
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Patrícia C. Pires | -- | 3219 | 2023-03-30 11:06:44 | | | |
| 2 | Lindsay Dong | Meta information modification | 3219 | 2023-03-31 05:23:53 | | |
Video Upload Options
Depression and anxiety are high incidence and debilitating psychiatric disorders, usually treated by antidepressant or anxiolytic drug administration, respectively. Three main strategies have been used to improve brain drug targeting: the intranasal route of administration, which allows the drug to be directly transported to the brain by neuronal pathways, bypassing the blood–brain barrier and avoiding the hepatic and gastrointestinal metabolism; the use of nanosystems for drug encapsulation, including polymeric and lipidic nanoparticles, nanometric emulsions, and nanogels; and drug molecule functionalization by ligand attachment, such as peptides and polymers. Pharmacokinetic and pharmacodynamic in vivo studies’ results have shown that intranasal administration can be more efficient in brain targeting than other administration routes, and that the use of nanoformulations and drug functionalization can be quite advantageous in increasing brain–drug bioavailability. These strategies could be the key to future improved therapies for depressive and anxiety disorders.
1. Pathophysiology and Treatment of Depression and Anxiety Disorders: Current Aspects and Limitations
2. Potential Strategies for Enhancing Brain Drug Targeting and Bioavailability
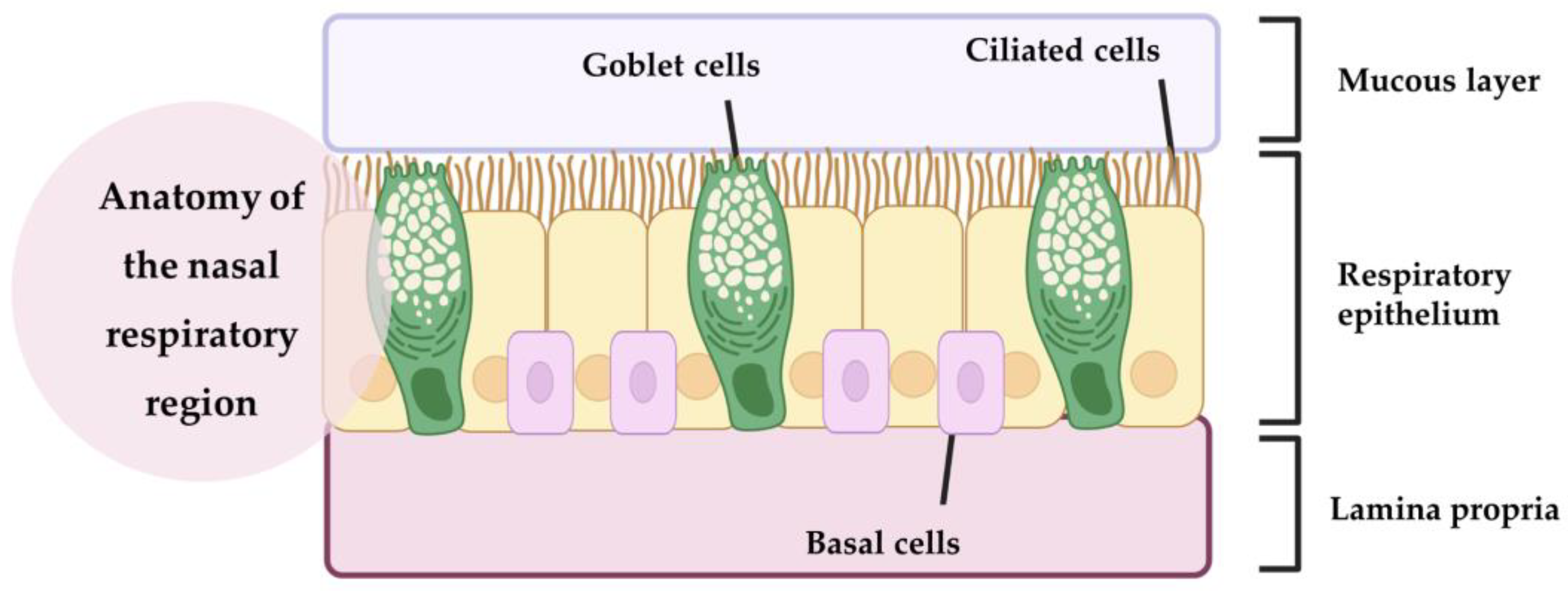
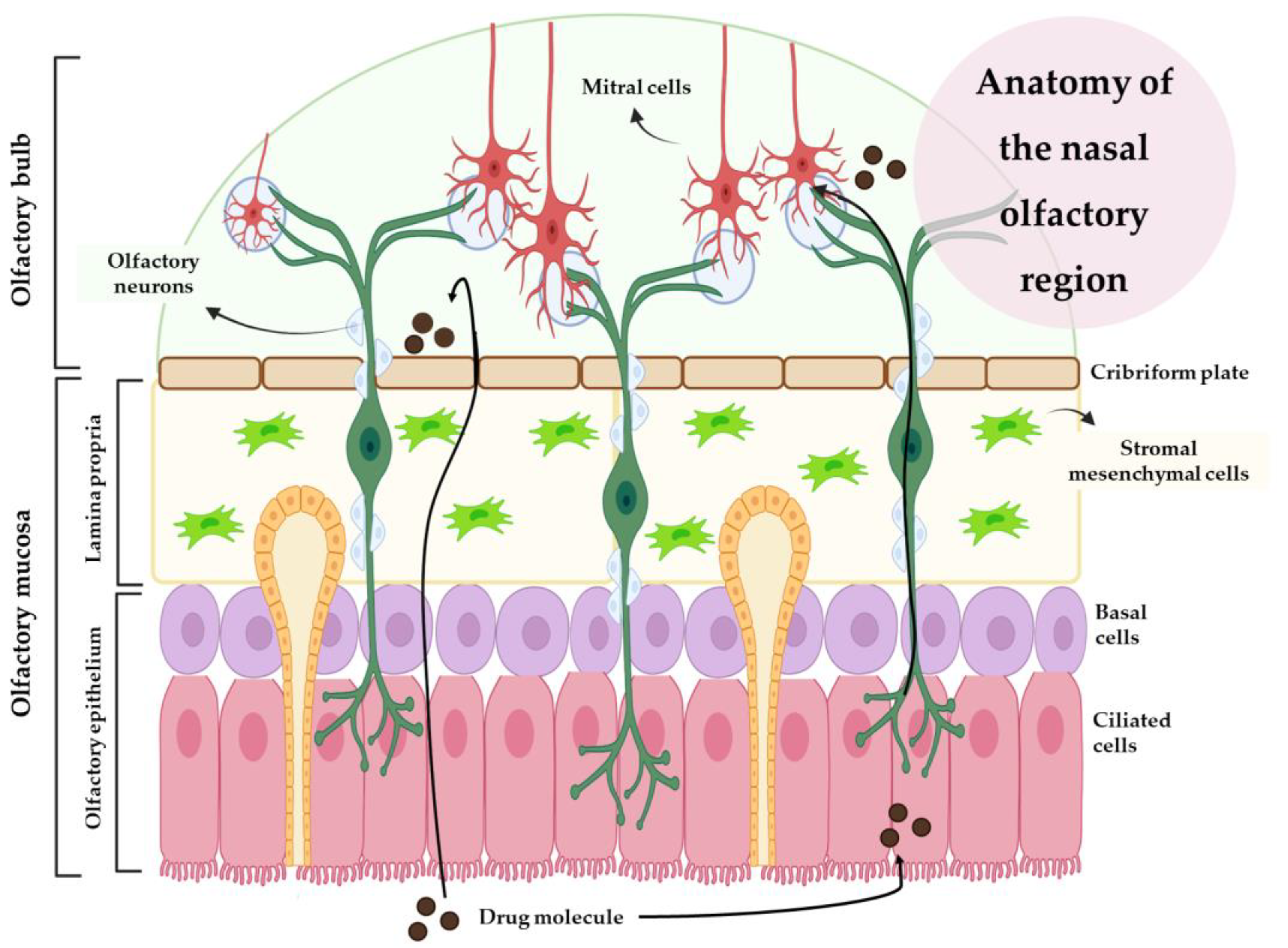
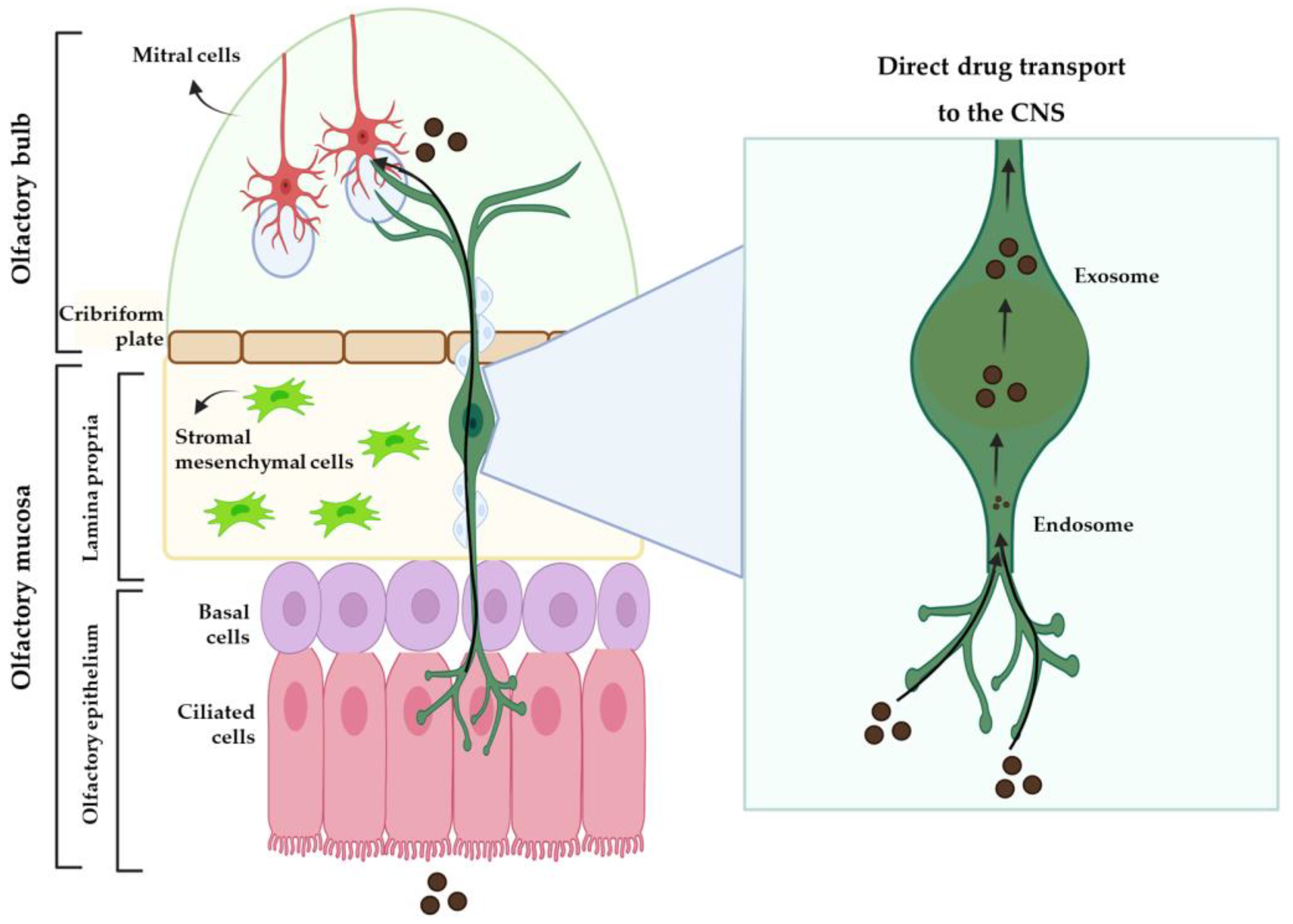
2.1. Intranasal Administration
2.2. Nanosystems
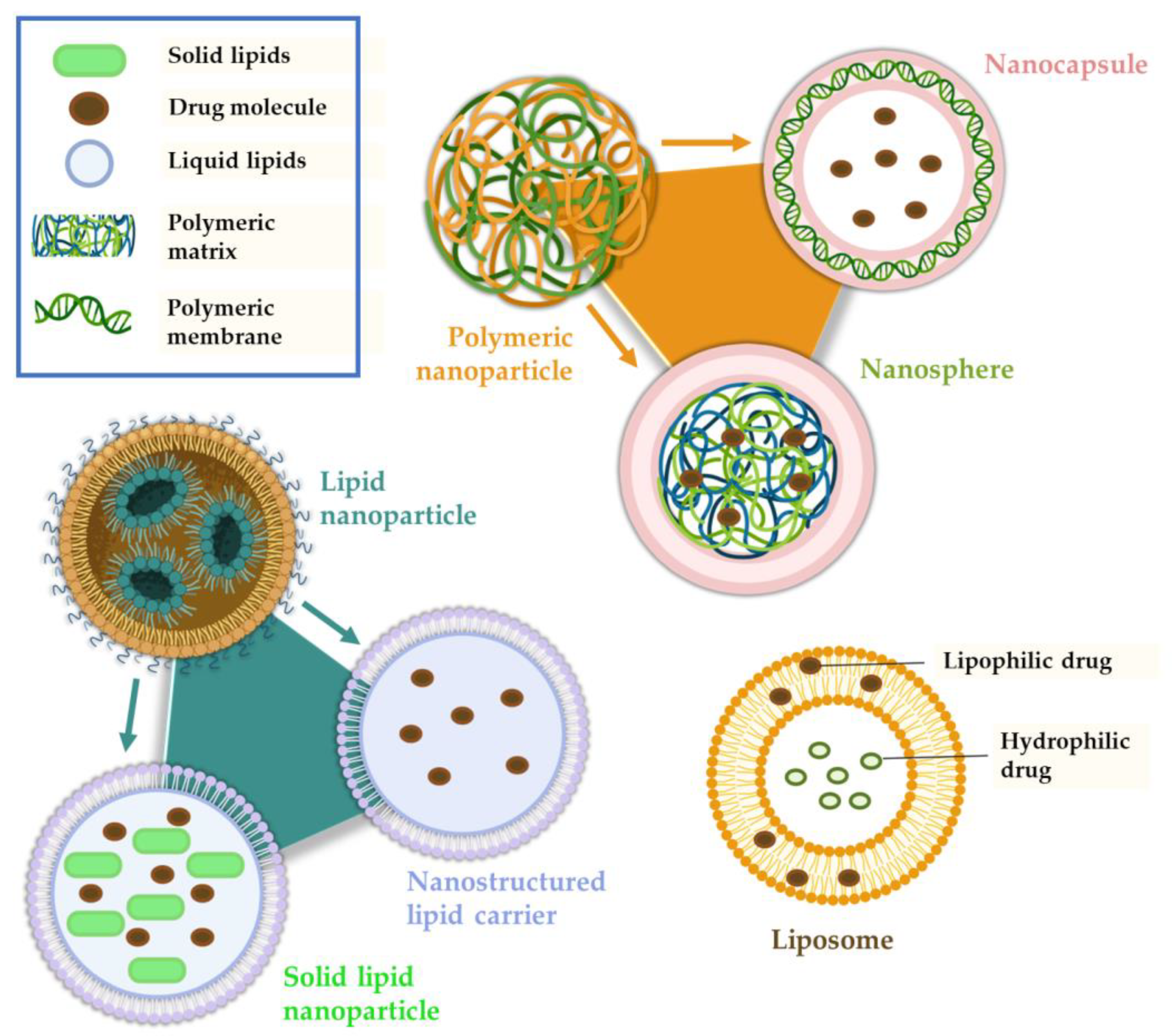
Polymeric Nanoparticles
Lipid Nanoparticles
Nanometric Emulsions
Nanogels
Liposomes
References
- Katzman, M.A.; Bleau, P.; Blier, P.; Chokka, P.; Kjernisted, K.; van Ameringen, M.; Antony, M.M.; Bouchard, S.; Brunet, A.; Flament, M.; et al. Canadian Clinical Practice Guidelines for the Management of Anxiety, Posttraumatic Stress and Obsessive-Compulsive Disorders. BMC Psychiatry 2014, 14, S1.
- Won, E.; Kim, Y.K. Neuroinflammation—Associated Alterations of the Brain as Potential Neural Biomarkers in Anxiety Disorders. Int. J. Mol. Sci. 2020, 21, 6546.
- Penninx, B.W.; Pine, D.S.; Holmes, E.A.; Reif, A. Anxiety Disorders. Lancet 2021, 397, 914–927.
- Nasir, M.; Trujillo, D.; Levine, J.; Dwyer, J.B.; Rupp, Z.W.; Bloch, M.H. Glutamate Systems in DSM-5 Anxiety Disorders: Their Role and a Review of Glutamate and GABA Psychopharmacology. Front. Psychiatry 2020, 11, 548505.
- Chesnut, M.; Harati, S.; Paredes, P.; Khan, Y.; Foudeh, A.; Kim, J.; Bao, Z.; Williams, L.M. Stress Markers for Mental States and Biotypes of Depression and Anxiety: A Scoping Review and Preliminary Illustrative Analysis. Chronic Stress 2021, 5, 1–17.
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858.
- Hu, P.; Lu, Y.; Pan, B.-X.; Zhang, W.-H. New Insights into the Pivotal Role of the Amygdala in Inflammation-Related Depression and Anxiety Disorder. Int. J. Mol. Sci. 2022, 23, 11076.
- GBD 2019 Diseases and Injuries Collaborators. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222.
- American Psychological Association Clinical Practice Guideline for the Treatment of Depression across Three Age Cohorts. 2019. Available online: https://www.apa.org/depression-guideline/guideline.pdf. (accessed on 1 February 2023).
- Zhao, Y.F.; Verkhratsky, A.; Tang, Y.; Illes, P. Astrocytes and Major Depression: The Purinergic Avenue. Neuropharmacology 2022, 220, 109252.
- Nemeroff, C.B. The State of Our Understanding of the Pathophysiology and Optimal Treatment of Depression: Glass Half Full or Half Empty? Am. J. Psychiatry 2020, 177, 671–685.
- Kircanski, K.; Joormann, J.; Gotlib, I.H. Cognitive Aspects of Depression. Wiley Interdiscip. Rev. Cogn. Sci. 2012, 3, 301–313.
- Gabriel, F.C.; de Melo, D.O.; Fraguas, R.; Leite-Santos, N.C.; da Silva, R.A.M.; Ribeiro, E. Pharmacological Treatment of Depression: A Systematic Review Comparing Clinical Practice Guideline Recommendations. PLoS ONE 2020, 15, 0231700.
- Nutt, D.J. Relationship of Neurotransmitters to the Symptoms of Major Depressive Disorder. J. Clin. Psychiatry 2008, 69, 4–7.
- Skelin, I.; Kovaèević, T.; Diksic, M. Neurochemical and Behavioural Changes in Rat Models of Depression. Croat. Chem. Acta 2011, 84, 287–299.
- Camkurt, M.A.; Findikli, E.; Izci, F.; Kurutaş, E.B.; Tuman, T.C. Evaluation of Malondialdehyde, Superoxide Dismutase and Catalase Activity and Their Diagnostic Value in Drug Naïve, First Episode, Non-Smoker Major Depression Patients and Healthy Controls. Psychiatry Res. 2016, 238, 81–85.
- Elsayed, O.H.; Ercis, M.; Pahwa, M.; Singh, B. Treatment-Resistant Bipolar Depression: Therapeutic Trends, Challenges and Future Directions. Neuropsychiatr. Dis. Treat 2022, 18, 2927–2943.
- Lenox, R.H.; Frazer, A. Mechanism of Action of Antidepressants and Mood Stabilizers. In Neuropsychopharmacology: The Fifth Generation of Progress; ACNP: Brentwood, TN, USA, 2002; pp. 1139–1163. ISBN 9780781728379.
- Kilts, C.D. Potential New Drug Delivery Systems for Antidepressants: An Overview. J. Clin. Psychiatry 2003, 64, 31–33.
- Pires, P.C.; Santos, A.O. Nanosystems in Nose-to-Brain Drug Delivery: A Review of Non-Clinical Brain Targeting Studies. J. Control. Release 2018, 270, 89–100.
- Lee, D.; Minko, T. Nanotherapeutics for Nose-to-Brain Drug Delivery: An Approach to Bypass the Blood Brain Barrier. Pharmaceutics 2021, 13, 2049.
- Xie, J.; Shen, Z.; Anraku, Y.; Kataoka, K.; Chen, X. Nanomaterial-Based Blood-Brain-Barrier (BBB) Crossing Strategies. Biomaterials 2019, 224, 119491.
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the Blood-Brain Barrier with Nanoparticles. J. Control. Release 2018, 270, 290–303.
- Ulbrich, K.; Knobloch, T.; Kreuter, J. Targeting the Insulin Receptor: Nanoparticles for Drug Delivery across the Blood-Brain Barrier (BBB). J. Drug Target. 2011, 19, 125–132.
- Teixeira, M.I.; Lopes, C.M.; Amaral, M.H.; Costa, P.C. Surface-Modified Lipid Nanocarriers for Crossing the Blood-Brain Barrier (BBB): A Current Overview of Active Targeting in Brain Diseases. Colloids Surf. B Biointerfaces 2023, 221, 112999.
- Bhattaccharjee, S.A.; Murnane, K.S.; Banga, A.K. Transdermal Delivery of Breakthrough Therapeutics for the Management of Treatment-Resistant and Post-Partum Depression. Int. J. Pharm. 2020, 591, 120007.
- Akil, H.; Gordon, J.; Hen, R.; Javitch, J.; Mayberg, H.; McEwen, B.; Meaney, M.J.; Nestler, E.J. Treatment Resistant Depression: A Multi-Scale, Systems Biology Approach. Neurosci. Biobehav. Rev. 2018, 84, 272–288.
- Misra, A.; Kher, G. Drug Delivery Systems from Nose to Brain. Curr. Pharm. Biotechnol. 2012, 13, 2355–2379.
- Erdó, F.; Bors, L.A.; Farkas, D.; Bajza, Á.; Gizurarson, S. Evaluation of Intranasal Delivery Route of Drug Administration for Brain Targeting. Brain Res. Bull. 2018, 143, 155–170.
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of Intranasal Drug Delivery Directly to the Brain. Life Sci. 2018, 195, 44–52.
- Keller, L.-A.; Merkel, O.; Popp, A. Intranasal Drug Delivery: Opportunities and Toxicologic Challenges during Drug Development. Drug Deliv. Transl. Res. 2022, 12, 735–757.
- Aderibigbe, B.; Naki, T. Design and Efficacy of Nanogels Formulations for Intranasal Administration. Molecules 2018, 23, 1241.
- Zha, S.; Wong, K.; All, A.H. Intranasal Delivery of Functionalized Polymeric Nanomaterials to the Brain. Adv. Healthc. Mater. 2022, 11, 2102610.
- Rohrer, J.; Lupo, N.; Bernkop-Schnürch, A. Advanced Formulations for Intranasal Delivery of Biologics. Int. J. Pharm. 2018, 553, 8–20.
- Casettari, L.; Illum, L. Chitosan in Nasal Delivery Systems for Therapeutic Drugs. J. Control. Release 2014, 190, 189–200.
- Alavian, F.; Shams, N. Oral and Intra-Nasal Administration of Nanoparticles in the Cerebral Ischemia Treatment in Animal Experiments: Considering Its Advantages and Disadvantages. Curr. Clin. Pharmacol. 2020, 15, 20–29.
- Pires, P.C.; Melo, D.; Santos, A.O. Intranasal Delivery of Antiseizure Drugs. In Drug Delivery Devices and Therapeutic Systems; Academic Press: Cambridge, MA, USA, 2021; pp. 623–646. ISBN 978-0-12-819838-4.
- Pires, P.C.; Rodrigues, M.; Alves, G.; Santos, A.O. Strategies to Improve Drug Strength in Nasal Preparations for Brain Delivery of Low Aqueous Solubility Drugs. Pharmaceutics 2022, 14, 588.
- Alberto, M.; Paiva-Santos, A.C.; Veiga, F.; Pires, P.C. Lipid and Polymeric Nanoparticles: Successful Strategies for Nose-to-Brain Drug Delivery in the Treatment of Depression and Anxiety Disorders. Pharmaceutics 2022, 14, 2742.
- Pires, P.C.; Paiva-Santos, A.C.; Veiga, F. Antipsychotics-Loaded Nanometric Emulsions for Brain Delivery. Pharmaceutics 2022, 14, 2174.
- Pires, P.C.; Paiva-Santos, A.C.; Veiga, F. Nano and Microemulsions for the Treatment of Depressive and Anxiety Disorders: An Efficient Approach to Improve Solubility, Brain Bioavailability and Therapeutic Efficacy. Pharmaceutics 2022, 14, 2825.
- Naqvi, S.; Panghal, A.; Flora, S.J.S. Nanotechnology: A Promising Approach for Delivery of Neuroprotective Drugs. Front. Neurosci. 2020, 14, 494.
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731.
- Volpatti, L.R.; Matranga, M.A.; Cortinas, A.B.; Delcassian, D.; Daniel, K.B.; Langer, R.; Anderson, D.G. Glucose-Responsive Nanoparticles for Rapid and Extended Self-Regulated Insulin Delivery. ACS Nano 2020, 14, 488–497.
- Ferreira, M.D.; Duarte, J.; Veiga, F.; Paiva-Santos, A.C.; Pires, P.C. Nanosystems for Brain Targeting of Antipsychotic Drugs: An Update on the Most Promising Nanocarriers for Increased Bioavailability and Therapeutic Efficacy. Pharmaceutics 2023, 15, 678.
- Zhang, W.; Mehta, A.; Tong, Z.; Esser, L.; Voelcker, N.H. Development of Polymeric Nanoparticles for Blood–Brain Barrier Transfer—Strategies and Challenges. Adv. Sci. 2021, 8, 2003937.
- Racine, L.; Texier, I.; Auzély-Velty, R. Chitosan-Based Hydrogels: Recent Design Concepts to Tailor Properties and Functions. Polym. Int. 2017, 66, 981–998.
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126.
- Jani, P.; Vanza, J.; Pandya, N.; Tandel, H. Formulation of Polymeric Nanoparticles of Antidepressant Drug for Intranasal Delivery. Ther. Deliv. 2019, 10, 683–696.
- Alsaab, H.O.; Alharbi, F.D.; Alhibs, A.S.; Alanazi, N.B.; Alshehri, B.Y.; Saleh, M.A.; Alshehri, F.S.; Algarni, M.A.; Almugaiteeb, T.; Uddin, M.N.; et al. PLGA-Based Nanomedicine: History of Advancement and Development in Clinical Applications of Multiple Diseases. Pharmaceutics 2022, 14, 2728.
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Novel Drug Delivery Systems: Applications, Advantages and Disadvantages. Res. Pharm. Sci. 2018, 13, 288.
- Bonferoni, M.; Rossi, S.; Sandri, G.; Ferrari, F.; Gavini, E.; Rassu, G.; Giunchedi, P. Nanoemulsions for “Nose-to-Brain” Drug Delivery. Pharmaceutics 2019, 11, 84.
- Kaur, P.; Garg, T.; Vaidya, B.; Prakash, A.; Rath, G.; Goyal, A.K. Brain Delivery of Intranasal in Situ Gel of Nanoparticulated Polymeric Carriers Containing Antidepressant Drug: Behavioral and Biochemical Assessment. J. Drug Target. 2015, 23, 275–286.
- Abdellatif, A.A.H.; Alsowinea, A.F. Approved and Marketed Nanoparticles for Disease Targeting and Applications in COVID-19. Nanotechnol. Rev. 2021, 10, 1941–1977.
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12.




