1. Introduction
Prebiotics are additional effective interventions through dietary supplements of indigestible food ingredients that improve host health by selectively stimulating the growth and activity of bacteria in one or a small number of colonies. In other words, prebiotics could be defined as substrates that are utilized by beneficial bacteria providing a health benefit to the host
[1]. The use of prebiotics may be a relevant option in human health
[2]. In fact, previous studies have investigated the efficacy of prebiotics treatment for nonalcoholic fatty liver disease (NAFLD)
[3]. For example, gellan gum had prebiotic activity, which is an anionic polysaccharide used as an additive in the food industry
[4]. Gellan gum may promote liver health through the modulation of gut homeostasis
[4]. In addition, low fiber consumption is associated with the prevalence of NAFLD
[5]. Patients with NAFLD have a low daily fiber consumption
[6]. Similarly, a high-fiber diet has been shown to be related to the recession of NAFLD
[7]. Furthermore, pectin could modulate the gut microbiome, which is a soluble fiber found in different fruits and vegetables. It has been shown that pectin could protect liver from metabolic injuries caused by alcohol and/or fatty diet
[6].
Mitochondrial activity is important for cell survival, proliferation, and/or differentiation
[8]. In addition, many diseases such as age-related pathological conditions have been related to an altered mitochondrial function
[9]. The importance of mitochondria has been emphasized in a variety of metabolic diseases including NAFLD
[10]. NAFLD is a term covering the conditions characterized by excessive fat accumulation in the liver that are not triggered by alcohol intake. Excessive hepatic fat accumulation might be a hallmark of NAFLD
[11]. NAFLD is the most common chronic liver disease affecting nearly 30% of the population
[12]. Deskbound lifestyles, dietary changes, epidemic obesity, and type 2 diabetes have been identified as risk factors contributing to the increase in NAFLD. Patients with NAFLD are at an increased risk of developing steatohepatitis and/or nonalcoholic steatohepatitis (NASH) and fibrosis. NASH may ultimately lead to complications such as cirrhosis or liver failure. Nowadays, NASH has become among the top leading indications for liver transplantation
[13]. Consequent complications of NASH or cirrhosis may include the development of hepatocellular carcinoma (HCC)
[14]. It is a multisystem illness affecting numerous extrahepatic organ-diseases including type 2 diabetes and/or cardiovascular disease, all of which contribute to increased mortality in patients with NAFLD/NASH
[15]. Recently, the redefinition of the NAFLD to metabolic-associated fatty liver disease (MAFLD) have been proposed to more accurately reflect the disease’s heterogeneity and pathogenesis
[16]. Therefore,
rwe
searchers hereafter used the term “MAFLD” instead of the term “NAFLD”. Mitochondrial dysfunction leads to the overproduction of ROS and hepatocyte apoptosis, which is closely related to MAFLD
[17]. To slow down the progression of the pathology of MAFLD, the high oxidative stress and the mitochondrial activity of hepatocytes should be at least improved. Mitophagy, a selective degradation of mitochondria by autophagy, could selectively degrade damaged mitochondria to reverse mitochondrial dysfunction and/or maintain mitochondrial function. Mutations in the genes that encode essential factors for mitophagy may result in impaired mitophagy, which could lead to MAFLD
[18]. Interestingly, it has been shown that the prebiotic administration of lactulose and/or trehalose could stimulate the autophagic pathway, which may result in the retrieval of learning in Alzheimer’s disease model mice
[19]. The conserved pathway of autophagy might be required for preventing and/or counteracting pathogenic actions which could lead to the prevention of MAFLD as well as of neurodegenerative diseases.
2. Valuable Components from Natural Foods for the Treatment of Metabolic Dysfunction-Associated Fatty Liver FLDisease
Cumulative evidence highlights a favorable role of the naturally derived compounds from various natural sources for the prevention and/or treatment of metabolic disorders including MAFLD
[20][69]. Currently, several composites with a liver-protective effect may be a potential alternative therapy for the complications of MAFLD due to their unique therapeutic properties and considerable safety
[21][70]. Additionally, there are a variety of natural compounds with known autophagy-modulating properties that also control the production of ROS
[22][23][71,72]. In particular, the potential of targeting mitophagy with phytochemicals for the possible management of MAFLD has been shown here, with a view providing a direction for finding some phytochemicals that target mitophagy to prevent and/or treat MAFLD
[24][73]. Prebiotics enhance the secretion of GLP-1, in part via the upregulation of the proglucagon gene expression in the distal intestine
[25][74]. Furthermore, the fermentation of prebiotics leads to the production of SCFAs
[26][75]. For example, prebiotic fibers could promote the growth of
Bifidobacterium spp. in the gut, which has been shown to reduce the metabolic endotoxemia associated with the consumption of a high-fat diet
[27][76]. In addition, inulin fiber has been used as a prebiotic to alleviate glucose and/or lipid metabolism disorders by modulating the gut microbiota
[28][77].
Resveratrol is a natural polyphenolic compound, which may show several helpful effects on the hepatocyte of MAFLD
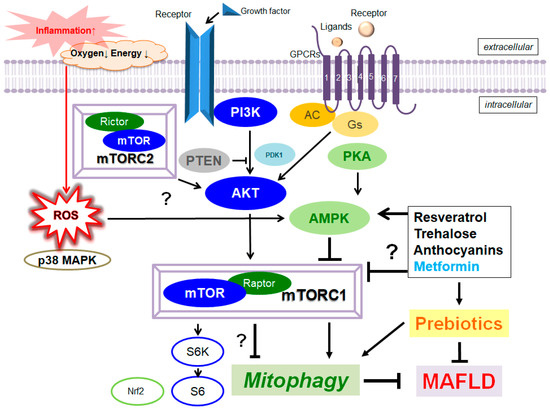
[29][78] through the possible activation of AMPK signaling
[30][79]. In general, dietary polyphenols may exhibit antioxidative, anti-inflammatory, and anticancer properties, which may lead to the restoration of the otherwise-impaired inflammatory diseases. Resveratrol could decrease the mTORC1 signaling for the stimulation of autophagy
[31][80] (
Figure 1). In addition, resveratrol could also encourage autophagy in embryonic stem cells, which may also be delivered by the associated suppression of the mTORC1
[32][81]. A prebiotic resveratrol could be a potential candidate for the treatment of obesity and MAFLD
[33][82]. Anthocyanins are common plant pigments, which could also stimulate autophagy
[34][83] with potential strong activity against MAFLD
[35][84]. Anthocyanins could induce autophagy via the AMPK-mTOR pathway
[36][85]. The positive effects of anthocyanins on the accumulation of hepatic lipid might be mediated via the activation of autophagy, suggesting a capability for the therapeutic tactics in MAFLD
[37][86]. In fact, it has been shown that resistant starch, including anthocyanins, could ameliorate insulin resistance, dyslipidemia and liver injury
[38][87]. Trehalose is a natural saccharide that could alter autophagy
[39][88], initiating lysosomal temporary damage
[40][89]. A health benefit for trehalose has been proposed in HepG2 cells
[41][90]. The action mechanisms of trehalose may involve the inhibition of glucose transporters leading to AMPK-activation, which could affect the autophagy
[42][91]. Thus, the trehalose could work as a fragile inhibitor of the lysosome via the inhibition of mTORC1
[43][92]. In addition, trehalose supplementation could act as a prebiotic to selectively promote the growth of beneficial bacteria
[44][93], which may attenuate hepatic steatosis
[45][94].
Figure 1. Several modulator molecules linked to the PI3K/AKT/mTOR/AMPK/mTORC1/ mTORC2 signaling pathway are demonstrated. Example compounds known to act on AMPK/mTOR and/or mitophagy/autophagy signaling are also shown on the right side. The arrowhead means stimulation, whereas hammerhead represents inhibition. Note that some critical events such as immune activation and/or antioxidant feedback have been omitted for clarity. Abbreviation: AMPK, adenosine monophosphate-activated protein kinase; mTOR, mammalian/mechanistic target of rapamycin; PI3K, phosphoinositide-3 kinase; PKA, protein kinase A; PTEN, phosphatase and tensin homologue deleted on chromosome 10; mTORC, mechanistic/mammalian target of rapamycin complex; MAFLD, metabolic dysfunction-associated fatty liver disease; mTOR, mechanistic/mammalian target of rapamycin; mTORC 1/2, mechanistic/mammalian target of rapamycin complex 1/2.
The aforementioned phytochemicals may have the capability to target putative molecular and/or biochemical actions in mitophagy. In view of the fact that more and more various phytochemicals would be applied to the treatment of MAFLD, it is necessary to have a widespread understanding of the effects and/or potential mechanisms of phytochemicals on MAFLD
[46][95]. In general, phytochemicals may be a pleiotropic molecule with the capability to interact with the different cellular targets involved in inflammation conditions
[47][96]. For example, it has been revealed that genistein could also downregulate the NF-κB signaling pathway, leading to a decrease in the expression of IL-1 and/or IL-6
[48][97]. On the other hand, genistein could also down-regulate Bcl-2 and up-regulates Bax, which might suppress cell growth and induces apoptosis
[49][98]. There is a need to further explore the underlying mechanisms. More research should focus on the regulatory mechanisms of mitophagy in MAFLD, and further search for the potential of targeting mitophagy with certain phytochemicals for the prevention and/or treatment of MAFLD
[50][99]. The use of such compounds could be an inaugural point for the new therapy of MAFLD.
3. Improved Mitophagy with the Modification of Gut Microbiota for the Treatment of Metabolic Dysfunction-Associated Fatty Liver FLDisease
Autophagic dysfunction may contribute to hepatic steatosis and accelerate the progression of MAFLD
[51][100]. Therefore, mitophagy activation might play a key role in improving the situation of MAFLD. In addition, mitophagy may account for the mitochondrial degradation occurring in the presence of stressful conditions, which is detected in several disorders such as Parkinson’s disease
[52][101]. The mitochondria dysfunctions suppress the β-oxidation of mitochondria and increase the production of the toxic lipid metabolism, which further adversely affects the mitochondrial dysfunction
[53][102]. Interestingly, the gut microbiota could produce short-chain fatty acids (SCFAs), which might act as signal molecules to regulate the autophagy
[54][55][103,104]. Therefore, the modulation of microbiota has been shown to control the machinery of autophagy against liver toxicity
[56][105]. The gut microbiota may regulate the autophagy progression through multiple mechanisms with regulating cellular metabolisms
[57][106] and/or epigenetically inhibiting histone deacetylases
[58][107]. SCFAs produced by the microbiota can be found in peripheral blood, where they are taken up by organs, as they act as signal molecules
[54][103]. Therefore, it is reasonable to consider that gut dysbiosis, resulting in the dysregulation of SCFA production, increases the susceptibility of liver injury. In fact, MAFLD pathogenesis is closely associated with gut dysbiosis
[59][108]. The gut dysbiosis could accelerate the development of MAFLD due to abnormal levels of gut microbial metabolites and/or their effects on gut epithelial permeability
[60][61][109,110]. Therefore, it has been shown that the gut microbiota might play an imperative role in the progression of MAFLD, and gut microbiota dysbiosis may be a significant factor in MAFLD pathogenesis
[62][63][111,112].
Many factors could affect the composition of gut microbiota, including the host age, sex, menopausal status, host immunity, exposure to antibiotics, and dietary behaviors
[64][113]. Specific dietary factors and/or the presence of specific bioactive compounds may affect the diversity of gut microbiota
[65][114]. In addition, insoluble fiber, fat, and protein contents may have important effects on the structure of gut microbiota
[66][115]. Metabolic diseases such as obesity and diabetes are also related with the gut microbiota
[67][116]. Therefore, restoring the gut microbiota with commensal bacteria may help improve the pathology
[68][117]. Interestingly, it has been shown that the amount of
Bacteroidetes may decrease in MAFLD, while those of
Firmicutes and
Proteobacteria may be increased
[69][118]. Gut microbiota has been shown to regulate key transcription factors, and enzymes related to mitochondrial biogenesis and metabolism. In addition, microbiota metabolites seem to directly affect mitochondrial oxidative stress and the formation of mitophagy, thereby regulating the activation and/or production of inflammatory cytokines. Furthermore, liver is exposed to endotoxins such as lipopolysaccharide (LPSs), which can be delivered to the liver or endogenously produced by gut microbiota
[70][119]. Yeast Fermentate Prebiotics may have beneficial roles in maintaining the health of the host
[71][120], which could be associated with balancing the gut microbial community, regulating immunity
[72][121]. In particular, prebiotics are used by microorganisms as food, which could exert a beneficial effect on the health of the host. Currently available prebiotics include lactulose, fibers, inulin derivatives, and milk oligosaccharides. In addition, phytochemicals may act as prebiotics in the gut lumen for autophagy inducers
[73][122].