Treviño, S.; Velázquez-Vázquez, D.; Sánchez-Lara, E.; Diaz-Fonseca, A.; Flores-Hernandez, J.Á.; Pérez-Benítez, A.; Brambila-Colombres, E.; González-Vergara, E. Metforminium Decavanadate as a Potential Metallopharmaceutical Drug for the Treatment of Diabetes Mellitus. Oxid. Med. Cell. Longev. 2016, 2016, 6058705.
- Xie, M.; Chen, D.; Zhang, F.; Willsky, G.R.; Crans, D.C.; Ding, W. Effects of vanadium (III, IV, V)-chlorodipicolinate on glycolysis and antioxidant status in the liver of STZ-induced diabetic rats. J. Inorg. Biochem. 2014, 136, 47–56.
- Usende, I.L.; Leitner, D.F.; Neely, E.; Connor, J.R.; Olopade, J.O. The Deterioration Seen in Myelin Related Morphophysiology in Vanadium Exposed Rats is Partially Protected by Concurrent Iron Deficiency. Niger. J. Physiol. Sci. 2016, 3, 11–22.
- Folarin, O.R.; Snyder, A.M.; Peters, D.G.; Olopade, F.; Connor, J.R.; Olopade, J.O. Brain Metal Distribution and Neuro-Inflammatory Profiles after Chronic Vanadium Administration and Withdrawal in Mice. Front. Neuroanat. 2017, 11, 58.
- Garcia, S.J.; Gellein, K.; Syversen, T.; Aschner, M. Iron Deficient and Manganese Supplemented Diets Alter Metals and Transporters in the Developing Rat Brain. Toxicol. Sci. 2007, 95, 205–214.
- Adekeye, A.O.; Irawo, G.J.; Fafure, A.A. Ficus exasperata Vahl leaves extract attenuates motor deficit in vanadium-induced parkinsonism mice. Anat. Cell Biol. 2020, 53, 183–193.
- Farina, M.; Avila, D.S.; da Rocha, J.B.T.; Aschner, M. Metals, oxidative stress and neurodegeneration: A focus on iron, manganese and mercury. Neurochem. Int. 2013, 62, 575–594.
- HaMai, D.; Campbell, A.; Bondy, S.C. Modulation of oxidative events by multivalent manganese complexes in brain tissue. Free Radic. Biol. Med. 2001, 31, 763–768.
- Latchoumycandane, C.; Anantharam, V.; Kitazawa, M.; Yang, Y.; Kanthasamy, A.; Kanthasamy, A.G. Protein Kinase Cδ Is a Key Downstream Mediator of Manganese-Induced Apoptosis in Dopaminergic Neuronal Cells. J. Pharmacol. Exp. Ther. 2005, 313, 46–55.
- Harischandra, D.S.; Ghaisas, S.; Zenitsky, G.; Jin, H.; Kanthasamy, A.; Anantharam, V.; Kanthasamy, A.G. Manganese-Induced Neurotoxicity: New Insights Into the Triad of Protein Misfolding, Mitochondrial Impairment, and Neuroinflammation. Front. Neurosci. 2019, 13, 654.
- Afeseh Ngwa, H.; Kanthasamy, A.; Anantharam, V.; Song, C.; Witte, T.; Houk, R.; Kanthasamy, A.G. Vanadium induces dopaminergic neurotoxicity via protein kinase Cdelta dependent oxidative signaling mechanisms: Relevance to etiopathogenesis of Parkinson’s disease. Toxicol. Appl. Pharmacol. 2009, 240, 273–285.
- Ohiomokhare, S.; Olaolorun, F.; Ladagu, A.; Olopade, F.; Howes, M.-J.R.; Okello, E.; Olopade, J.; Chazot, P.L. The Pathopharmacological Interplay between Vanadium and Iron in Parkinson’s Disease Models. Int. J. Mol. Sci. 2020, 21, 6719.
- Bush, A.I. The metallobiology of Alzheimer’s disease. Trends Neurosci. 2003, 26, 207–214.
- Markesbery, W.; Lovell, M. Four-Hydroxynonenal, a Product of Lipid Peroxidation, is Increased in the Brain in Alzheimer’s Disease. Neurobiol. Aging 1998, 19, 33–36.
- Zhao, Y.; Zhao, B. Oxidative Stress and the Pathogenesis of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2013, 2013, 316523.
- Montiel-Flores, E.; Mejía-García, O.A.; Ordoñez-Librado, J.L.; Gutierrez-Valdez, A.L.; Espinosa-Villanueva, J.; Dorado-Martínez, C.; Reynoso-Erazo, L.; Tron-Alvarez, R.; Rodríguez-Lara, V.; Avila-Costa, M.R. Alzheimer-like cell death after vanadium pentoxide inhalation. Heliyon 2021, 7, e07856.
- He, Z.; You, G.; Liu, Q.; Li, N. Alzheimer’s Disease and Diabetes Mellitus in Comparison: The Therapeutic Efficacy of the Vanadium Compound. Int. J. Mol. Sci. 2021, 22, 11931.
- Dong, Y.; Stewart, T.; Zhang, Y.; Shi, M.; Tan, C.; Li, X.; Yuan, L.; Mehrotra, A.; Zhang, J.; Yang, X. Anti-diabetic vanadyl complexes reduced Alzheimer’s disease pathology independent of amyloid plaque deposition. Sci. China Life Sci. 2019, 62, 126–139.
- He, L.; Wang, X.; Zhu, D.; Zhao, C.; Du, W. Methionine oxidation of amyloid peptides by peroxovanadium complexes: Inhibition of fibril formation through a distinct mechanism. Metallomics 2015, 7, 1562–1572.
- He, Z.; Han, S.; Zhu, H.; Hu, X.; Li, X.; Hou, C.; Wu, C.; Xie, Q.; Li, N.; Du, X.; et al. The Protective Effect of Vanadium on Cognitive Impairment and the Neuropathology of Alzheimer’s Disease in APPSwe/PS1dE9 Mice. Front. Mol. Neurosci. 2020, 13, 21.
- Ścibior, A. Overview of Research on Vanadium-Quercetin Complexes with a Historical Outline. Antioxidants 2022, 11, 790.
- Crans, D.C.; Kostenkova, K. Open questions on the biological roles of first-row transition metals. Commun. Chem. 2020, 3, 104.
- Ghalichi, F.; Ostadrahimi, A.; Saghafi-Asl, M. Vanadium and biomarkers of inflammation and oxidative stress in diabetes: A systematic review of animal studies. Health Promot. Perspect. 2022, 12, 122–130.
- Kaur, M.; Kaushal, R. Synthesis and in-silico molecular modelling, DFT studies, antiradical and antihyperglycemic activity of novel vanadyl complexes based on chalcone derivatives. J. Mol. Struct. 2022, 1252, 132176.
- Zhang, B.; Li, Y.; Fei, Y.; Cheng, Y. Novel Pathway for Vanadium(V) Bio-Detoxification by Gram-Positive Lactococcus raffinolactis. Environ. Sci. Technol. 2021, 55, 2121–2131.
More
Information
Subjects:
Biochemistry & Molecular Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
849
Revisions:
4 times
(View History)
Update Date:
31 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




