Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Irene Dini | -- | 2889 | 2023-03-30 18:27:10 | | | |
| 2 | Peter Tang | Meta information modification | 2889 | 2023-03-31 03:12:19 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Dini, I.; Mancusi, A. Food Peptides for the Nutricosmetic Industry. Encyclopedia. Available online: https://encyclopedia.pub/entry/42668 (accessed on 14 January 2026).
Dini I, Mancusi A. Food Peptides for the Nutricosmetic Industry. Encyclopedia. Available at: https://encyclopedia.pub/entry/42668. Accessed January 14, 2026.
Dini, Irene, Andrea Mancusi. "Food Peptides for the Nutricosmetic Industry" Encyclopedia, https://encyclopedia.pub/entry/42668 (accessed January 14, 2026).
Dini, I., & Mancusi, A. (2023, March 30). Food Peptides for the Nutricosmetic Industry. In Encyclopedia. https://encyclopedia.pub/entry/42668
Dini, Irene and Andrea Mancusi. "Food Peptides for the Nutricosmetic Industry." Encyclopedia. Web. 30 March, 2023.
Copy Citation
Biopeptides are considered interesting for industrial application since they show numerous functional properties (e.g., anti-aging, antioxidant, anti-inflammatory, and antimicrobial properties) and technological properties (e.g., solubility, emulsifying, and foaming). Moreover, they have fewer side effects than synthetic drugs. Nevertheless, some challenges must be overcome before their administration via the oral route. The gastric, pancreatic, and small intestinal enzymes and acidic stomach conditions can affect their bioavailability and the levels that can reach the site of action. Some delivery systems have been studied to avoid these problems (e.g., microemulsions, liposomes, solid lipid particles).
food antioxidant peptides
food analytical methods
large-scale biopeptide production
supplements
delivery systems
nutricosmetic
cosmeceutical
circular economy
waste recycling
anti-aging
skincare market
1. Introduction
The cosmetic industry considers food peptides as innovative bioactive compounds for cosmetics market growth. According to the Food and Drug Administration (FDA; responsible for health products’ regulation in the USA), peptides are defined as amino acid polymers with a specific sequence and less than 40 amino acids in total [1]. According to their intended action mechanism, cosmetic peptides can be categorized into: signal peptides (which stimulate matrix protein production, cell growth, and other cell metabolic functions); carrier peptides (which help transport of active or trace elements inside the cell); neurotransmitter-inhibiting peptides (which inhibit acetylcholine release that may lead to expression wrinkles); and enzyme-inhibiting peptides (which decrease the activity of enzymes related to skin aging) [2]. Peptides have gained worldwide attention for their sustainability, with no toxic side effects [3]. The global bioactive peptide market was USD 4960.4 million in 2022 with an expected compound annual growth rate (CAGR) of 9.4% in 2022–2030 [4]. The growing number of biopeptides listed in the “European glossary of common ingredient names for use in the labeling of cosmetic products” (there were 2698 entries with the word peptide [5] in the 2022 revision compared to 848 entries in the 2019 revision [6]) demonstrates the market interest in these bioactive compounds. As a result, much research has been performed to optimize biopeptide production from natural sources (e.g., food products and protein-rich by-products of the food industries) and to examine their bioactivity in vitro (cell culture and biochemical assays) and in vivo (animal and human tests). Traditional medicine and modern scientific research consider bioactive peptides useful for formulating food supplements and cosmetic products. Bioactivity, interaction with skin cells by multiple mechanisms, high potency at a low dosage, and size compatible with penetration into the upper skin layers seem to confirm this hypothesis [7].
2. Production Methods for Natural Biopeptides
Natural peptides can be obtained by enzymatic hydrolysis, fermentation, and chemical-physical processes (alkaline or acidic treatments and use of microwaves, ultrasonics, hydrostatic pressure, and pulsed electric fields). Electrophoresis, membrane separation, or chromatography techniques (gel permeation chromatography, ion-exchange chromatography, reversed-phase high-performance liquid chromatography, etc.) can be used for their isolation and spectroscopic technologies (i.e., MS or NMR) as characterization techniques (Figure 1).
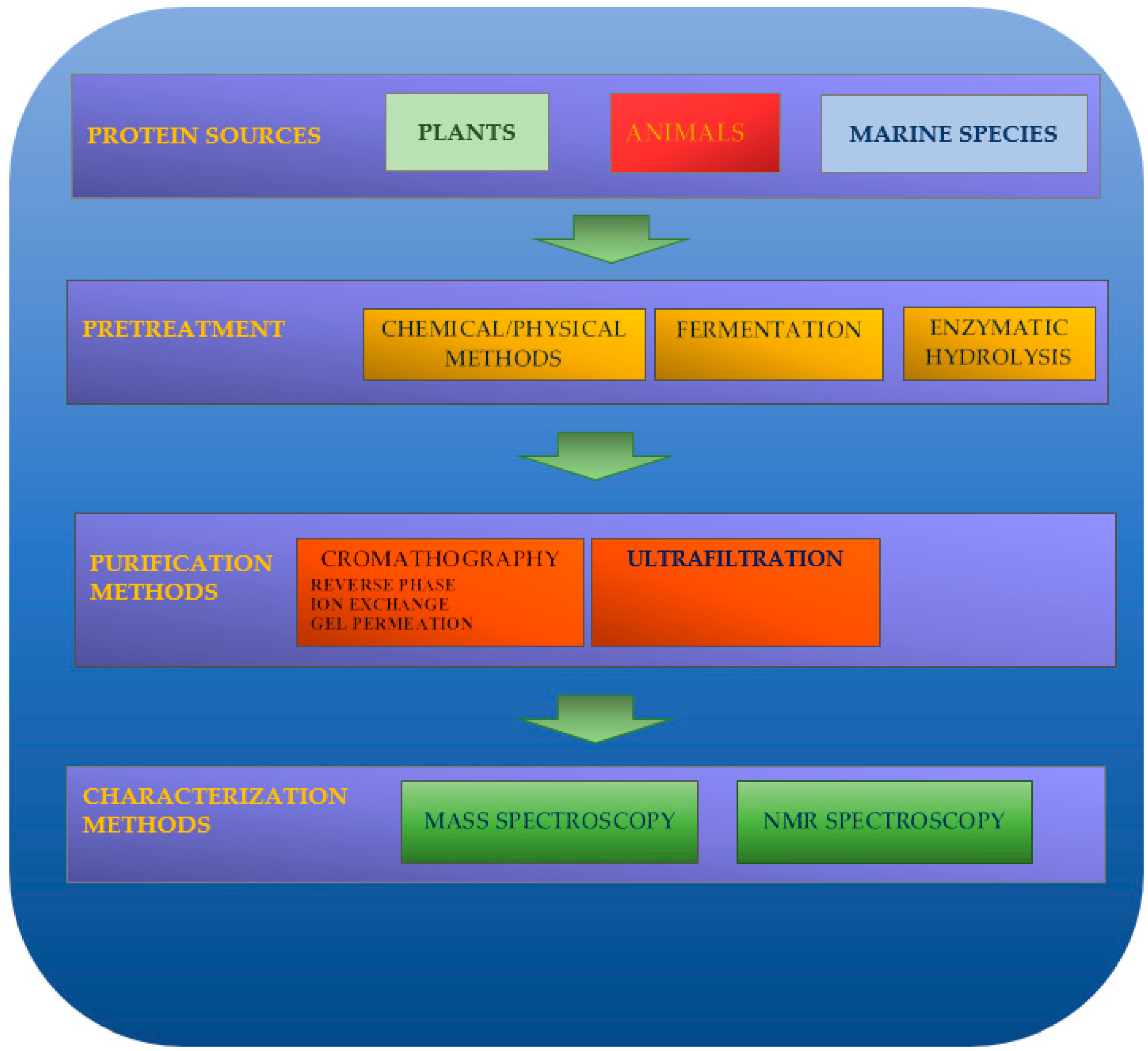
Figure 1. Production of natural peptides.
3. The Human Skin
The human skin is a complex organ with an exceptional structure. It is made up of diverse cell types and compartments with distinctive functions. The epidermis (outermost layer) contains four sublayers (strata corneum, granulosum, spinosum, and basalis) and four major cell types (melanocytes, keratinocytes, Langerhans and Merkel cells). The epidermal–dermal junction (border between the epidermis and dermis) constitutes the basement membrane (an aggregation of proteins and structures). Under the basement membrane is the underlying dermis, which contains dendritic cells, mast cells, macrophages, fibroblasts, elastic fibers, collagen, hair follicles, blood vessels, nerves, lymph vessels, and sweat glands.
The dermis allows nutrients to reach the skin and has a structural support function [8]. Aging affects all skin layers, altering their structure and function [8]. The organism’s aging is inevitably a progressive process. The consequences of aging are changes at the tissue and cellular levels. During physiological skin aging, the number of keratinocytes at the epidermis level gradually decreases. The epithelium layers atrophy. The reproductive layer cell division activity, Langerhans cell and melanocyte numbers decrease. The dermis’ connective tissue also atrophies (its cellular and extracellular matrix components diminish). The fibroblasts synthesize collagen, but the fibers are less elastic and efficient, and protein fibers, already existing, are subject to degeneration. Only the corneocyte number in the dead stratum corneum shows no changes. The accumulation of damage reduces cells’ ability to renew [9]. The decrease in lipid and CD44 glycoprotein (regulator of keratinocyte proliferation) levels, the loss of hyaluronic acid homeostasis, and the reduced cell proliferation in the basal layer contribute to this decline [10][11].
Moreover, the contact surface area between the epidermis and dermis becomes thinner, resulting in a weakened epidermis nutrition supply and a further decline in basal cell proliferation ability [12][13]. The epidermal–dermal junction and dermis also become thinner, causing wrinkle formation since there are fewer cells, with less oxygen and less nutrition. The dermal extracellular matrix (ECM) accumulates type I and type III collagens [14], and there is a decrease in the synthesis of type I/III [15], altering the elastic fiber organization [16]. The low fibroblast levels increase wrinkling and reduce elasticity [17]. Skin aging is associated with extrinsic (external) factors, e.g., UVA, UVB, temperature, environmental pollution, nutritional factors, cigarette smoke, lack of sleep, and stress [18] and with intrinsic (endogenous) factors, e.g., genetic factors, chronological time, hormones, decreased age-related antioxidant capacity, and increase in reactive oxygen species [19][20]. The principal consequences are blemished, dry, pale skin with rugged texture, visible pores, redness, small actinic keratomes, gradual loss of elasticity, and fine wrinkles [21]. The intrinsic clinical skin aging signs caused by intrinsic factors are xerosis (dry skin), fine lines, and laxity [22]. The aging signs caused by extrinsic factors are irregular pigmentation, coarse wrinkles, and lentigines (or age spots). The photo-exposed areas (e.g., the face, hands, and neck) show a more visible occurrence of these changes. The duration and intensity of exposure to environmental factors and the skin type affect the occurrence of extrinsic skin aging signs [22]. Skin aging impacts human aesthetics and increases susceptibility to infections and chronic wounds (e.g., venous, pressure, or diabetic foot ulcers, dermatitis, and melanoma) [23][24].
4. Biopeptides’ Potential in Cosmeceutical Applications
The increased demand for natural cosmetics has led to the formulation of a new generation of cosmetics based on active compounds obtained from natural sources such as biopeptides. Biopeptides can enhance skin health (acting against aging-related enzymes) and decrease the harmful effect of agents that produce skin injuries (acting as antioxidant, antimicrobial, and anti-inflammatory agents). Multifunctional biopeptides, which can simultaneously start, modulate, or impede multiple physiological pathways, are preferred to single-activity peptides [25]. The problems associated with using biopeptides in cosmetics concern the yields of the techniques with which biopeptides are produced (in terms of production quantity and concentration of biopeptides capable of expressing desirable bioactivity) and the biopeptides’ structural stability and bioactivity during product manufacturing and storage. Biopeptide activity is affected by pH, interactions with other components, temperature, water activity, and formulation processes (e.g., concentration, delivery of the active compounds, and packaging) [26][27].
For example, when used in gels, creams, or lotions, the parameters to consider are sensitivity to temperature and pH to guarantee the peptide bioactivity at the action site. Moreover, it is essential to realize that only the bioactive peptides with low molecular weight penetrate the skin. Biopeptides with high molecular weight, hydrophobic character, and poor aqueous solubility at high concentrations require carriers to permit their release when needed [28]. When biopeptides are administrated orally, their bioavailability (integrity during digestion, intestinal absorption, and transport) must be controlled [28] since they are exposed to gastric, pancreatic, small intestinal enzymes, and acidic stomach conditions, meaning that only minimal biopeptide levels (nano-molar or pico-molar concentrations) reach the action site [29]. Finally, some protein-derived peptides have a bitter taste. Therefore, they must be subjected to processes to debitter them and/or mask the bitter taste to enhance the sensory properties of the final product [30]. Transport systems studied to overcome these problems include liposomes, biopolymer microgel emulsions, and solid–lipid nanoparticles [31]. Some natural lipid-based systems (e.g., chitosan fabricated nanocarriers, soy lecithin-derived nanoliposome, and microgels from alginates and methacrylate) were suggested as potential inclusion complexes for biopeptides [32]. Another limitation is the risk of allergens since most peptide preparations are produced as unpurified mixtures of several components. Plant hydrolysates may contain allergens and potentially toxic contaminating compounds (environmental pollutants) [33]. Therefore, peptide preparations from plant tissue cultures (grown in the laboratory, under axenic and controlled conditions) are preferred today [34].
5. Peptide Delivery Systems
Chemical, physical, and biological variability can degrade biopeptides, decrease storage life, and limit their application in different formulations. Chemical instability is due to oxidation reactions, deamination, etc. Physical instability is mainly produced by aggregation, denaturation, and surface adsorption. Biological instability is due to cell enzymes, which may cause degradation or inactivation of the active molecule and loss of biological activity [35]. Using nanocarriers can enhance the biopeptide’s stability and limit side effects. Nanocarriers (e.g., liposomes, niosomes, novasomes, transferosomes, ethosomes, cubosomes, ultrasomes, photosomes, polymerosomes, nanofibres, metal nanoparticles, dendrimers, nanocrystals, carbon nanotubes, fullerene, cyclodextrin nanosponges, solid lipid nanoparticles), hydrogels, and nanoemulsions are carrier systems used for biopeptide delivery.
Liposomes are sphere-shaped vesicles with a hydrophilic core enclosed by at least one phospholipid bilayer. They can enter the skin by merging with the lipids of the stratum corneum or via the sebaceous glands [36]. The liposomes can be made with food-grade materials (biodegradable and non-toxic) and can encapsulate nonpolar, polar, and amphiphilic amino acids [37][38]. Mechanical methods (e.g., sonication, film formation, microfluidization, and extrusion), solvent replacement methods (reverse phase evaporation, injecting ethanol, and proliposome techniques), or detergent removal methods can be used to produce them [39]. The liposomes are used in lipsticks, antiperspirants, creams, deodorants, moisturizers, and hair care formulations. They are employed to improve the solubility of vitamins (e.g., A, E, and K), antioxidants (e.g., lycopene, coenzyme Q10, carotenoids, etc.), and other active biomolecules in water, facilitate the skin’s hydration and restore the skin’s epidermal layers by incorporating lipid compounds (e.g., cholesterols, and ceramides) [40]. They can deliver biopeptides in moisturizing, anti-aging creams, body sprays, deodorants, lotions, sunscreens, fragrances, shampoos, conditioning agents, etc. High production cost and osmotic stability limit their use in cosmetic products [41].
Niosomes contain one to seven bi-lipid layers, a non-ionic surfactant (spans, tweens, alkyl amides, brijs, polyoxyethylene alkyl ethers, and sorbitan esters), and an amorphous central core [42][43]. They are obtained by mixing free fatty acids, cholesterol, and a non-phospholipid surfactant.
Novasomes can deliver hydrophilic and hydrophobic molecules, have a lower production cost than liposomes [38], improve the biopeptides residence time on the dermal layers and skin penetration, decrease the horny layer barrier’s resistance and the biopeptides’ systemic absorption [44]. Novasomes have high molecule entrapment efficiency and a much lower production cost than liposomes. They have a little higher deposition volume on the skin than niosomes [45]. Moreover, they are stable at pH changes between 2 and 13 and temperatures between 0 °C and 100 °C.
Ethosomes are vesicles containing phospholipids with a high concentration of ethanol (20–50%) which improve the bioactive peptides’ permeation across the skin, mediating the disruption of the skin’s lipid layers. Ethasomes with niacinamide are used to decrease aging, pigmentation, skin blotches, and acne [46][47].
Transferosomes are deformable vesicles containing phospholipids and an edge activator (e.g., sodium chlorate, tween 80, and span 80). They can be used as curcumin, capsaicin, and resveratrol vehicles in transdermal skin layers [48], in antiwrinkle [49], and anti-aging cosmetics [50].
Cubosomes are self-assembling honeycomb-shaped liquid crystalline lipid nanoparticles (3D structures obtained from a bi-continuous cubic liquid phase with two aqueous channels divided by a surfactant bilayer) which can contain lipophilic, hydrophilic, and amphiphilic molecules [51][52]. They are used to absorb pollutants and as stabilizers for oil-in-water emulsions [48].
Ultrasomes are liposomes that contain a UV-endonuclease enzyme that repairs UV-damaged DNA and decreases the expression of pro-inflammatory cytokines [53].
Photosomes are liposomal formulations of photolyase. They are incorporated in sunscreen products [54][55].
Polymersomes are artificial vesicular systems containing block copolymers encapsulating lipophilic and/or lipophobic molecules. They have higher stability than liposomes because of their thick and rigid bilayer structure [56][57]. They enhance skin elasticity and increase the skin cells’ activation energy [58].
Biopolymer microgels are small particles comprising a cross-linked polymer molecule network [59]. They can contain natural, synthetic, or bio-polymers (e.g., chitosan, hyaluronic acid, collagen, gelatin, and polyvinyl alcohol), polyacrylamide, xanthan gum, polyethylene glycol, pectin, starch, cellulose, alginate). They can be obtained by coacervation, antisolvent precipitation, and emulsion. Unfortunately, porous microgels can diffuse small peptides. Biopolymer hydrogels are used to produce “beauty masks” [60][61].
Solid lipid particles (SLN) are a colloidal delivery system formed by crystallized lipid particles in an aqueous medium [62]. SLNs are used in cosmetic creams, lotions, and sunscreens [61].
Nanostructured lipid carriers (NLCs) are a mixture of solid and liquid lipids with a less ordered structure that load more active molecules than SLN into their pockets. NLCs are suitable carriers for volatile essential oils [63].
Nanofibers are one-dimensional nanomaterials (e.g., collagen, silk, PVP, and PVA) having a high surface area to volume ratio, high bioactive loading capacity, small diameters, and excellent absorbing capacity. They can be used for production of cleansers, face masks, and skin healing products [64].
Inorganic nanocosmetics are nanoparticles containing metals (e.g., gold, silver, aluminum, platinum, titanium) or metalloids (e.g., silica and selenium). Among metal-based nanoparticles, gold and silver are the most used. Gold has high stability and penetrability, is inert, and is non-cytotoxic. Gold nanoparticles have antioxidant and anti-aging effects, enhance skin elasticity, skin firmness, and blood circulation, and have antibacterial, antifungal, and antiseptic properties [65].
Silver has antimicrobial properties against many microbial species and is an anti-inflammatory agent. Silver nanoparticles (AgNPs) are used in lotions, skin cleansers, creams, shampoos, deodorants, and toothpaste [66].
Inorganic metalloid silica and selenium are the most used in the cosmetic field. Silica has a feel-good texture and excellent penetrability and can enclose hydrophilic and hydrophobic molecules. Silicone-based vesicles are used to deliver vitamins A, C, and E and oils such as jojoba and lanolin, in emollients and creams [69][70].
Silica nanoparticles are employed in lipsticks to homogenize lipstick pigments, in anti-aging/anti-wrinkle creams, and in hair and nail cosmetic products. They can improve cosmetic products’ texture, effectiveness, and shelf-life and act as an anti-caking agent. Moreover, they have high photostability and protect against UV radiation [71].
Dendrimers are macromolecular organic nanocarriers with a network of symmetric branches (the number of branches required determines the production process) arising from a central core, with functional groups attached at their terminal ends [41]. Polyvalence, solubility, monodispersity, low cytotoxicity, self-assembling, chemical stability, and electrostatic interactions are key factors responsible for their high selectivity and precision in the biopeptides’ delivery [72]. Biodegradable polymers (e.g., polysaccharides, poly α-esters, poly alkyl cyanoacrylates, and poly amidoamine dendrimers) are used in cosmetic formulations to benefit hair (e.g., hair-styling gels and shampoos), skin (e.g., anti-acne cream) and nails (e.g., nail polishes), and as sunscreens. Dendrimers were developed to improve resveratrol and vitamins A and B6 (PAMAM dendrimer) solubility and skin infiltration [39] and give a glossy appearance to the skin and hair (carbosiloxane dendrimer able to resist oil and water) [73].
Nanocrystals are clusters of thousands of active agents linked together in a fixed pattern to form a group (sizes ranging from 10 to 400 nm) having a very high surface area to volume ratio and high solubility and bioavailability. They facilitate biopeptide absorption into the skin by creating a high biological adhesion and concentration gradient on the skin surface for long periods. They are usually utilized to administer poorly soluble active compounds [74]. Undissolved nanocrystals can aggregate in hair follicles to produce an active molecules reservoir in addition to intracellular and intercellular pathways [75][76][77].
Fullerenes (or buckyballs) are spherical structures with many carbon atoms [78]. They can deliver biopeptides in cosmetics (e.g., anti-wrinkle, anti-acne, lightening toner, pore reduction, and moisturizing creams) and sunscreen [79][80].
Cyclodextrin nanosponges are natural oligosaccharides (containing 6–8 glucopyranose molecules) with a truncated cone-shaped structure [81]. Cyclodextrin’s lipophilic cavity can encapsulate aromatic molecules, aliphatic hydrocarbons, and vitamins [82]. They are used in perfumes, tanning products, deodorants, laundry detergents, odor removers, underarm odor shields, etc. [83].
Microemulsions (diameter 10 to 100 nm) are classified as water-in-oil (W/O) and oil-in-water (O/W) based on the predominant system’s components. The W/O microemulsions are thermodynamically stable, have noninvasive administration, high solubilization capacity, and are easily formulated but require high concentrations of surfactants to stabilize them [62] and can be only employed in oral formulations that contain mainly oil (e.g., oil-filled soft capsules). Water-dispersible forms can be formulated by homogenizing the W/O microemulsion with water and a hydrophilic emulsifier to form a W/O/W type system. Mortazavi et al. used W/O microemulsion to encapsulate PKEK, a tetrapeptide that can decrease the pigmentation process [84].
The O/W microemulsion can encapsulate hydrophobic biopeptides mixed with a hydrophobic surfactant and a co-surfactant [85][86].
Water-in-oil-in-water (W/O/W) systems are used to encapsulate the water-soluble peptides. They are multicompartment liquid dispersions where the dispersed phase is an emulsion [87]. The double emulsion can mask flavor and odor and regulate bioactive ingredients released during digestion. The type of oil used significantly affects the formation and structure of multiple emulsions and the skin barrier function [88]. Their use is limited by instability [89]. The W1/O/W2 double emulsion system is a helpful delivery matrix for hydrophilic biopeptides, as shown by Ying et al. [90], who prepared applications of W1/O/W2 double emulsions containing soy peptides by a two-step emulsification process and Giroux et al. [91] who encapsulated β-lactoglobulin hydrolysate using a W1/O/W2 emulsion system, obtaining a peptides’ release inversely correlated to the oil’s viscosity and peptides’ hydrophobicity.
References
- Cosmetic Ingredient Review. Safety Assessment of Soy Proteins and Peptides as Used in Cosmetics. Available online: https://www.cir-safety.org/sites/default/files/soypep092015final.pdf (accessed on 30 April 2020).
- Ferreira, M.S.; Magalhães, M.C.; Sousa-Lobo, J.M.; Almeida, I.F. Trending Anti-Aging Peptides. Cosmetics 2020, 7, 91.
- Tkaczewska, J. Peptides and protein hydrolysates as food preservatives and bioactive components of edible films and coatings—A review. Trends Food Sci. Technol. 2020, 106, 298–311.
- Bioactive Peptides Market Analysis. Available online: https://www.coherentmarketinsights.com/market-insight/bioactive-peptide-market-3018 (accessed on 1 September 2022).
- European Commission. Commission Decision (EU) 2022/677 of 29 April 2022 establishing a glossary of common ingredient names for use in the labelling of cosmetic products. Off. J. Eur. Union 2022, 127, 1–448.
- European Commission. Commission Decision (EU) 2019/701 of 5 April 2019 establishing a glossary of common ingredient names for use in the labelling of cosmetic products. Off. J. Eur. Union 2019, 121, 1–370.
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Plant protein-derived antioxidant peptides: Isolation, identification, mechanism of action and application in food systems: A review. Trends Food Sci. Technol. 2020, 105, 308–322.
- Barbieri, J.S.; Wanat, K.; Seykora, J. Skin: Basic Structure and Function; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 9780123864567.
- Bonté, F.; Girard, D.; Archambault, J.C.; Desmoulière, A. Skin Changes during Ageing. Subcell Biochem. 2019, 91, 249–280.
- Jensen, J.-M.; Förl, M.; Winoto-Morbach, S.; Seite, S.; Schunck, M.; Proksch, E.; Schütze, S. Acid and neutral sphingomyelinase, ceramide synthase, and acid ceramidase activities in cutaneous aging. Exp. Dermatol. 2005, 14, 609–618.
- Kaya, G.; Tran, C.; Sorg, O.; Hotz, R.; Grand, D.; Carraux, P.; Didierjean, L.; Stamenkovic, I.; Saurat, J.-H. Hyaluronate Fragments Reverse Skin Atrophy by a CD44-Dependent Mechanism. PLoS Med. 2006, 3, e493.
- Makrantonaki, E.; Zouboulis, C.C.; William, J. Cunliffe Scientific Awards. Characteristics and pathomechanisms of endogenously aged skin. Dermatology 2007, 214, 352–360.
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217.
- Lovell, C.R.; Smolenski, K.A.; Duance, V.C.; Light, N.D.; Young, S.; Dyson, M. Type I and III collagen content and fibre distribution in normal human skin during ageing. Br. J. Dermatol. 1987, 117, 419–428.
- Autio, P.; Risteli, J.; Haukipuro, K.; Risteli, L.; Oikarinen, A. Collagen synthesis in human skin in vivo: Modulation by aging, ultraviolet B irradiation and localization. Photodermatol. Photoimmunol. Photomed. 1994, 10, 212–216.
- Csekes, E.; Račková, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641.
- Wang, A.S.; Dreesen, O. Biomarkers of cellular senescence and skin aging. Front. Genet. 2018, 9, 247.
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161.
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036.
- Dąbrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The relationship between skin function, barrier properties, and body-dependent factors. Ski. Res. Technol. 2018, 24, 165–174.
- Law, M.H.; Medland, S.E.; Zhu, G.; Yazar, S.; Viñuela, A.; Wallace, L.; Shekar, S.N.; Duffy, D.L.; Bataille, V.; Glass, D.; et al. Genome-wide association shows that pigmentation genes play a role in skin aging. J. Investig. Dermatol. 2017, 137, 1887–1894.
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-Induced (Extrinsic) Skin Aging: Exposomal Factors and Underlying Mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103.
- Blume-Peytavi, U.; Kottner, J.; Sterry, W.; Hodin, M.W.; Griffiths, T.W.; Watson, R.E.B.; Hay, R.J.; Griffiths, C.E.M. Age-Associated Skin Conditions and Diseases: Current Perspectives and Future Options. Gerontologist 2016, 56, S230–S242.
- Kurban, R.S.; Kurban, A.K. Common skin disorders of aging: Diagnosis and treatment. Geriatrics 1993, 48, 30–31, 35–36, 39–42.
- Aguilar-Toalá, J.; Santiago-López, L.; Peres, C.; Garcia, H.; Vallejo-Cordoba, B.; González-Córdova, A.; Hernández-Mendoza, A. Assessment of multifunctional activity of bioactive peptides derived from fermented milk by specific Lactobacillus plantarum strains. J. Dairy Sci. 2017, 100, 65–75.
- Amigo, L.; Hernández-Ledesma, B. Current Evidence on the Bioavailability of Food Bioactive Peptides. Molecules 2020, 25, 4479.
- Sedighi, M.; Shrestha, N.; Mahmoudi, Z.; Khademi, Z.; Ghasempour, A.; Dehghan, H.; Talebi, S.F.; Toolabi, M.; Préat, V.; Chen, B.; et al. Multifunctional Self-Assembled Peptide Hydrogels for Biomedical Applications. Polymers 2023, 15, 1160.
- Udenigwe, C.C.; Fogliano, V. Food matrix interaction and bioavailability of bioactive peptides: Two faces of the same coin? J. Funct. Foods 2017, 35, 9–12.
- Li-Chan, E.C.Y. Bioactive peptides and protein hydrolysates may pass into the systemic circulation: Research trends and challenges for application as nutraceuticals and functional food ingredients. Curr. Opin. Food Sci. 2015, 1, 28–37.
- Ying, X.; Agyei, D.; Udenigwe, C.; Adhikari, B.; Wang, B. Manufacturing of Plant-Based Bioactive Peptides Using Enzymatic Methods to Meet Health and Sustainability Targets of the Sustainable Development Goals. Front. Sustain. Food Syst. 2021, 5, 769028.
- Manzanares, P.; Gandía, M.; Garrigues, S.; Marcos, J.F. Improving Health-Promoting Effects of Food-Derived Bioactive Peptides through Rational Design and Oral Delivery Strategies. Nutrients 2019, 11, 2545.
- McClements, D.J. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: A review. Adv. Colloid Interfac. Sci. 2018, 253, 1–22.
- Patil, P.J.; Usman, M.; Zhang, C.N.; Mehmood, A.; Zhou, M.C.; Teng, C.; Li, X.T. An updated review on food-derived bioactive peptides: Focus on the regulatory requirements, safety, and bioavailability. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1732–1776.
- Apone, F.; Barbulova, A.; Colucci, M.G. Plant and Microalgae Derived Peptides Are Advantageously Employed as Bioactive Compounds in Cosmetics. Front. Plant Sci. 2019, 10, 756.
- Swain, S.; Mondal, D.; Beg, S.; Niranjan Patra, C.; Chandra Dinda, S.; Sruti, J.; Eswara Bhanoji Rao, M. Stabilization and delivery approaches for protein and peptide pharmaceuticals: An extensive review of patents. Recent Pat. Biotechnol. 2013, 7, 28–46.
- Barua, S.; Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 2014, 9, 223–243.
- Liu, X.; Wang, P.; Zou, Y.X.; Luo, Z.G.; Tamer, T.M. Co-encapsulation of Vitamin C and beta-Carotene in liposomes: Storage stability, antioxidant activity, and in vitro gastrointestinal digestion. Food Res. Int. 2020, 136, 109587.
- Ge, X.; Wei, M.; He, S.; Yuan, W.-E. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics 2019, 11, 55.
- Dubey, S.K.; Dey, A.; Singhvi, G.; Pandey, M.M.; Singh, V.; Kesharwani, P. Emerging trends of nanotechnology in advanced cosmetics. Colloids Surf. B Biointerfaces 2022, 214, 112440.
- Yasamineh, S.; Yasamineh, P.; Ghafouri Kalajahi, H.; Gholizadeh, O.; Yekanipour, Z.; Afkhami, H.; Eslami, M.; Hossein Kheirkhah, A.; Taghizadeh, M.; Yazdani, Y.; et al. A State-of-the-Art Review on the Recent Advances of Niosomes as a Targeted Drug Delivery System. Int. J. Pharm. 2022, 624, 121878.
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of nanotechnology in cosmeceuticals: A review of recent advances. J. Pharm. 2018, 2018, 3420204.
- Trucillo, P.; Campardelli, R.; Reverchon, E. Liposomes: From Bangham to Supercritical Fluids. Processes 2020, 8, 1022.
- Sguizzato, M.; Pepe, A.; Baldisserotto, A.; Barbari, R.; Montesi, L.; Drechsler, M.; Mariani, P.; Cortesi, R. Niosomes for Topical Application of Antioxidant Molecules: Design and In Vitro Behavior. Gels 2023, 9, 107.
- Kaur, I.P.; Agrawal, R. Nanotechnology: A new paradigm in cosmeceuticals. Recent Pat. Drug Deliv. Formul. 2007, 1, 171–182.
- Mosallam, S.; Ragaie, M.H.; Moftah, N.H.; Elshafeey, A.H.; Abdelbary, A.A. Use of novasomes as a vesicular carrier for improving the topical delivery of terconazole: In vitro characterization, in vivo assessment and exploratory clinical experimentation. Int. J. Nanomed. 2021, 16, 119–132.
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A promising nanoencapsulation technique for transdermal drug delivery. Pharmaceutics 2020, 12, 855.
- Saraf, G.J.S. Topical Delivery of Curcuma longa Extract Loaded Nanosized Ethosomes to Combat Facial Wrinkles Research Article. J. Pharm. Drug Deliv. Res. 2014, 3, 1.
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Ansari, M.J.; Aldawsari, M.F.; Alalaiwe, A.S.; Mirza, M.A.; Iqbal, Z. Nanotechnology in Cosmetics and Cosmeceuticals—A Review of Latest Advancements. Gels 2022, 8, 173.
- Wu, P.-S.; Li, Y.-S.; Kuo, Y.-C.; Tsai, S.-J.J.; Lin, C.-C. Preparation and Evaluation of Novel Transfersomes Combined with the Natural Antioxidant Resveratrol. Molecules 2019, 24, 600.
- Yang, J.; Kim, B. Synthesis and characterization of ethosomal carriers containing cosmetic ingredients for enhanced transdermal delivery of cosmetic ingredients. Korean J. Chem. Eng. 2018, 35, 792–797.
- Yaghmur, A.; Mu, H. Recent advances in drug delivery applications of cubosomes, hexosomes, and solid lipid nanoparticles. Acta Pharm. Sin. B 2021, 11, 871–885.
- Fytianos, G.; Rahdar, A.; Kyzas, G.Z. Nanomaterials in Cosmetics: Recent Updates. Nanomaterials 2020, 10, 979.
- Kim, B.; Cho, H.-E.; Moon, S.H.; Ahn, H.-J.; Bae, S.; Cho, H.-D.; An, S. Transdermal delivery systems in cosmetics. Biomed. Dermatol. 2020, 4, 10.
- Decome, L.; De Méo, M.; Geffard, A.; Doucet, O.; Duménil, G.; Botta, A. Evaluation of photolyase (Photosome®) repair activity in human keratinocytes after a single dose of ultraviolet B irradiation using the comet assay. J. Photochem. Photobiol. B Biol. 2005, 79, 101–108.
- Patravale, V.B.; Mandawgade, S.D. Novel cosmetic delivery systems: An application update. Int. J. Cosmet. Sci. 2008, 30, 19–33.
- Zhang, X.-Y.; Zhang, P.-Y. Polymersomes in nanomedicine—A review. Curr. Nanosci. 2017, 13, 124–129.
- Souto, E.B.; Fernandes, A.R.; Martins-Gomes, C.; Coutinho, T.E.; Durazzo, A.; Lucarini, M.; Souto, S.B.; Silva, A.M.; Santini, A. Nanomaterials for Skin Delivery of Cosmeceuticals and Pharmaceuticals. Appl. Sci. 2020, 10, 1594.
- Bermudez, H.; Brannan, A.K.; Hammer, D.A.; Bates, F.S.; Discher, D.E. Molecular weight dependence of polymersome membrane structure, elasticity, and stability. Macromolecules 2002, 35, 8203–8208.
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427.
- Allamraju, K.V. Green Hydrogels. Green Compos. 2021, 225–249.
- Pramanik, B.; Ahmed, S. Peptide-Based Low Molecular Weight Photosensitive Supramolecular Gelators. Gels 2022, 8, 533.
- Perry, S.L.; McClements, D.J. Recent Advances in Encapsulation, Protection, and Oral Delivery of Bioactive Proteins and Peptides using Colloidal Systems. Molecules 2020, 25, 1161.
- Nair, A.; Mallya, R.; Suvarna, V.; Khan, T.A.; Momin, M.; Omri, A. Nanoparticles—Attractive Carriers of Antimicrobial Essential Oils. Antibiotics 2022, 11, 108.
- Rasouli, R.; Barhoum, A.; Bechelany, M.; Dufresne, A. Nanofibers for Biomedical and Healthcare Applications. Macromol. Biosci. 2018, 19, e1800256.
- Haddada, M.B.; Gerometta, E.; Chawech, R.; Sorres, J.; Bialecki, A.; Pesnel, S.; Morel, A.L. Assessment of antioxidant and dermoprotective activities of gold nanoparticles as safe cosmetic ingredient. Colloids Surf. B Biointerfaces 2020, 189, 110855.
- Mondéjar-López, M.; López-Jiménez, A.J.; Abad-Jordá, M.; Rubio-Moraga, A.; Ahraz, O.; Gómez-Gómez, L.; Niza, E. Biogenic Silver Nanoparticles from Iris tuberosa as Potential Preservative in Cosmetic Products. Molecules 2021, 26, 4696.
- Lee, C.-C.; Lin, Y.-H.; Hou, W.-C.; Li, M.-H.; Chang, J.-W. Exposure to ZnO/TiO2 Nanoparticles Affects Health Outcomes in Cosmetics Salesclerks. Int. J. Environ. Res. Public Health 2020, 17, 6088.
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292.
- Walters, K.A.; Lane, M.E. Dermal and Transdermal Drug Delivery Systems. In Dermal Drug Delivery, 1st ed.; Ghosh, T.K., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 1–60.
- Gugleva, V.; Ivanova, N.; Sotirova, Y.; Andonova, V. Dermal Drug Delivery of Phytochemicals with Phenolic Structure via Lipid-Based Nanotechnologies. Pharmaceuticals 2021, 14, 837.
- Morais, R.P.; Hochheim, S.; de Oliveira, C.C.; Riegel-Vidotti, I.C.; Marino, C.E.B. Skin interaction, permeation, and toxicity of silica nanoparticles: Challenges and recent therapeutic and cosmetic advances. Int. J. Pharm. 2022, 614, 121439.
- Bilal, M.; Iqbal, H.M.N. New Insights on Unique Features and Role of Nanostructured Materials in Cosmetics. Cosmetics 2020, 7, 24.
- Pentek, T.; Newenhouse, E.; O’Brien, B.; Singh Chauhan, A. Development of a Topical Resveratrol Formulation for Commercial Applications Using Dendrimer Nanotechnology. Molecules 2017, 22, 137.
- Alkilani, A.Z.; Nasereddin, J.; Hamed, R.; Nimrawi, S.; Hussein, G.; Abo-Zour, H.; Donnelly, R.F. Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems. Pharmaceutics 2022, 14, 1152.
- Zhou, H.; Luo, D.; Chen, D.; Tan, X.; Bai, X.; Liu, Z.; Yang, X.; Liu, W. Current Advances of Nanocarrier Technology-Based Active Cosmetic Ingredients for Beauty Applications. Clin. Cosmet. Investig. Dermatol. 2021, 14, 867–887.
- Eckert, R.W.; Wiemann, S.; Keck, C.M. Improved dermal and transdermal delivery of curcumin with smartfilms and nanocrystals. Molecules 2021, 26, 1633.
- Wadhawan, J.; Parmar, P.K.; Bansal, A.K. Nanocrystals for improved topical delivery of medium soluble drug: A case study of acyclovir. J. Drug Deliv. Sci. Technol. 2021, 65, 102662.
- Kazemzadeh, H.; Mozafari, M. Fullerene-based delivery systems. Drug Discov. Today 2019, 24, 898–905.
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133.
- Miljkovic, S.; Jeftic, B.; Stankovic, I.; Stojiljkovic, N.; Koruga, D. Mechanisms of skin moisturization with hyperharmonized hydroxyl modified fullerene substance. J. Cosmet. Dermatol. 2021, 20, 3018–3025.
- Krabicová, I.; Appleton, S.L.; Tannous, M.; Hoti, G.; Caldera, F.; Rubin Pedrazzo, A.; Cecone, C.; Cavalli, R.; Trotta, F. History of Cyclodextrin Nanosponges. Polymers 2020, 12, 1122.
- Torne, S.J.; Ansari, K.A.; Vavia, P.R.; Trotta, F.; Cavalli, R. Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded nanosponges. Drug Deliv. 2010, 17, 419–425.
- Trotta, F.; Cavalli, R.; Martina, K.; Biasizzo, M.; Vitillo, J.; Bordiga, S.; Vavia, P.; Ansari, K. Cyclodextrin nanosponges as effective gas carriers. J. Incl. Phenom. Macrocycl. Chem. 2011, 71, 189–194.
- Mortazavi, S.M.; Moghimi, H.R. Skin permeability, a dismissed necessity for anti-wrinkle peptide performance. Int. J. Cosmet. Sci. 2022, 44, 232–248.
- Engelskirchen, S.; Maurer, R.; Levy, T.; Berghaus, R.; Auweter, H.; Glatter, O. Highly concentrated emulsified microemulsions as solvent-free plant protection formulations. J. Colloid Interface Sci. 2012, 388, 151–161.
- Yaghmur, A.; de Campo, L.; Sagalowicz, L.; Leser, M.E.; Glatter, O. Emulsified microemulsions and oil-containing liquid crystalline phases. Langmuir 2005, 21, 569–577.
- Dini, I. Contribution of Nanoscience Research in Antioxidants Delivery Used in Nutricosmetic Sector. Antioxidants 2022, 11, 563.
- Sabri, F.; Raphael, W.; Berthomier, K.; Fradette, L.; Tavares, J.R.; Virgilio, N. One-Step Processing of Highly Viscous Multiple Pickering Emulsions. J. Colloid Interface Sci. 2020, 560, 536–545.
- Lin, C.; Debeli, D.K.; Gan, L.; Deng, J.; Hu, L.; Shan, G. Polyether-modified siloxane stabilized dispersion system on the physical stability and control release of double (W/O/W) emulsions. Food Chem. 2020, 332, 127381.
- Ying, X.; Gao, J.; Lu, J.; Ma, C.; Lv, J.; Adhikari, B.; Wang, B. Preparation and drying of water-in-oil-in-water (W/O/W) double emulsion to encapsulate soy peptides. Food Res. Int. 2021, 141, 110148.
- Giroux, H.J.; Shea, R.; Sabik, H.; Fustier, P.; Robitaille, G.; Britten, M. Effect of oil phase properties on peptide release from water-in-oil-in-water emulsions in gastrointestinal conditions. LWT 2019, 109, 429–435.
More
Information
Subjects:
Chemistry, Medicinal
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.9K
Entry Collection:
Peptides for Health Benefits
Revisions:
2 times
(View History)
Update Date:
31 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




