Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hasniza Zaman Huri | -- | 1590 | 2023-03-29 09:49:51 | | | |
| 2 | Camila Xu | Meta information modification | 1590 | 2023-03-29 09:56:00 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Perumalsamy, S.; Huri, H.Z.; Abdullah, B.M.; Mazlan, O.; Wan Ahmad, W.A.; Vethakkan, S.R.D.B. IR and Atherosclerosis in T2DM with CAD. Encyclopedia. Available online: https://encyclopedia.pub/entry/42605 (accessed on 06 March 2026).
Perumalsamy S, Huri HZ, Abdullah BM, Mazlan O, Wan Ahmad WA, Vethakkan SRDB. IR and Atherosclerosis in T2DM with CAD. Encyclopedia. Available at: https://encyclopedia.pub/entry/42605. Accessed March 06, 2026.
Perumalsamy, Sangeetha, Hasniza Zaman Huri, Bashar Mudhaffar Abdullah, Othman Mazlan, Wan Azman Wan Ahmad, Shireene Ratna D. B. Vethakkan. "IR and Atherosclerosis in T2DM with CAD" Encyclopedia, https://encyclopedia.pub/entry/42605 (accessed March 06, 2026).
Perumalsamy, S., Huri, H.Z., Abdullah, B.M., Mazlan, O., Wan Ahmad, W.A., & Vethakkan, S.R.D.B. (2023, March 29). IR and Atherosclerosis in T2DM with CAD. In Encyclopedia. https://encyclopedia.pub/entry/42605
Perumalsamy, Sangeetha, et al. "IR and Atherosclerosis in T2DM with CAD." Encyclopedia. Web. 29 March, 2023.
Copy Citation
Type 2 diabetes mellitus (T2DM) is characterized by impaired insulin secretion on a background of insulin resistance (IR). IR and T2DM are associated with atherosclerotic coronary artery disease (CAD). The mechanisms of IR and atherosclerosis are known to share similar genetic and environmental roots.
insulin resistance
type 2 diabetes mellitus
atherosclerosis
endothelial dysfunction
1. Background
Type 2 diabetes mellitus (T2DM) accounts for over 90 per cent of all patients with diabetes [1]. T2DM shares several risk factors in common with coronary artery diseases (CAD), such as aging, hypertension, dyslipidemia, obesity, lack of physical activity, genetics, and stress. In addition, an increase in the prevalence of diabetes indirectly escalates the risk of CAD [2]. T2DM is primarily caused by insulin resistance (IR), in which insulin cannot promote glucose uptake in skeletal muscle and adipose tissue and suppress hepatic glucose output [3][4].
Endothelial dysfunction (ED), the failure of endothelium to maintain vascular homeostasis, is present at the early stages of IR. It may be the origin of the initiation and progression of atherosclerosis [5]. Atherosclerosis that affects the coronary arteries can cause coronary artery disease (CAD) [6]. CAD is a type of cardiovascular disease (CVD) that is often asymptomatic in T2DM patients until the onset of myocardial infarction (MI) or sudden cardiac death [7].
IR may be associated with ED via various mechanisms such as disturbances of the subcellular signaling pathways that involve insulin action and nitric oxide (NO) production, oxidative stress, endothelin, the secretion of hormones and cytokines, as well as the renin–angiotensin–aldosterone system [8]. Changes in the subcellular signaling pathways convert the anti-atherogenic property of NO into pro-atherogenic and cause the development of atherosclerosis and subsequently CAD [9].
ED has been linked to several factors such as diabetes, hypertension, smoking, a high-fat diet, as well genetic factors [10]. The genetic factor of ED involves the dysregulation of the endothelial NO synthase (eNOS) gene followed by disruption of the endothelial vascular homeostasis [8]. It has been reported that the polymorphism caused by four or five repeats of a 27-base-pair sequence in intron 4 of the eNOS gene is associated with the risk of CAD [11]. Another study of the eNOS gene also found that intron 4 polymorphism was associated with T2DM [12]. Thus, the genetic factor of ED is linked to both T2DM and CAD [13].
The eNOS gene expression pathways involve the activation of the pro-inflammatory markers related to IR, atherosclerotic CAD and T2DM [14]. These genes and their single-nucleotide polymorphisms (SNPs) may act as the possible identifiers of IR and atherosclerosis in T2DM patients with CAD.
2. How Does IR Result in T2DM?
Insulin is a peptide hormone secreted by the β cells of the pancreatic islets of Langerhans. It maintains normal blood glucose levels by facilitating cellular glucose uptake, regulating carbohydrate, lipid, and protein metabolism, and promoting cell division and growth through its mitogenic effects [15]. Insulin secretion may be influenced by alterations in synthesis at the level of gene transcription, translation, and post-translational modification in the Golgi among other factors influencing insulin release from the secretory granules [15][16].
The actions of insulin are influenced by the interplay of other hormones such as growth hormone, IGF-1, glucagon, glucocorticoids, and catecholamines [17]. Excessive hormone production might have contributed to IR in some circumstances, though com-promised insulin signaling is thought to have a greater role at the cellular level [18]. As such, IR can be considered as a state of chronic, low-level inflammation [19]. Several states or mechanisms result in IR, such as fatty acid-induced IR [20] and lipid accumulation in skeletal muscle and liver [21].
The beginning of IR frequently results in insulin paucity and a gradually dwindling blood glucose regulation, hyperinsulinemia, and increased levels of free fatty acid (FFA) circulation [22][23]. The circulating FFAs are the main substrate for production of hepatic triglycerides in the form of very low-density lipoprotein (VLDL) [24][25][26]. These changes are trailed by a subsequent decline in plasma glucose control, which manifests as elevated fasting plasma glucose levels with intermittent and persistent hyperglycemia leading to a diagnosis of T2DM (Figure 1) [27]. Therefore, in terms of pathogenesis, glucolipotoxicity is stated as an essential determinant of T2DM. Glucolipotoxicity is the combination of glucotoxicity (hyperglycemia or elevated blood glucose levels) and lipotoxicity (high lipid levels, especially FFAs) [28].
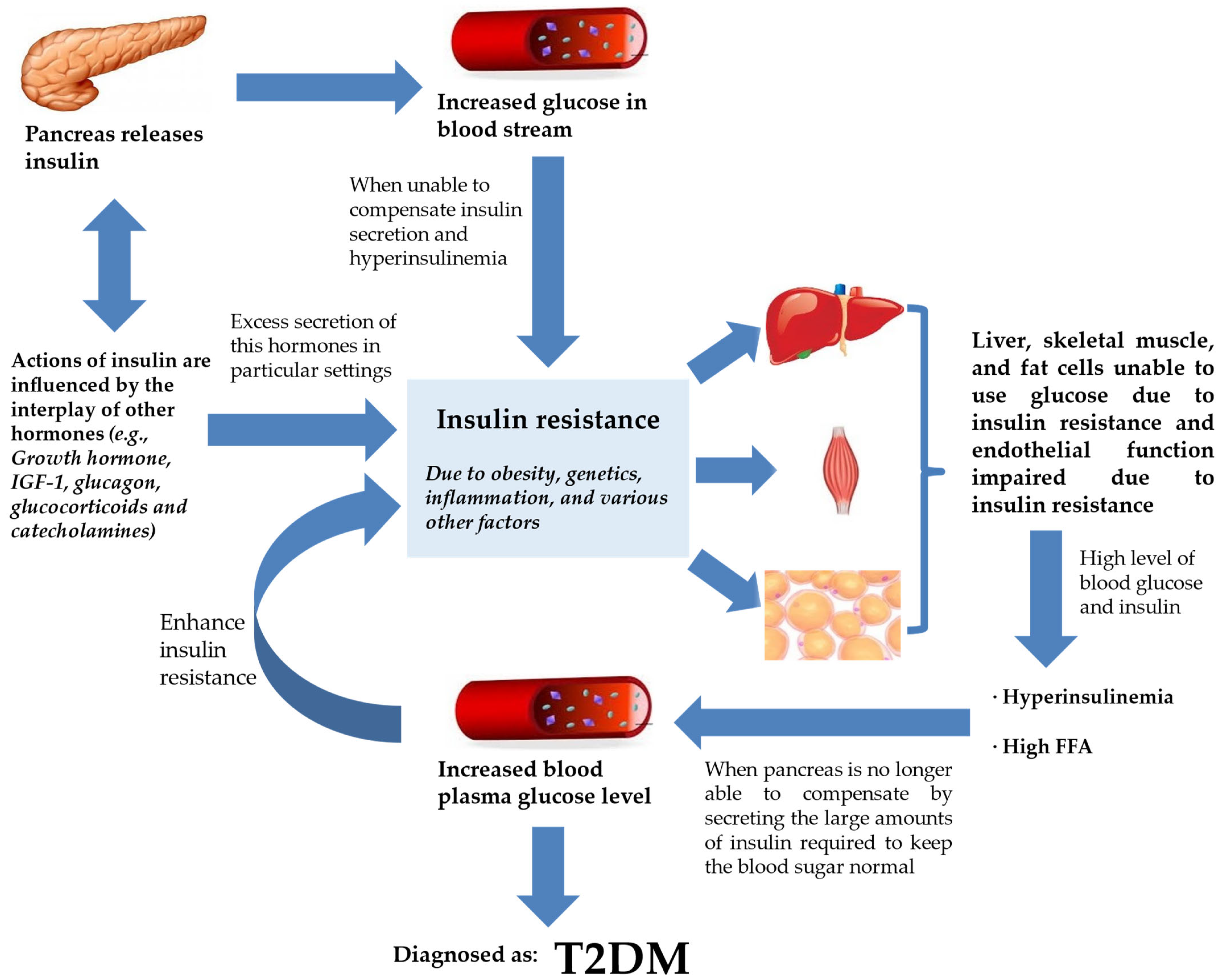
Figure 1. IR and T2DM. Insulin secretion by the pancreas is influenced by the interplay of various hormones (hormone, IGF-1, glucagon, glucocorticoids, and catecholamines) in order to regulate glucose levels in the bloodstream. However, insulin failure to manage blood glucose will lead to the progression of IR. IR will compromise endothelial function, rendering liver, skeletal muscle, and fat cells unable to use glucose. This worsens hyperinsulinemia, and the pancreas is already incapable of keeping normal blood glucose despite secreting large amounts of insulin. Blood plasma glucose levels keep increasing and the feedback loop enhances IR further. At this stage, the patient is diagnosed with T2DM. Abbreviations: ED, endothelial dysfunction; FFA, free fatty acid; IGF-1, insulin-like growth factor 1; IR, insulin resistance; T2DM, type 2 diabetes mellitus.
From Figure 1, T2DM can be seen as end-stage IR in a genetically susceptible individual. The effects of insulin, insulin shortage, and IR, however, vary depending on the physiological function of the tissues and organs and their reliance on insulin for metabolic activities [29]. The direct and indirect effects in tissues sensitive to insulin are linked to glucose homeostasis in the liver, skeletal muscle, and fat tissues [30]. Another site of insulin action and the manifestation of IR and T2DM is the endothelium [31]. With ED being observed at the early phase of atherosclerosis, the vascular endothelial cells may play critical roles in various facets of cardiovascular biology [8].
IR is strongly prevalent in the pathogenesis of T2DM [32]. A study in the United Kingdom found that almost 8% of 6500 participants were diagnosed with diabetes throughout the course of the study’s 10 years of follow-up [33]. There was a significant decline in insulin sensitivity in the five years before the diabetes diagnosis in comparison to those who were not diagnosed with diabetes.
3. Atherosclerosis and Its Relationship with IR, T2DM, and CAD
T2DM and atherosclerotic CVD share many common factors. In T2DM, inflammatory processes play a part in the cause of atherosclerotic CVD [34]. The markers of inflammation predict CAD and the levels of markers are raised in patients with T2DM [35]. ED is considered as an early marker for atherosclerosis, and it plays a prominent role in the development of IR and T2DM [5]. IR and impaired insulin secretion are central to the pathogenesis of T2DM, but it is unclear how these abnormalities are related to accelerated atherosclerosis [36]. The precise mechanisms for the susceptibility and advancement of atherosclerosis in patients with T2DM are undetermined.
Yet, there are many indications linking lipoproteins with atherosclerosis [37]. Lipoproteins are the combinations of fats and protein. The lipoproteins are proved to play an important role in atherosclerosis, and they interact with the artery wall to trigger the chain of events that leads to atherosclerosis. Low-density lipoprotein (LDL) is the most common lipoprotein that is associated with atherosclerosis, but other lipoproteins such as VLDL may also be atherogenic. An important component of these atherogenic lipoproteins is Apolipoprotein B (Apo B) that promotes the accumulation of LDL in the intima initiates atherosclerosis [38]. This is mediated by increased endothelial permeability and raised intimal retention of LDL [39]. Moreover, diabetes (T1DM and T2DM) is associated with increased hepatic production of triglyceride-rich lipoproteins, which leads to increased formation of atherogenic VLDL [40]. The atherogenic index is defined as the ratio of LDL-C (low-density lipoprotein cholesterol) to HDL-C (high-density lipoprotein cholesterol). In one study, researchers discovered that hyperlipidemic rats had a higher atherogenic ratio (8.06 mg/dL) than the control group (1.09 mg/dL) [41].
Metabolic syndrome, pre-diabetes, and T2DM, which all co-segregate with IR, accelerate the progression of atherosclerosis and the consequential disease [34].
Silent atherosclerosis and cardiovascular complications start to commence during the pre-diabetic period in genetically susceptible people [42]. During the onset of diabetes, hyperglycemia and hyperinsulinemia are present in IR and lead to acceleration of atherosclerosis and CAD (Figure 2) [43][44]. Still, despite the fact that diabetic patients developed severe lesion formation, patients with and without diabetes often have identical atherosclerotic lesions [36]. Areas of necrotic lesions accumulate lipid, cholesterol crystals, and inflammatory cells, which leads to thickening of the arterial wall. Complications can arise from erosion or calcification, causing a thrombus to form and obstruct the lumen, resulting in health defects such as CAD [8][44].
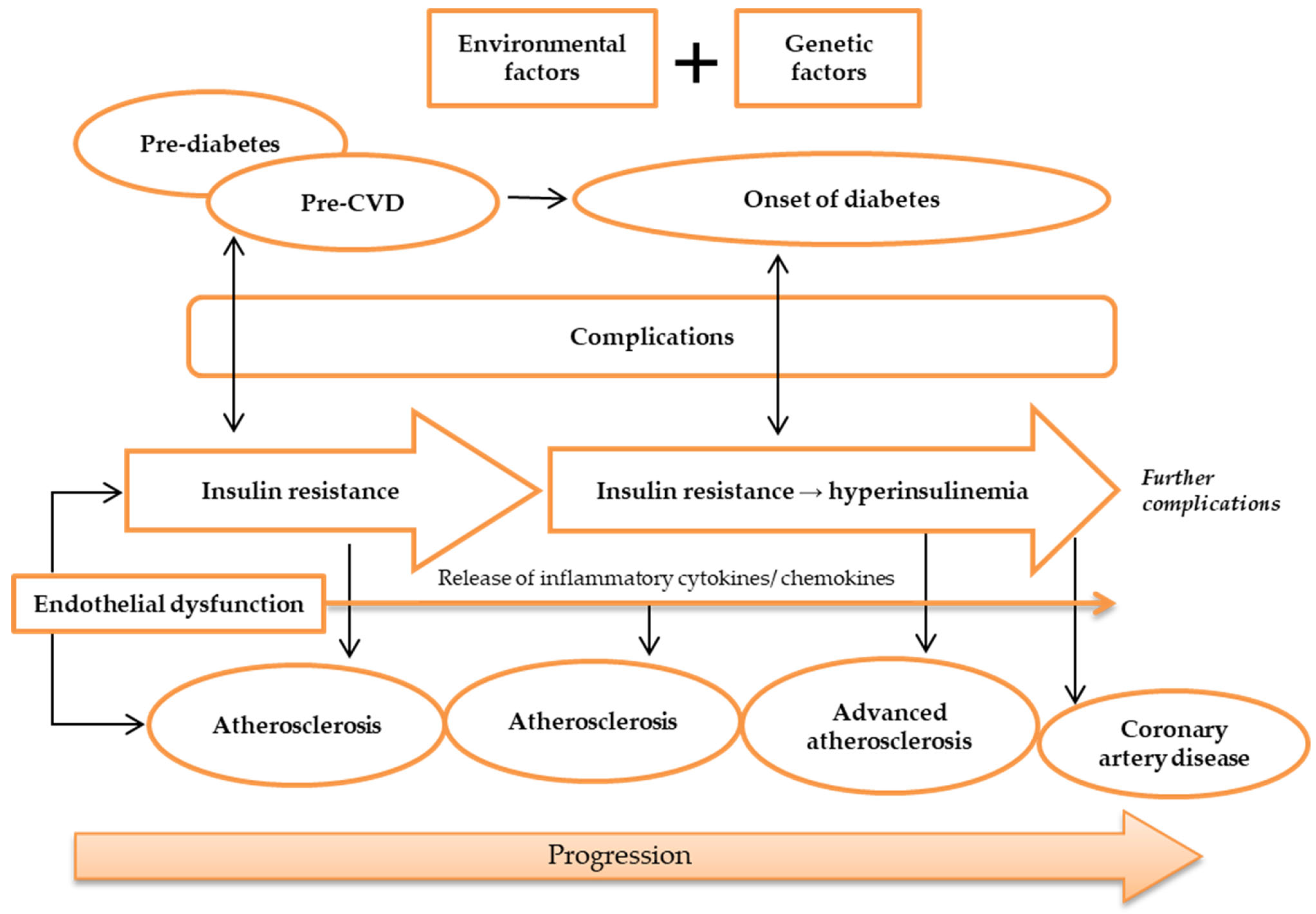
Figure 2. Pathogenesis of atherosclerosis. Atherosclerosis may be caused by environmental and genetic factors. The onset of atherosclerosis is during ED initial occurrence, which is also associated with the onset of IR. These incidences reflect pre-diabetes and pre-CVD phases, and symptoms of complications may appear. Ongoing atherosclerosis development is incessantly supported by ED, linked to the release and regulation of inflammatory cytokines and chemokines, as well as IR, which develops into hyperinsulinemia. At this stage, patients may have developed advanced atherosclerosis and diabetes, while exhibiting evident complications. Further ED, IR, and atherosclerosis advancement will result in more apparent complications and CAD development. Abbreviations: CAD, coronary artery disease; CVD, cardiovascular disease; ED, endothelial dysfunction; IR, insulin resistance.
Moreover, genetic factors have been investigated to modulate atherosclerosis development. Candidate gene and linkage analysis studies have failed to identify previously unknown pathways in the pathogenesis of atherosclerosis [45][46]. The publication of the HapMap has made possible genome-wide association studies aimed at probing the pathogenesis of atherosclerosis. Genome-wide association studies have reproducibly identified several loci involved in the pathogenesis of atherosclerosis, and most of the identified genes are newly implicated in the disease process. APOE- or LDLR-deficient mice are widely used models to study the pathogenesis of atherosclerosis.
References
- IDF. Type 2 diabetes. In IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; p. 14.
- Grant, P.J.; Cosentino, F.; Marx, N. Diabetes and coronary artery disease: Not just a risk factor. Heart 2020, 106, 1357–1364.
- Boersma, G.J.; Johansson, E.; Pereira, M.J.; Heurling, K.; Skrtic, S.; Lau, J.; Katsogiannos, P.; Panagiotou, G.; Lubberink, M.; Kullberg, J.; et al. Altered Glucose Uptake in Muscle, Visceral Adipose Tissue, and Brain Predict Whole-Body Insulin Resistance and may Contribute to the Development of Type 2 Diabetes: A Combined PET/MR Study. Horm. Metab. Res. 2018, 50, 627–639.
- Honka, M.J.; Latva-Rasku, A.; Bucci, M.; Virtanen, K.A.; Hannukainen, J.C.; Kalliokoski, K.K.; Nuutila, P. Insulin-stimulated glucose uptake in skeletal muscle, adipose tissue and liver: A positron emission tomography study. Eur. J. Endocrinol. 2018, 178, 523–531.
- Takeda, Y.; Matoba, K.; Sekiguchi, K.; Nagai, Y.; Yokota, T.; Utsunomiya, K.; Nishimura, R. Endothelial Dysfunction in Diabetes. Biomedicines 2020, 8, 182.
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823.
- Singh, K.B.; Nnadozie, M.C.; Abdal, M.; Shrestha, N.; Abe, R.A.M.; Masroor, A.; Khorochkov, A.; Prieto, J.; Mohammed, L. Type 2 Diabetes and Causes of Sudden Cardiac Death: A Systematic Review. Cureus 2021, 13, e18145.
- Xu, S.; Ilyas, I.; Little, P.J.; Li, H.; Kamato, D.; Zheng, X.; Luo, S.; Li, Z.; Liu, P.; Han, J.; et al. Endothelial Dysfunction in Atherosclerotic Cardiovascular Diseases and Beyond: From Mechanism to Pharmacotherapies. Pharmacol. Rev. 2021, 73, 924–967.
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346.
- Poredos, P.; Poredos, A.V.; Gregoric, I. Endothelial Dysfunction and Its Clinical Implications. Angiology 2021, 72, 604–615.
- Syed, R.; Biyabani, M.U.; Prasad, S.; Deeba, F.; Jamil, K. Correlation and Identification of Variable number of Tandem repeats of eNOS Gene in Coronary artery disease (CAD). Saudi J. Biol. Sci. 2010, 17, 209–213.
- Rai, H.; Parveen, F.; Kumar, S.; Kapoor, A.; Sinha, N. Association of Endothelial Nitric Oxide Synthase Gene Polymorphisms with Coronary Artery Disease: An Updated Meta-Analysis and Systematic Review. PLoS ONE 2014, 9, e113363.
- Dong, J.; Ping, Y.; Wang, Y.; Zhang, Y. The roles of endothelial nitric oxide synthase gene polymorphisms in diabetes mellitus and its associated vascular complications: A systematic review and meta-analysis. Endocrine 2018, 62, 412–422.
- Król, M.; Kepinska, M. Human Nitric Oxide Synthase—Its Functions, Polymorphisms, and Inhibitors in the Context of Inflammation, Diabetes and Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 56.
- Riaz, S. Obesity as a Risk Factor for Diabetes Mellitus in the Local Population of Pakistan. Univers. J. Clin. Med. 2014, 2, 58–64.
- Fu, Z.; Gilbert, E.R.; Liu, D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013, 9, 25–53.
- Rogowicz-Frontczak, A.; Majchrzak, A.; Zozulińska-Ziółkiewicz, D. Insulin resistance in endocrine disorders—Treatment options. Endokrynol. Polska 2017, 68, 334–351.
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191.
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150.
- Xin, Y.; Wang, Y.; Chi, J.; Zhu, X.; Zhao, H.; Zhao, S.; Wang, Y. Elevated free fatty acid level is associated with insulin-resistant state in nondiabetic Chinese people. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 139–147.
- Da Silva Rosa, S.C.; Nayak, N.; Caymo, A.M.; Gordon, J.W. Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol. Rep. 2020, 8, e14607.
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic syndrome and insulin resistance: Underlying causes and modification by exercise training. Compr. Physiol. 2013, 3, 1–58.
- Wondmkun, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3611–3616.
- Ebbert, J.O.; Jensen, M.D. Fat depots, free fatty acids, and dyslipidemia. Nutrients 2013, 5, 498–508.
- Khound, R.; Taher, J.; Baker, C.; Adeli, K.; Su, Q. GLP-1 Elicits an Intrinsic Gut–Liver Metabolic Signal to Ameliorate Diet-Induced VLDL Overproduction and Insulin Resistance. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 2252–2259.
- Petersen, K.S.; Bowen, K.J.; Tindall, A.M.; Sullivan, V.K.; Johnston, E.A.; Fleming, J.A.; Kris-Etherton, P.M. The Effect of Inflammation and Insulin Resistance on Lipid and Lipoprotein Responsiveness to Dietary Intervention. Curr. Dev. Nutr. 2020, 4, nzaa160.
- Tohidi, M.; Baghbani-Oskouei, A.; Ahanchi, N.S.; Azizi, F.; Hadaegh, F. Fasting plasma glucose is a stronger predictor of diabetes than triglyceride–glucose index, triglycerides/high-density lipoprotein cholesterol, and homeostasis model assessment of insulin resistance: Tehran Lipid and Glucose Study. Acta. Diabetol. 2018, 55, 1067–1074.
- Akash, M.S.; Rehman, K.; Chen, S. Role of inflammatory mechanisms in pathogenesis of type 2 diabetes mellitus. J. Cell. Biochem. 2013, 114, 525–531.
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022, 7, 216.
- Chadt, A.; Al-Hasani, H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflügers Arch.-Eur. J. Physiol. 2020, 472, 1273–1298.
- Zhou, M.S.; Wang, A.; Yu, H. Link between insulin resistance and hypertension: What is the evidence from evolutionary biology? Diabetol. Metab. Syndr. 2014, 6, 12.
- Roden, M.; Petersen, K.; Shulman, G. Insulin Resistance in Type 2 Diabetes. In Textbook of Diabetes; John Wiley & Sons Ltd.: West Sussex, UK, 2017; pp. 174–186.
- Tabák, A.G.; Jokela, M.; Akbaraly, T.N.; Brunner, E.J.; Kivimäki, M.; Witte, D.R. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: An analysis from the Whitehall II study. Lancet 2009, 373, 2215–2221.
- Di Pino, A.; DeFronzo, R.A. Insulin Resistance and Atherosclerosis: Implications for Insulin-Sensitizing Agents. Endocr. Rev. 2019, 40, 1447–1467.
- Aboonabi, A.; Meyer, R.R.; Singh, I. The association between metabolic syndrome components and the development of atherosclerosis. J. Hum. Hypertens. 2019, 33, 844–855.
- Mariana, M.; Lijun, M.; Barry, I.F. Genetic and Environmental Factors Associated With Type 2 Diabetes and Diabetic Vascular Complications. Rev. Diabet. Stud. 2012, 9, 6–22.
- Nigro, J.; Osman, N.; Dart, A.M.; Little, P.J. Insulin Resistance and Atherosclerosis. Endocr. Rev. 2006, 27, 242–259.
- Sniderman, A.D.; Thanassoulis, G.; Glavinovic, T.; Navar, A.M.; Pencina, M.; Catapano, A.; Ference, B.A. Apolipoprotein B Particles and Cardiovascular Disease: A Narrative Review. JAMA Cardiol. 2019, 4, 1287–1295.
- Bornfeldt, K.E.; Tabas, I. Insulin Resistance, Hyperglycemia, and Atherosclerosis. Cell Metab. 2011, 14, 575–585.
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus–Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835.
- Raut, P.; Dhawale, S.; Kulkarni, D.; Pekamwar, S.; Shelke, S.; Panzade, P.; Paliwal, A. Pharmacodynamic findings for the usefulness of Luffa cylindrica (L.) leaves in atherosclerosis therapy with supporting antioxidant potential. Future J. Pharm. Sci. 2021, 7, 38.
- Chopra, S.; Peter, S. Screening for coronary artery disease in patients with type 2 diabetes mellitus: An evidence-based review. Indian J. Endocrinol. Metab. 2012, 16, 94–101.
- Upadhyay, R.K. Emerging Risk Biomarkers in Cardiovascular Diseases and Disorders. J. Lipids 2015, 2015, 971453.
- Sakakura, K.; Nakano, M.; Otsuka, F.; Ladich, E.; Kolodgie, F.D.; Virmani, R. Pathophysiology of Atherosclerosis Plaque Progression. Heart Lung Circ. 2013, 22, 399–411.
- Mota, R.; Homeister, J.W.; Willis, M.S.; Bahnson, E.M. Atherosclerosis: Pathogenesis, Genetics and Experimental Models. In eLS.; John Wiley & Sons Ltd.: Chichester, UK, 2017; pp. 1–10.
- Poznyak, A.V.; Grechko, A.V.; Wetzker, R.; Orekhov, A.N. In Search for Genes Related to Atherosclerosis and Dyslipidemia Using Animal Models. Int. J. Mol. Sci. 2020, 21, 2097.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
840
Revisions:
2 times
(View History)
Update Date:
29 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





