
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Satoru Matsuda | -- | 2018 | 2023-03-29 19:23:06 | | | |
| 2 | Jessie Wu | + 8 word(s) | 2026 | 2023-03-30 05:23:46 | | |
Video Upload Options
Mitophagy/autophagy plays a protective role in various forms of liver damage, by renovating cellular metabolism linking to sustain liver homeostasis. A characterized pathway for mitophagy is the phosphatase and tensin homolog (PTEN)-induced putative kinase 1 (PINK1)/Parkin-dependent signaling pathway. In particular, PINK1-mediated mitophagy could play an indispensable role in improving the metabolic dysfunction-associated fatty liver disease (MAFLD) which could precede to steatohepatitis (NASH), fibrosis, and hepatocellular carcinoma. In addition, the PI3K/AKT/mTOR pathway might regulate the various characteristics of cellular homeostasis including energy metabolism, cell proliferation, and/or cell protection. Therefore, targeting mitophagy with the alteration of PI3K/AKT/mTOR or PINK1/Parkin-dependent signaling to eliminate impaired mitochondria might be an attractive strategy for the treatment of MAFLD. In particular, the efficacy of prebiotics for the treatment of MAFLD has been suggested to be useful via the modulation of the PI3K/AKT/mTOR/AMPK pathway. Additionally, several edible phytochemicals could activate mitophagy for the improvement of mitochondrial damages, which could also be a promising option to treat MAFLD with providing liver protection.
1. Introduction
2. Valuable Components from Natural Foods for the Treatment of Metabolic Dysfunction-Associated Fatty Liver Disease
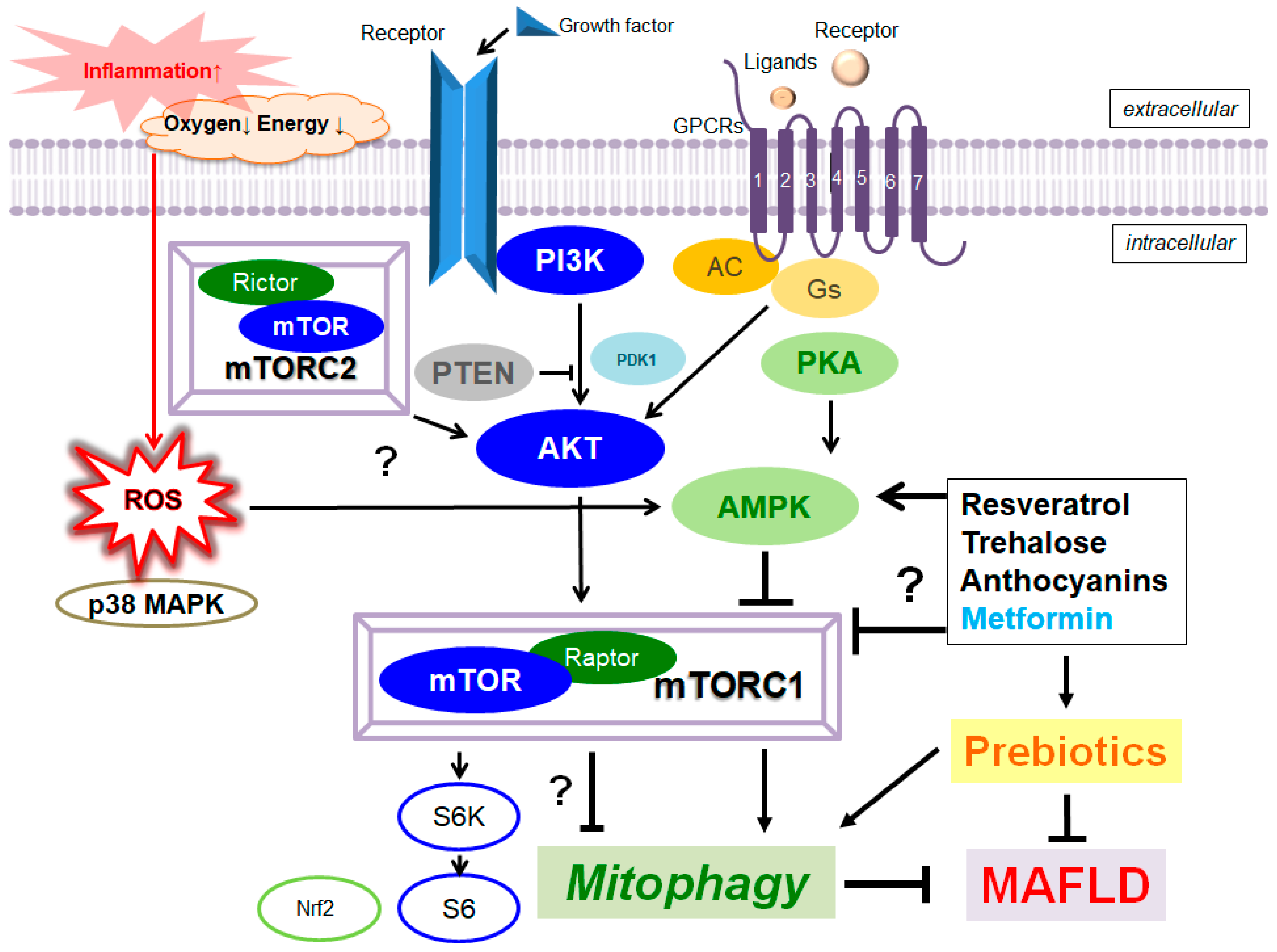
3. Improved Mitophagy with the Modification of Gut Microbiota for the Treatment of Metabolic Dysfunction-Associated Fatty Liver Disease
References
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502.
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host. Microb. 2018, 23, 705–715.
- Khalesi, S.; Johnson, D.W.; Campbell, K.; Williams, S.; Fenning, A.; Saluja, S.; Irwin, C. Effect of probiotics and synbiotics consumption on serum concentrations of liver function test enzymes: A systematic review and meta-analysis. Eur. J. Nutr. 2018, 57, 2037–2053.
- Do, M.H.; Lee, H.H.L.; Lee, J.E.; Park, M.; Oh, M.J.; Lee, H.B.; Park, J.H.; Jhun, H.; Kim, J.H.; Kang, C.H.; et al. Gellan gum prevents non-alcoholic fatty liver disease by modulating the gut microbiota and metabolites. Food Chem. 2023, 400, 134038.
- Papandreou, D.; Karabouta, Z.; Pantoleon, A.; Rousso, I. Investigation of anthropometric, biochemical and dietary parameters of obese children with and without non-alcoholic fatty liver disease. Appetite 2012, 59, 939–944.
- Dreher, M.L. Whole Fruits and Fruit Fiber Emerging Health Effects. Nutrients 2018, 10, 1833.
- Alferink, L.J.M.; Erler, N.S.; de Knegt, R.J.; Janssen, H.L.A.; Metselaar, H.J.; Darwish Murad, S.; Kiefte-de Jong, J.C. Adherence to a plant-based, high-fibre dietary pattern is related to regression of non-alcoholic fatty liver disease in an elderly population. Eur. J. Epidemiol. 2020, 35, 1069–1085.
- Harms, M.J.; Li, Q.; Lee, S.; Zhang, C.; Kull, B.; Hallen, S.; Thorell, A.; Alexandersson, I.; Hagberg, C.E.; Peng, X.R.; et al. Mature Human White Adipocytes Cultured under Membranes Maintain Identity, Function, and Can Transdifferentiate into Brown-like Adipocytes. Cell Rep. 2019, 27, 213–225.e5.
- Sorrentino, V.; Menzies, K.J.; Auwerx, J. Repairing Mitochondrial Dysfunction in Disease. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 353–389.
- Nassir, F.; Ibdah, J.A. Role of mitochondria in alcoholic liver disease. World J. Gastroenterol. 2014, 20, 2136–2142.
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689.
- Rinella, M.E. Nonalcoholic fatty liver disease: A systematic review. J. Am. Med. Assoc. 2015, 313, 2263–2273.
- Younossi, Z.M.; Stepanova, M.; Ong, J.; Trimble, G.; AlQahtani, S.; Younossi, I.; Ahmed, A.; Racila, A.; Henry, L. Nonalcoholic Steatohepatitis Is the Most Rapidly Increasing Indication for Liver Transplantation in the United States. Clin. Gastroenterol. Hepatol. 2021, 19, 580–589.e5.
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428.
- Fotbolcu, H.; Zorlu, E. Nonalcoholic fatty liver disease as a multi-systemic disease. World J. Gastroenterol. 2016, 22, 4079–4090.
- Méndez-Sánchez, N.; Bugianesi, E.; Gish, R.G.; Lammert, F.; Tilg, H.; Nguyen, M.H.; Sarin, S.K.; Fabrellas, N.; Zelber-Sagi, S.; Fan, J.G.; et al. Global multi-stakeholder endorsement of the MAFLD definition. Lancet Gastroenterol. Hepatol. 2022, 7, 388–390.
- Farzanegi, P.; Dana, A.; Ebrahimpoor, Z.; Asadi, M.; Azarbayjani, M.A. Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur. J. Sport Sci. 2019, 19, 994–1003.
- Dabravolski, S.A.; Bezsonov, E.E.; Baig, M.S.; Popkova, T.V.; Nedosugova, L.V.; Starodubova, A.V.; Orekhov, A.N. Mitochondrial Mutations and Genetic Factors Determining NAFLD Risk. Int. J. Mol. Sci. 2021, 22, 4459.
- Lee, Y.S.; Lai, D.M.; Huang, H.J.; Lee-Chen, G.J.; Chang, C.H.; Hsieh-Li, H.M.; Lee, G.C. Prebiotic Lactulose Ameliorates the Cognitive Deficit in Alzheimer’s Disease Mouse Model through Macroautophagy and Chaperone-Mediated Autophagy Pathways. J. Agric. Food Chem. 2021, 69, 2422–2437.
- Zobeiri, M.; Parvizi, F.; Kalhori, M.R.; Majnooni, M.B.; Farzaei, M.H.; Abdollahi, M. Targeting miRNA by Natural Products: A Novel Therapeutic Approach for Nonalcoholic Fatty Liver. Evid. Based Complement Alternat. Med. 2021, 2021, 6641031.
- Shi, L.; Karrar, E.; Liu, R.; Chang, M.; Wang, X. Comparative effects of sesame lignans (sesamin, sesamolin, and sesamol) on oxidative stress and lipid metabolism in steatosis HepG2 cells. J. Food Biochem. 2022, 46, e14180.
- Chen, N.; Qi, Y.; Ma, X.; Xiao, X.; Liu, Q.; Xia, T.; Xiang, J.; Zeng, J.; Tang, J. Rediscovery of Traditional Plant Medicine: An Underestimated Anticancer Drug of Chelerythrine. Front. Pharmacol. 2022, 13, 906301.
- Hsu, C.M.; Yen, C.H.; Wang, S.C.; Liu, Y.C.; Huang, C.T.; Wang, M.H.; Chuang, T.M.; Ke, Y.L.; Yeh, T.J.; Gau, Y.C.; et al. Emodin Ameliorates the Efficacy of Carfilzomib in Multiple Myeloma Cells via Apoptosis and Autophagy. Biomedicines 2022, 10, 1638.
- Su, Z.; Guo, Y.; Huang, X.; Feng, B.; Tang, L.; Zheng, G.; Zhu, Y. Phytochemicals: Targeting Mitophagy to Treat Metabolic Disorders. Front. Cell Dev. Biol. 2021, 9, 686820.
- Delzenne, N.M.; Cani, P.D.; Neyrinck, A.M. Modulation of glucagon-like peptide 1 and energy metabolism by inulin and oligofructose: Experimental data. J. Nutr. 2007, 137 (Suppl. 11), 2547S–2551S.
- Zhou, J.; Martin, R.J.; Tulley, R.T.; Raggio, A.M.; McCutcheon, K.L.; Shen, L.; Danna, S.C.; Tripathy, S.; Hegsted, M.; Keenan, M.J. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1160–E1166.
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103.
- Song, X.; Zhong, L.; Lyu, N.; Liu, F.; Li, B.; Hao, Y.; Xue, Y.; Li, J.; Feng, Y.; Ma, Y.; et al. Inulin Can Alleviate Metabolism Disorders in ob/ob Mice by Partially Restoring Leptin-related Pathways Mediated by Gut Microbiota. Genom. Proteom. Bioinform. 2019, 17, 64–75.
- Yuan, W.; Zhang, M.; Wang, C.; Li, B.; Li, L.; Ye, F.; Xu, C. Resveratrol Attenuates High-Fat Diet-Induced Hepatic Lipotoxicity by Upregulating Bmi-1 Expression. J. Pharmacol. Exp. Ther. 2022, 381, 96–105.
- Zhang, Y.; Chen, M.L.; Zhou, Y.; Yi, L.; Gao, Y.X.; Ran, L.; Chen, S.H.; Zhang, T.; Zhou, X.; Zou, D.; et al. Resveratrol improves hepatic steatosis by inducing autophagy through the cAMP signaling pathway. Mol. Nutr. Food Res. 2015, 59, 1443–1457.
- Hecht, J.T.; Coustry, F.; Veerisetty, A.C.; Hossain, M.G.; Posey, K.L. Resveratrol Reduces COMPopathy in Mice Through Activation of Autophagy. JBMR Plus 2021, 5, e10456.
- Suvorova, I.I.; Knyazeva, A.R.; Petukhov, A.V.; Aksenov, N.D.; Pospelov, V.A. Resveratrol enhances pluripotency of mouse embryonic stem cells by activating AMPK/Ulk1 pathway. Cell Death Discov. 2019, 5, 61.
- Hu, D.; Yang, W.; Mao, P.; Cheng, M. Combined Amelioration of Prebiotic Resveratrol and Probiotic Bifidobacteria on Obesity and Nonalcoholic Fatty Liver Disease. Nutr. Cancer 2021, 73, 652–661.
- Kim, Y.K.; Yoon, H.H.; Lee, Y.D.; Youn, D.Y.; Ha, T.J.; Kim, H.S.; Lee, J.H. Anthocyanin Extracts from Black Soybean (Glycine max L.) Protect Human Glial Cells Against Oxygen-Glucose Deprivation by Promoting Autophagy. Biomol. Ther. 2012, 20, 68–74.
- Mehmood, A.; Zhao, L.; Wang, Y.; Pan, F.; Hao, S.; Zhang, H.; Iftikhar, A.; Usman, M. Dietary anthocyanins as potential natural modulators for the prevention and treatment of non-alcoholic fatty liver disease: A comprehensive review. Food Res. Int. 2021, 142, 110180.
- Li, J.; Zhao, R.; Zhao, H.; Chen, G.; Jiang, Y.; Lyu, X.; Wu, T. Reduction of Aging-Induced Oxidative Stress and Activation of Autophagy by Bilberry Anthocyanin Supplementation via the AMPK-mTOR Signaling Pathway in Aged Female Rats. J. Agric. Food Chem. 2019, 67, 7832–7843.
- Chu, Q.; Zhang, S.; Chen, M.; Han, W.; Jia, R.; Chen, W.; Zheng, X. Cherry Anthocyanins Regulate NAFLD by Promoting Autophagy Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 4825949.
- Thongnak, L.; Jaruan, O.; Pengrattanachot, N.; Promsan, S.; Phengpol, N.; Sutthasupha, P.; Jaikumkao, K.; Sriyotai, W.; Mahatheeranont, S.; Lungkaphin, A. Resistant starch from black rice, Oryza sativa L. var. ameliorates renal inflammation, fibrosis and injury in insulin resistant rats. Phytother. Res. 2022, 37, 935–948, Online ahead of print.
- Hosseinpour-Moghaddam, K.; Caraglia, M.; Sahebkar, A. Autophagy induction by trehalose: Molecular mechanisms and therapeutic impacts. J. Cell. Physiol. 2018, 233, 6524–6543.
- Rusmini, P.; Cortese, K.; Crippa, V.; Cristofani, R.; Cicardi, M.E.; Ferrari, V.; Vezzoli, G.; Tedesco, B.; Meroni, M.; Messi, E.; et al. Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Autophagy 2019, 15, 631–651.
- van Laar, A.; Grootaert, C.; Van Nieuwerburgh, F.; Deforce, D.; Desmet, T.; Beerens, K.; Van Camp, J. Metabolism and Health Effects of Rare Sugars in a CACO-2/HepG2 Coculture Model. Nutrients 2022, 14, 611.
- DeBosch, B.J.; Heitmeier, M.R.; Mayer, A.L.; Higgins, C.B.; Crowley, J.R.; Kraft, T.E.; Chi, M.; Newberry, E.P.; Chen, Z.; Finck, B.N.; et al. Trehalose inhibits solute carrier 2A (SLC2A) proteins to induce autophagy and prevent hepatic steatosis. Sci. Signal. 2016, 9, ra21.
- Jeong, S.J.; Stitham, J.; Evans, T.D.; Zhang, X.; Rodriguez-Velez, A.; Yeh, Y.S.; Tao, J.; Takabatake, K.; Epelman, S.; Lodhi, I.J.; et al. Trehalose causes low-grade lysosomal stress to activate TFEB and the autophagy-lysosome biogenesis response. Autophagy 2021, 17, 3740–3752.
- Wu, Y.T.; Yang, W.Y.; Wu, Y.H.S.; Chen, J.W.; Chen, Y.C. Modulations of growth performance, gut microbiota, and inflammatory cytokines by trehalose on Salmonella Typhimurium-challenged broilers. Poult. Sci. 2020, 99, 4034–4043.
- Stachowicz, A.; Wiśniewska, A.; Kuś, K.; Kiepura, A.; Gębska, A.; Gajda, M.; Białas, M.; Totoń-Żurańska, J.; Stachyra, K.; Suski, M.; et al. The Influence of Trehalose on Atherosclerosis and Hepatic Steatosis in Apolipoprotein E Knockout Mice. Int. J. Mol. Sci. 2019, 20, 1552.
- Han, H.; Xue, T.; Li, J.; Guo, Y.; Li, X.; Wang, L.; Pei, L.; Zheng, M. Plant sterol ester of α-linolenic acid improved non-alcoholic fatty liver disease by attenuating endoplasmic reticulum stress-triggered apoptosis via activation of the AMPK. J. Nutr. Biochem. 2022, 107, 109072.
- Zhao, L.; Wang, Y.; Liu, J.; Wang, K.; Guo, X.; Ji, B.; Wu, W.; Zhou, F. Protective Effects of Genistein and Puerarin against Chronic Alcohol-Induced Liver Injury in Mice via Antioxidant, Anti-inflammatory, and Anti-apoptotic Mechanisms. J. Agric. Food Chem. 2016, 64, 7291–7297.
- Du, Z.R.; Feng, X.Q.; Li, N.; Qu, J.X.; Feng, L.; Chen, L.; Chen, W.F. G protein-coupled estrogen receptor is involved in the anti-inflammatory effects of genistein in microglia. Phytomedicine 2018, 43, 11–20.
- Li, Z.; Li, J.; Mo, B.; Hu, C.; Liu, H.; Qi, H.; Wang, X.; Xu, J. Genistein induces cell apoptosis in MDA-MB-231 breast cancer cells via the mitogen-activated protein kinase pathway. Toxicol. In Vitro 2008, 22, 1749–1753.
- Sabir, U.; Irfan, H.M.; Alamgeer; Umer, I.; Niazi, Z.R.; Asjad, H.M.M. Phytochemicals targeting NAFLD through modulating the dual function of forkhead box O1 (FOXO1) transcription factor signaling pathways. Naunyn. Schmiedebergs. Arch. Pharmacol. 2022, 395, 741–755.
- Inami, Y.; Yamashina, S.; Izumi, K.; Ueno, T.; Tanida, I.; Ikejima, K.; Watanabe, S. Hepatic steatosis inhibits autophagic proteolysis via impairment of autophagosomal acidification and cathepsin expression. Biochem. Biophys. Res. Commun. 2011, 412, 618–625.
- Galindo, M.F.; Solesio, M.E.; Atienzar-Aroca, S.; Zamora, M.J.; Jordán Bueso, J. Mitochondrial dynamics and mitophagy in the 6-hydroxydopamine preclinical model of Parkinson’s disease. Parkinsons Dis. 2012, 2012, 131058.
- Nassir, F.; Ibdah, J.A. Role of mitochondria in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 8713–8742.
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478.
- Omar, N.N.; Mosbah, R.A.; Sarawi, W.S.; Rashed, M.M.; Badr, A.M. Rifaximin Protects against Malathion-Induced Rat Testicular Toxicity: A Possible Clue on Modulating Gut Microbiome and Inhibition of Oxidative Stress by Mitophagy. Molecules 2022, 27, 4069.
- Dinić, M.; Lukić, J.; Djokić, J.; Milenković, M.; Strahinić, I.; Golić, N.; Begović, J. Lactobacillus fermentum Postbiotic-induced Autophagy as Potential Approach for Treatment of Acetaminophen Hepatotoxicity. Front. Microbiol. 2017, 8, 594.
- Pugin, B.; Barcik, W.; Westermann, P.; Heider, A.; Wawrzyniak, M.; Hellings, P.; Akdis, C.A.; O’Mahony, L. A wide diversity of bacteria from the human gut produces and degrades biogenic amines. Microb. Ecol. Health Dis. 2017, 28, 1353881.
- Oh, M.; Choi, I.K.; Kwon, H.J. Inhibition of histone deacetylase1 induces autophagy. Biochem. Biophys. Res. Commun. 2008, 369, 1179–1183.
- Tang, W.; Yuan, M.; Li, Z.; Lin, Q.; Zhen, Y.; Li, Z.; Zhou, H.; Xia, F. Polyphenol-Rich Liupao Tea Extract Prevents High-Fat Diet-Induced MAFLD by Modulating the Gut Microbiota. Nutrients 2022, 14, 4930.
- Song, L.; Li, Y.; Qu, D.; Ouyang, P.; Ding, X.; Wu, P.; Guan, Q.; Yang, L. The regulatory effects of phytosterol esters (PSEs) on gut flora and faecal metabolites in rats with NAFLD. Food Funct. 2020, 11, 977–991.
- Hu, H.; Lin, A.; Kong, M.; Yao, X.; Yin, M.; Xia, H.; Ma, J.; Liu, H. Intestinal microbiome and NAFLD: Molecular insights and therapeutic perspectives. J. Gastroenterol. 2020, 55, 142–158.
- Li, S.; Chen, F.; Zou, Y.; Ning, L.; Zhang, G.; Zhang, S.; Yao, Q. Yinzhihuang oral liquid protects against non-alcoholic steatohepatitis via modulation of the gut-liver axis in mice. Ann. Transl. Med. 2022, 10, 631.
- Yao, N.; Yang, Y.; Li, X.; Wang, Y.; Guo, R.; Wang, X.; Li, J.; Xie, Z.; Li, B.; Cui, W. Effects of Dietary Nutrients on Fatty Liver Disease Associated With Metabolic Dysfunction (MAFLD): Based on the Intestinal-Hepatic Axis. Front. Nutr. 2022, 9, 906511.
- Gómez-Hurtado, I.; Santacruz, A.; Peiró, G.; Zapater, P.; Gutiérrez, A.; Pérez-Mateo, M.; Sanz, Y.; Francés, R. Gut microbiota dysbiosis is associated with inflammation and bacterial translocation in mice with CCl4-induced fibrosis. PLoS ONE 2011, 6, e23037.
- Nadal, I.; Santacruz, A.; Marcos, A.; Warnberg, J.; Garagorri, J.M.; Moreno, L.A.; Martin-Matillas, M.; Campoy, C.; Martí, A.; Moleres, A.; et al. Shifts in clostridia, bacteroides and immunoglobulin-coating fecal bacteria associated with weight loss in obese adolescents. Int. J. Obes. 2009, 33, 758–767.
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270.
- Chen, J.; Vitetta, L. Mitochondria could be a potential key mediator linking the intestinal microbiota to depression. J. Cell. Biochem. 2020, 121, 17–24.
- Chen, J.; Vitetta, L. Gut Microbiota Metabolites in NAFLD Pathogenesis and Therapeutic Implications. Int. J. Mol. Sci. 2020, 21, 5214.
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297.
- An, L.; Wirth, U.; Koch, D.; Schirren, M.; Drefs, M.; Koliogiannis, D.; Nieß, H.; Andrassy, J.; Guba, M.; Bazhin, A.V.; et al. The Role of Gut-Derived Lipopolysaccharides and the Intestinal Barrier in Fatty Liver Diseases. J. Gastrointest. Surg. 2022, 26, 671–683.
- Pinheiro, I.; Robinson, L.; Verhelst, A.; Marzorati, M.; Winkens, B.; den Abbeele, P.V.; Possemiers, S. A yeast fermentate improves gastrointestinal discomfort and constipation by modulation of the gut microbiome: Results from a randomized double-blind placebo-controlled pilot trial. BMC Complement. Altern. Med. 2017, 17, 441.
- Klaenhammer, T.R.; Kleerebezem, M.; Kopp, M.V.; Rescigno, M. The impact of probiotics and prebiotics on the immune system. Nat. Rev. Immunol. 2012, 12, 728–734.
- Martel, J.; Ojcius, D.M.; Ko, Y.F.; Young, J.D. Phytochemicals as Prebiotics and Biological Stress Inducers. Trends Biochem. Sci. 2020, 45, 462–471.




