Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jeyakumar Rajesh Banu | -- | 3554 | 2023-03-27 11:21:04 | | | |
| 2 | Sirius Huang | Meta information modification | 3554 | 2023-03-28 03:00:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Gopalakrishnan, G.; Jeyakumar, R.B.; Somanathan, A. Mechanism of Advanced Oxidation Processes. Encyclopedia. Available online: https://encyclopedia.pub/entry/42556 (accessed on 07 March 2026).
Gopalakrishnan G, Jeyakumar RB, Somanathan A. Mechanism of Advanced Oxidation Processes. Encyclopedia. Available at: https://encyclopedia.pub/entry/42556. Accessed March 07, 2026.
Gopalakrishnan, Ginni, Rajesh Banu Jeyakumar, Adishkumar Somanathan. "Mechanism of Advanced Oxidation Processes" Encyclopedia, https://encyclopedia.pub/entry/42556 (accessed March 07, 2026).
Gopalakrishnan, G., Jeyakumar, R.B., & Somanathan, A. (2023, March 27). Mechanism of Advanced Oxidation Processes. In Encyclopedia. https://encyclopedia.pub/entry/42556
Gopalakrishnan, Ginni, et al. "Mechanism of Advanced Oxidation Processes." Encyclopedia. Web. 27 March, 2023.
Copy Citation
Advanced oxidation processes (AOPs) involves the generation of powerful oxidizing radical groups, such as hydroxyl radicals, which function as oxidizing agents and mineralize organic chemical substances into CO2 and H2O. AOPs such as photocatalysis and photo-Fenton have been widely considered to be very effective in removing persistent organic pollutants.
advanced oxidation processes
solar photo-Fenton
solar photocatalysis
biological process
1. Introduction
One of the essential needs of all living things, including humans, is safe and uncontaminated drinking water. However, these days, finding it has become a big issue. The water used by humans for most activities produces wastewater. Wastewater accounts for over 80% of the water provided for domestic use [1]. About 5–10 billion tons of industrial waste are generated each year and discharged untreated into the environment [2]. If released directly into the environment without treatment, the wastewater generated from various industries can cause significant environmental problems [3]. Therefore, removing emerging pollutants is essential to making the water safe for drinking, and the release of wastewater should not cause any adverse effect on the environment. Moreover, recycling and reusing treated wastewater is necessary to reduce freshwater consumption. There are physical, chemical, and biological treatment methods; these are the most commonly employed wastewater treatment technologies. In the case of physical treatment, the removal of organics from the wastewater is challenging. The existing biological and chemical treatment methods include ozonation, electrochemical treatment, photocatalysis, aerobic treatment, activated sludge, anaerobic treatment, coagulation–flocculation treatment, etc. [4]. Aerobic biological activities require a lot of energy and generate a significant amount of biomass. Anaerobic biological processes are susceptible to shock loading, which requires further treatment of the wastewater produced before the final release. Although biological processes are efficient and cost-effective, they primarily need a vast area and have a significant energy demand (for aeration) and a substantial quantity of produced sludge [5]. Chemical treatments can quickly oxidize and totally breakdown the organic contaminants, making them an effective wastewater treatment approach [6]. As a result, the development of advanced oxidation processes (AOPs), which have an excellent potential for ultimately destroying a variety of resistant pollutants, has shown to be a promising alternative [7].
2. Mechanism of Advanced Oxidation Processes
There are two types of AOPs: homogeneous processes and heterogeneous processes. The homogeneous processes in the AOP are defined by the chemical alterations resulting solely from the interactions between the chemical reagents and the target substances [8]. In heterogeneous processes, the reactants and products adsorb and desorb on the catalyst’s active sites. As the reaction occurs, the desorption of products and the absorption of new species takes place on the active sites; thereby, the efficiency is affected by the surface characteristics and the pore structure of the catalyst [9]. The different methods for the production of hydroxyl radicals take place via several combinations, such as the Fenton process (Fe2+/H2O2), the solar photo-Fenton process (solar/Fe2+/H2O2), solar photocatalysis (solar/TiO2/H2O2), peroxone (Ozone/H2O2), the combination of peroxone with ultraviolet (Ozone/H2O2), Ozone/UV, and photolysis (H2O2/UV). The oxidation by-product formation mechanisms in the various AOPs are discussed.
2.1. Solar Photo-Fenton Process
Among the AOPs, the solar photo-Fenton process is particularly interesting for treating a large variety of hazardous pollutants and is one of the most environmentally benign treatment systems [10]. Due to the potential utilization of sunlight, this technique has drawn more attention. As a result, it may be considered to be an affordable AOP as sunlight is always available in a tropical country such as India [11][12]. Figure 1 shows the schematic diagram of the SPF.
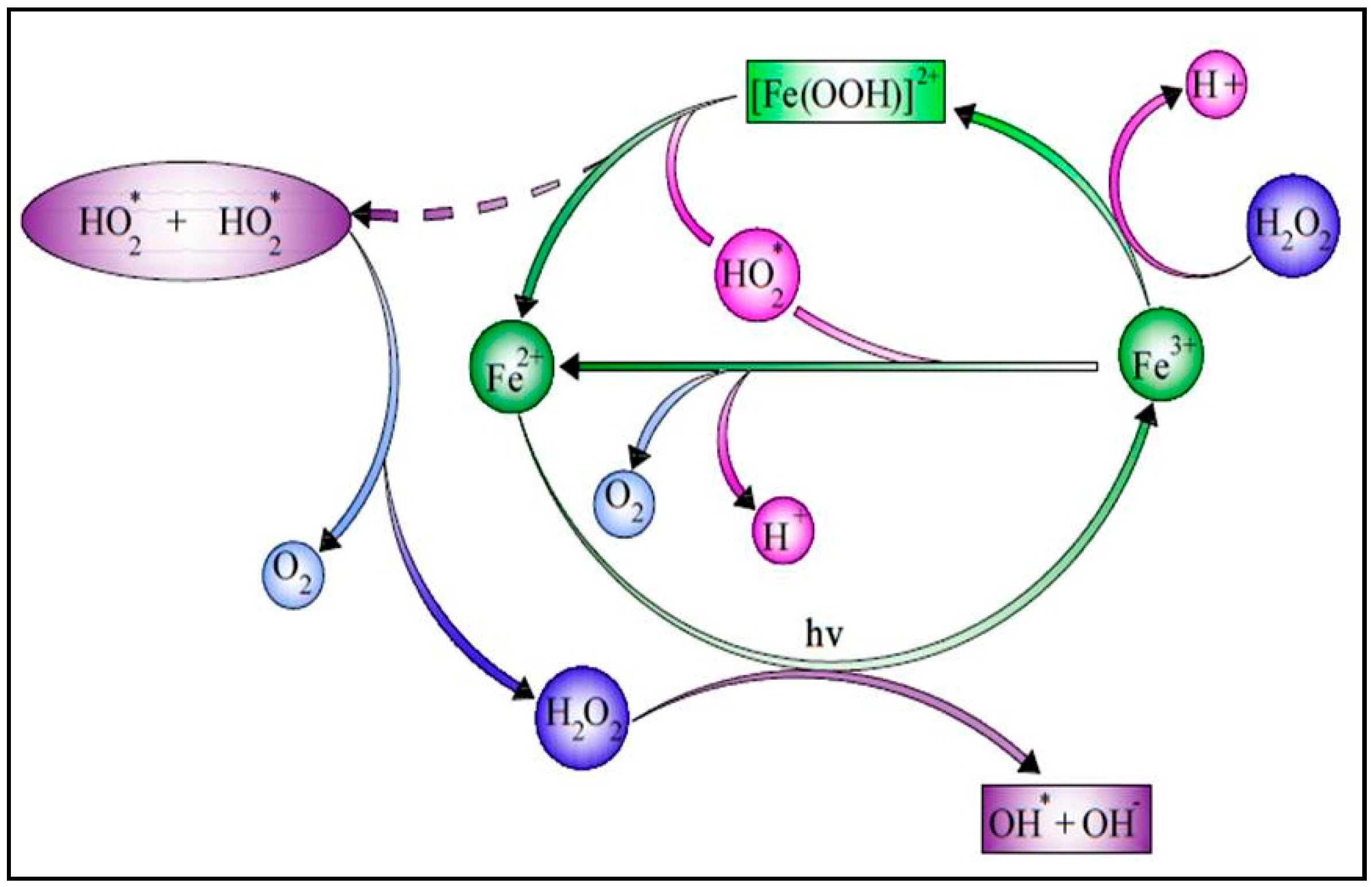
Figure 1. Schematic diagram of a solar photo-Fenton process.
As shown in Equation (1), catalysis by ferrous iron breaks down hydrogen peroxide in an acidic medium to produce highly reactive species such as OH● radicals without requiring high pressure or temperature. When solar irradiation is used, Fe3+ hydroxy complexes (Fe(OH)2+) and Fe3+ organic complexes (FeOOCR2+) can perform the photo-reduction in Equation (2) and the photodecarboxylation in Equation (3), enabling the regeneration of iron and generating extra OH● radicals [13]. The hydroxyl radicals break down the complex organic structures into simpler compounds, and the partial oxidation of the non-biodegradable organics contributes to the biodegradability of the wastewater.
Fe2+ + H2O2 → Fe3+ + OH● + HO
[Fe(OH)]2+ + hν → Fe2+ + OH● + H+
Fe(OOCR)2+ + hν → Fe2+ + CO2 + R•
In the solar photo-Fenton process, the significant characteristic of iron is that it undergoes cyclic oxidation and reduction. Table 1 shows the treatment of wastewater by various solar photo-Fenton processes.
Xu et al. (2007) [14] used the solar photo-Fenton technique to treat a pulp and paper mill’s bleaching wastewater. According to the reports, the TOC removal was usually faster for 15 min using a cost-effective source of solar light irradiation. After that, there was a slow increase for 3 h. Sheik et al. (2008) [15] explored the photocatalytic oxidation of organic contaminants such as p-nitroaniline, p-aminophenols, and acetanilide. It was found that the organic compounds were entirely oxidized and degraded into CO2 and H2O. Karimi et al. (2011) [16] focused on the discoloration of wastewater from a paper mill by the photo-Fenton process. The research revealed that after 15 min of photo-Fenton treatment, the soda effluent had dropped nearly 65% of its original color.
Lucas et al. (2012) [17] reported experimentally that about 90% of DOC degradation was attained with 5 mg/L of Fe2+ and 50 mM of H2O2 for the treatment of pulp and paper mill wastewater. The same DOC degradation can be achieved by solar photo-Fenton using less H2O2 and a shorter time. Bernabeu et al. (2012) [18] employed the photo-Fenton process to degrade emerging pollutants. According to the findings, emerging contaminants at high concentrations are removed at an acidic pH rather than a neutral pH. Trovo et al. (2012) [19] evaluated the photodegradation of paracetamol using ferrous sulphate and potassium ferrioxalate under artificial solar light. In contrast to FeOx, FeSO4 decreased Fe3+ to Fe2+, enhancing the decomposition by generating higher concentrations of hydroxylated intermediates. Garcia and Buitron (2013) [13] proposed the photo-Fenton process and obtained 97% decolorization of each azo dye and the development of chemometric tools, a strategy that reduced the peroxide from 33% to 65%. Turbay et al. (2013) [20] examined the photo-Fenton reaction-based tetracycline antibiotic degradation under varying hydrogen peroxide doses. The outcome demonstrated the improved effectiveness of the treatment and a more effective use of the hydroxyl radicals generated in the reaction medium.
Rocha et al. (2014) [21] treated extracted petroleum wastewater utilizing sunlight as the irradiation source. After 7 h of exposure to sunlight, the experimental results showed a reduction of about 92.7% and 96.2% of the polycyclic aromatic hydrocarbons and the aromaticity, respectively. Rodriguez et al. (2014) [22] explored the potential of the photo-Fenton process to decolorize and mineralize simulated wastewaters from wool dyeing tanks. The results indicated a high level of efficiency in decreasing TOC, COD, and BOD5. Velegraki and Mantzavinos (2015) [23] studied the solar photo-Fenton technique for the treatment of winery effluent and achieved mineralization at a low catalyst dose (Fe2+ = 5 mg/L) and relied on an oxidant consumption of 500 mg/L. Chueca et al. (2015) [24] assessed the efficacy of a mild solar photo-Fenton system for disinfecting the actual effluents containing fecal bacteria. Despite the complexity and variety of the pollutants in the effluents, the tests conducted on the real effluents yielded highly encouraging results. Soares et al. (2015) [25] treated the textile dye wastewater using ferric organic ligands with the solar photo-Fenton process. It was reported that 87% of the mineralization was achieved at neutral pH values with biodegradability enhancement. Guzman et al. (2016) [26] assessed solar photo-Fenton treatment and various ozone-based processes for the pretreatment of synthetic samples of citrus wastewater. According to the findings, the photo-Fenton method removes COD and DOC at rates of 76.9% and 53.3%, respectively, which are higher than those of ozone-based systems.
Benitez and Penuela (2018) [27] examined the complete removal of benzophenone and the biodegradability increase using the solar photo-Fenton process under simulated solar irradiation. Costa et al. (2018) [28] treated biodiesel effluent, employing the solar photo-Fenton approach to decrease COD by adding oxalate at 50 kJ m−2 of accumulated UVA radiation. About 72% of COD and 76% of BOD removal were achieved using 1 mmol L−1 of ferrioxalate. Garcia et al. (2021) [29] investigated an advanced method for post-treating laundry effluent using the solar photo-Fenton process. It was found that a high removal efficiency was achieved, reaching 89% and 96%, respectively, in just 8 min. Arka et al. (2021) [30] investigated the solar photo-Fenton method for the treatment of institutional wastewater. According to the study, the solar UV/Fe2+/H2O2 process was very efficient in treating the wastewater from institutions, achieving a greater pollutant removal rate. The removal of six representative pharmaceuticals (PHCs) in raw hospital wastewater was investigated by Lumbaque et al. (2021) [31]. It was observed how the molar ratios (1:1 and 1:2) affected the breakdown of PHCs and the production of their transformation products during the treatments. The primary findings showed that PHC degradation is greatly favored in Fe3+:EDDS (1:2) due to the overall H2O2 consumption and the enhanced iron stability, with degradation efficiencies of above 77% in most PHCs.
Lin and Lin (2021) [32] used a solar photo-Fenton technique to remove cytostatic medicines. In comparison to three other Fe(III)-ligand complexes, the addition of EDDS caused the degradation of cyclophosphamide and 5-fluorouracil to occur more quickly. Gualda-Alonso et al. (2022) [33] carried out a continuous flow solar photo-Fenton process, employing a 100 m2 raceway pond reactor (RPR), for the elimination of emerging contaminants. The RPR was run in continuous flow mode with 0.1 mM FeSO4 and 1.47 mM H2O2 at an acidic pH and 1 h of hydraulic residence time. The liquid depth was fixed at 10 cm for winter and 18 cm for summer, removing >85% of the CEC in both cases. Pandey et al. (2023) [34] treated contaminated water by solar photo-Fenton using Fe-rich catalysts and obtained 82% COD removal.
The discussions mentioned above provide a general overview of the refractory compounds degraded by the photo-Fenton process and the effectiveness of the mineralization of these chemical pollutants under observed optimal conditions.
Table 1. Treatment of wastewaters by various solar photo-Fenton processes.
| S. No. | Wastewater | Process | Operational Conditions | Degradation Efficiency | Reference |
|---|---|---|---|---|---|
| 1 | Pulp and paper mill | Solar photo-Fenton | Fe(II) concentration from 31 to 310 mg L−1 (initial pH 3.0, 30 °C), initial H2O2 concentration from 0.5 to 3 Dth | TOC—82% Time—120 min |
Xu et al., 2007 [14] |
| 2 | p-nitroaniline | Solar photo-Fenton | Sheik et al. (2008) [15] | ||
| 3 | Soda pulping effluent | Fenton and photo-Fenton | FeSO4—1 mM H2O2—570 μL |
Color—65% | Karimi et al. (2011) [16] |
| 4 | Pulp and paper mill | Solar photo-Fenton | 5 mg/L of Fe2+ and H2O2 of 50 mM | 90% of DOC mineralization | Lucas et al. (2012) [17] |
| 5 | Azo dye, Acid Blue 161 | Solar photo-Fenton | H2O2/Fe2+ = 12, pH = 2.5 to 4.0 | Degradation 40 % | Trovo et al., 2016 [19] |
| 6 | Sulfonated azo dyes | Solar photo-Fenton | Fe2+ = 5 to 38 mg/L, H2O2 = 98 to 828 mg/L | Color—97% | Garcia and Buitron (2013) [13] |
| 7 | Petroleum extraction | Photo-Fenton | 485.3 mmol/L of H2O2 | Hydrocarbons and aromaticity of about 92.7% and 96.2% | Rocha et al. (2014) [21] |
| 8 | Winery effluent | Photo-Fenton | (Fe2+ = 5 mg/L) | - | Velegraki and Mantzavinos (2015) [23] |
| 9 | Citrus wastewater | Photo-Fenton | H2O2 = 1017 mg/L, pH = 7 | COD—77% | Guzman et al., 2016 [26] |
| 10 | Biodiesel effluent | Solar photo-Fenton | 1 mmol L−1 of ferrioxalate | 72% of COD and 76% of BOD | Costa et al. (2018) [28] |
| 11 | Laundry effluent | Solar photo-Fenton | H2O2 (50–400 mg/L), Fe2+ (2.75–10 mg/L) | SDS removal—96% | Garcia et al. (2021) [29] |
| 12 | Industrial effluent | Solar photo-Fenton | (H2O2)—0.25 to 1.25 g/L, Fe2+—0.005 to 0.12 g/L, pH (2 to 10), time (30 to 180 min) | color (91%), turbidity (90%), and COD—86%) | Arka et al. (2021) [30] |
| 13 | Hospital wastewater | Solar photo-Fenton | Fe3+:EDDS = 1:2, H2O2 (230 mg L−1) |
Degradation = 77% | Lumbaque et al., 2021 [31] |
| 14 | Cytostatic drugs | Solar photo-Fenton | pH 3.0–8.5, 0.1 mM Fe(III)-EDDS, 1 mM H2O2 | Lin and Lin, 2021 [32] | |
| 15 | Emerging contaminants | Solar photo-Fenton | 0.1 mM FeSO4 and 1.47 mM H2O2 at an acidic pH and 1 h of hydraulic residence time | >85% | Gualda-Alonso et al., 2022 [33] |
| 16 | Paraquat (PQ)-contaminated water | Photo-Fenton | Fe-containing industrial waste | COD—82% | Pandey et al., 2023 [34] |
2.2. Solar Photocatalytic Process
Solar photocatalysis has been widely used to degrade organic compounds. According to numerous studies, many organic contaminants have been totally oxidized in irradiated semiconductor solutions [35]. Photocatalysts remove pollutants from wastewater primarily through hydroxyl radical (OH●) attacks by transforming them into harmless compounds such as water and CO2 [36]. The photocatalyst that has been researched the most is TiO2. However, its usage is restricted to ultraviolet light because of the wide energy band gap (3.2 eV) [37]. When exposed to ultraviolet light, a semiconductor catalyst such as TiO2 or another transition metal oxide is initiated by the photon absorption with enough energy to be equal to or greater than the catalyst’s bandgap energy, stimulating an electron to move from the valence band to the conduction band, creating a hole in the valence band while using just 4% of the solar radiation [38][39][40].
TiO2 photocatalysis uses UV-A radiation (λ < 387 nm) in the presence of oxygen and water to produce hydroxyl radicals for the excitation of the photocatalyst [41]. The overall rates of pollutant degradation are significantly influenced by the surface area of the TiO2 catalysts that contains active sites. Thus, increasing the TiO2-specific surface area effectively increases the pollutant adsorption capacity on the catalyst. As a result, a larger surface area with a more significant number of active sites will lead to a faster and more extensive reaction [42].
The activation Equation (4) can be written as
TiO2 + hν → h+ + e−
It is possible to express the oxidation and reduction reactions as given in Equations (5) and (6)
h+ + OH− → -OH●
e− + O2ads− → O2ads−
Another critical factor in the process of photocatalytic oxidation is the pH level. In alkaline media, the reaction of OH● with holes on the TiO2 surface causes the formation of hydroxyl radicals. Thus, hydroxyl radicals accelerate photocatalytic oxidation at higher pH solutions and vice versa [43]. Figure 2 shows the schematic diagram of the SPC.
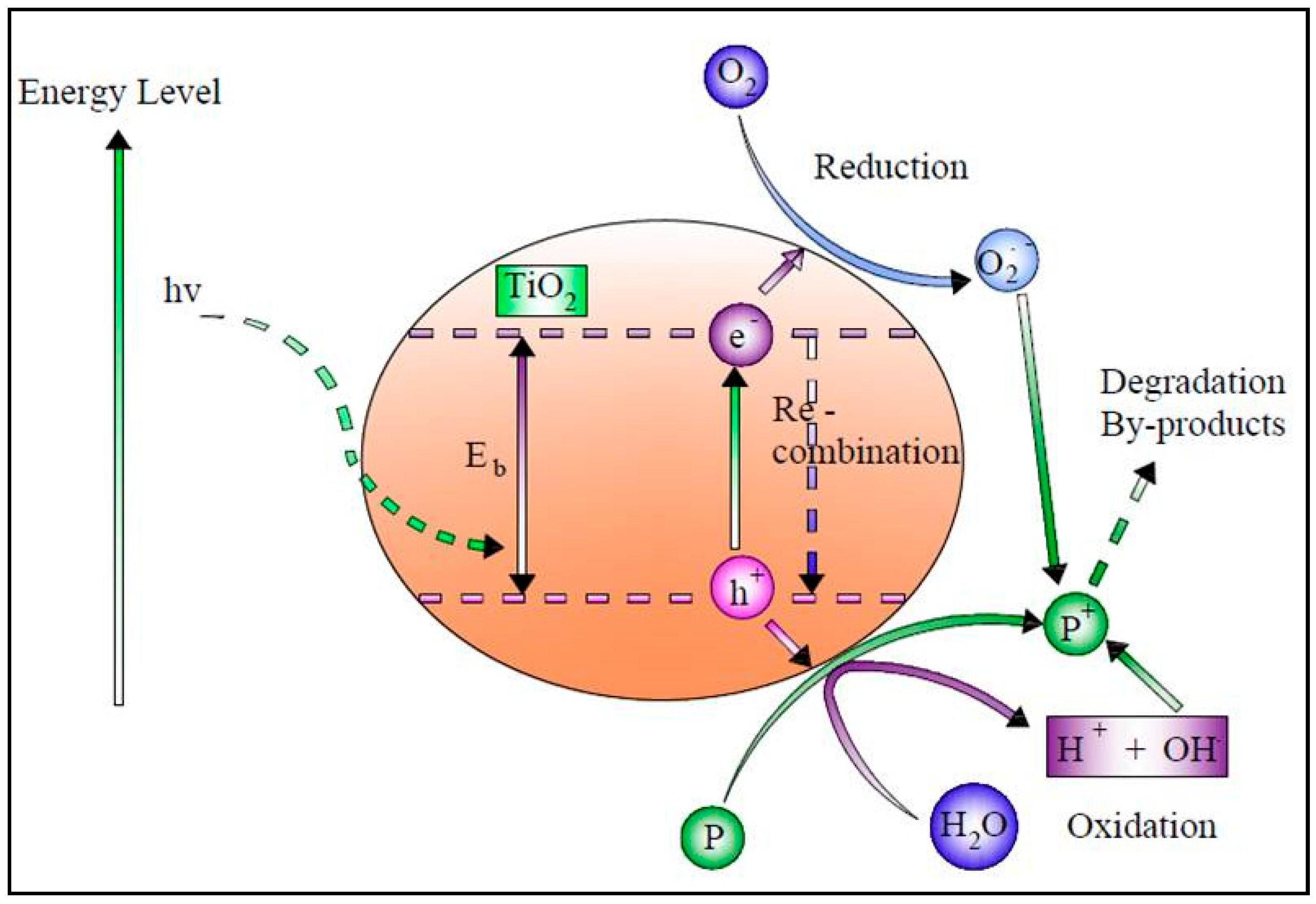
Figure 2. Schematic diagram of the solar photocatalytic process.
Adish and Kanmani (2010) [44] used a single baffle reactor to photo-catalyze H2O2 to treat phenolic wastewater. According to reports, the solar/Fe2+/H2O2 process degrades phenol two to three times more quickly than the solar/TiO2 process. Kumar et al. (2011) [45] studied the degradation of pulp and paper mill wastewater by a photocatalytic process. The findings showed that the increase in the BOD/COD ratio after photocatalytic oxidation was 0.09 for the primary wastewater with the removal of the COD (57.9%), BOD (42.9%), and color (89.2%). Dimitrakopoulou et al. (2012) [46] exploited eight different TiO2 catalysts for the degradation of amoxicillin, and it was reported that Degussa P25 was the most active of the catalysts, with 93% mineralization after 25 and 95 min of reaction time at the amoxicillin concentrations of 10 mg/L and 250 mg/L of titania. Singh et al. (2013) [43] examined the photocatalytic oxidation of the organic content in synthetic wastewater at the pH values 2, 4, 6, 8, and 10 and at normal pH. The outcomes demonstrated clearly that an 86% reduction in COD was achieved at a normal pH level (pH = 6.8), and it was reported that an acidic pH was unfavorable for the reduction of organic content. Nagpure et al. (2013) [47] studied the photocatalytic decomposition of paper mill wastewater with ultraviolet and catalysts such as TiO2. The degradation efficiency was observed as 91.34% using TiO2 as a photocatalyst, with an optimum catalyst dose of 80 mg/50 mL for an irradiation time of 8 h under continuous stirring with maximum intensity. Ruiz et al. (2013) [48] studied the photodegradation of trimethoprim by various nanoparticles deposited on TiO2. It was reported that the degradation of trimethoprim was only 50% of the organic matter mineralization with pure TiO2-P25.
Shao et al. (2013) [49] produced mesoporous TiO2 nanotubes with a large diameter for the photodecomposition of effluent from paper manufacturing. The result indicated that after 12 h of photodegradation, the chemical oxygen demand and chroma percent degradations of the paper manufacturing wastewater were approximately 73% and 99.5%, respectively. Metribuzin, a common herbicide, was transformed and mineralized in extensive detail by TiO2-driven photocatalysis under simulated sunlight, as described by Antonopoulou et al. (2014) [50]. The results pointed out that the complete transformation of metribuzin was attained within 40 min at a metribuzin concentration of 10 mg/L, a TiO2 concentration of 100 mg/L, and I = 750 W/m2, whereas 80% mineralization was achieved within 300 min of irradiation.
Thomas et al. (2014) [51] compared the efficiency of TiO2 and few-layer graphene nanocomposites for degrading rhodamine B under solar radiation. The titania incorporation on the few-layer graphene improved titania’s visible light photocatalytic activity, decreased electron–hole recombination, and increased electron–hole mobility. Fernandez et al. (2015) [41] investigated the photocatalytic treatment of polluted wastewater. The effects of many factors, including the type of microorganism, the water temperature, the dissolved oxygen concentration, and the water matrix composition were studied, and the results showed that this process has great potential for the chemical reduction and disinfection of pathogens. Murgolo et al. (2015) [52] investigated a novel photocatalyst based on nanosized TiO2 upon radiation by both UV and solar-simulated light, resulting in degradation efficiencies ranging from 9 to 87% and 9 to 96%, respectively.
Solano et al. (2018) [53] evaluated the removal efficiency of hexavalent chromium (Cr6+) and divalent zinc (Zn2+) using heterogeneous photocatalysis with TiO2. The result revealed that this process was efficient for the total removal of a Cr6+ concentration of 5 mg/L and was less effective for higher concentrations (15, 25 ppm); however, this technology was ineffective for Zn2+ removal. Yang and Yang (2018) [54] addressed the decomposition of rhodamine B using photocatalyst TiO2. The result indicated that the catalyst reuse was found to be efficient up to 8 times with the same degradation efficiency. Aljouboury et al., 2021 [55] studied the ideal conditions for natural petroleum wastewater, utilizing TiO2/ZnO/Fenton/solar and TiO2/ZnO/air/solar. According to the reports, the TiO2/ZnO/air/solar process achieved the highest treatment effectiveness with the COD removal of 74% and the TOC removal of 99% under ideal circumstances.
Kader et al. (2022) [56] investigated the synthesis of TiO2-based photocatalysts, the production of TiO2-based immobilized borosilicate glass reactors, and the use of the reactors to the treatment of methyl orange dye under UV light. The results revealed that the photocatalyst effectiveness dropped with the increase in pH and the initial dye concentrations. Total organic halogen was utilized as an analytical tool by Abusallout and Hua (2022) [57] to assess the effectiveness of solar TiO2 photocatalysis in dehalogenating disinfection by-products in the water. Compared to pH 5, the TOX dehalogenation was improved at pH 9, and the addition of hydrogen peroxide slightly improved the TOX elimination. The findings demonstrated that compared to the anatase and rutile TiO2 particles, the mixed-phase TiO2 (Aeroxide P25) was significantly more efficient at removing TOX.
To immobilize TiO2 for the removal of paraben from wastewater, Martins et al., 2022 [58] tested the suitability of utilizing polymeric supports. According to the results, polydimethylsiloxane is a suitable material to support TiO2 for wastewater treatment by solar photocatalytic oxidation. Using two nanocomposites (TiO2 and TiO2 (Ag) doped), Behera et al. (2022) [59] compared the photocatalytic activity on the breakdown of phenol from water. The nanocomposites were created using the UV photo-reduction method with silver loadings of 0.25, 0.5, 0.75, and 1% (w/w). At pH 7, about 98% of the phenol was degraded in 180 min with 0.5 g L−1 photo-catalyst TiO2 (Ag-1.0) EY. Rueda-Marquez et al., 2020 [60] examined the solar photocatalytic process using immobilized TiO2 for treating urban wastewater and obtained a maximum removal of greater than 40% of the pharmaceutically active compounds. Gai et al., 2021 [61] synthesized potassium and iodide TiO2 and observed the enhancement of photocatalytic activity under simulated sunlight. Giang et al., 2023 [62] synthesized sulfur-doped TiO2 for the degradation of crystal violet. The findings revealed the potential uses of the material in environmental treatments. Rapti et al., 2022 [63] treated hospital wastewater using TiO2 with the catalyst loading between 150 mg/L and 200 mg/L and attained the degradation efficiency of 73%.
Although numerous research studies on the photocatalytic degradation of wastewater pollutants using TiO2 as a semiconductor have been reported, its industrial application has some constraints, including the recombination of the photogenerated electron–hole pair, the lack of and the inability to produce a catalyst with high photon efficiency and low cost that can absorb a broader range of the solar spectrum, and the surface structure [38]. Moreover, the aggregation of the TiO2 catalyst reduces the surface area and hence the effectiveness of the catalyst [64]. Because of the large amounts of chemicals required for proper performance, photocatalysis is only sometimes considered a viable treatment to be used alone; however, their combination with biological processes may improve the overall process efficiency, increasing their viability. Table 2 shows the treatment of wastewater by various solar photocatalytic processes.
Table 2. Treatment of wastewater by various solar photocatalytic processes.
| S. No. | Wastewater | Process | Operational Conditions | Degradation Efficiency | Reference |
|---|---|---|---|---|---|
| 1 | Pulp and paper mill | Solar photocatalytic (TiO2) | - | COD—57.9%, BOD—42.9%, color—89.2% | Kumar et al. (2011) [45] |
| 2 | Amoxicillin | Solar photocatalytic (TiO2) | TiO2 catalysts 100 to 750 mg/L | 93% mineralization | Dimitrakopoulou et al. (2012) [46] |
| 3 | Synthetic wastewater | Photocatalytic oxidation | pH values 2, 4, 6, 8, 10 | COD—86% | Singh et al. (2013) [43] |
| 4 | Trimethoprim | Photocatalytic oxidation | - | 50% mineralization | Ruiz et al. (2013) [48] |
| 5 | Pulp and paper mill | Photocatalytic oxidation | - | COD—73%, chroma percent—95% | Shao et al. (2013) [49] |
| 6 | Metribuzin | Photocatalytic oxidation | TiO2—100 mg/L and I = 750 W/m2 |
80% mineralization Reaction time—300 min |
Antonopoulou et al. (2014) [50] |
| 7 | Organic pollutants | Nanosized TiO2 supported on single-wall carbon nanotubes | - | UV—9 to 87%, simulated solar light—9 to 96% | Murgolo et al. (2015) [52] |
| 8 | Hexavalent chromium | Solar photocatalytic (TiO2) | pH = 2, 6 and 10, TiO2—2.0, 2.5, and 3.0 g/L | Complete removal for 5 mg/L Cr6+ | Solano et al. (2018) [53] |
| 9 | Rhodamine B | Solar photocatalytic (TiO2) | Film thickness = 361 nm Specific surface area of 47.72 m2 g−1 | 98.33% in 30 min | Yang and Yang (2018) [54] |
| 10 | Urban wastewater | Immobilized TiO2 | High removal (>40%) | Rueda-Marquez et al., 2020 [60] | |
| 11 | Petroleum wastewater | TiO2/ZnO/Fenton/Solar and TiO2/ZnO/Air/Solar | ZnO dosage of 54 g/L and TiO2 dosage 50 g/L | COD—74%, TOC—99% | Aljouboury et al., 2021 [55] |
| 12 | Organic pollutant | Solar TiO2 photocatalysis | Perfect acid and alkali resistance | Gai et al., 2021 [61] | |
| 13 | Methyl orange dye | TiO2-based photocatalysts | pH—7.0, reaction time—5.5 h of UV irradiation, photocatalyst (0.12 g) | Degradation—75.8% | Kader et al. (2022) [56] |
| 14 | Dehalogenation disinfection by-products | Solar TiO2 photocatalysis | pH 9, TiO2—100 mg/L | Effective removal of TOX | Abusallout and Hua (2022) [57] |
| 15 | Pharmaceutical residues | Solar TiO2 photocatalysis | TiO2—200 and 300 mg L−1 | Higher than 56% | Rapti et al. (2022) [63] |
| 16 | Crystal violet | Titanium dioxide/graphene aerogel-doped sulfur | Enhanced photo-degradation | Giang et al., 2023 [62] |
References
- Renuka, R.; Mohan, M.S.; Sowmiya, B.; Raj, A.S. Performance evaluation of panelled anaerobic baffle-cum-filter reactor in treating municipal wastewater. Ecol. Eng. 2016, 97, 1–12.
- Nandkumar, P. Studies on the effluent generated during the pulping process in paper industry. Curr. World Environ. 2008, 3, 189–193.
- Subramonian, W.; Wu, T.Y.; Chai, S.P. A comprehensive study on coagulant performance and floc characterization of natural Cassia obtusifolio seed gum in treatment of raw pulp and paper mill effluent. Ind. Crops Prod. 2014, 61, 317–324.
- Assalin, M.R.; Almeida, E.S.; Duran, N. Combined System of Activated Sludge and Ozonation for the Treatment of Kraft E1 Effluent. Int. J. Environ. Res. Public Health 2009, 6, 1145–1154.
- Davarnejad, R.; Nasiri, S. Slaughterhouse wastewater treatment using an advanced oxidation process: Optimization study. Environ. Pollut. 2016, 223, 1–10.
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104.
- Barcelo, M.A.; Lopez, M.I.P.; Lucena, F.; Jofre, J.; Ibanez, P.F. Solar Advanced Oxidation Processes as disinfection tertiary treatments for real wastewater: Implications for water reclamation. Appl. Catal. B Environ. 2013, 136–137, 341–350.
- Aramyan, S.M.; Moussavi, M. Advances in Fenton and Fenton based Oxidation processes for industrial effluent contaminants control—A review. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 555594.
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51.
- Gomez, E.; Martin, M.M.B.; Carratala, A.P.; Ibanez, F.; Pérez, J.A.S.; Pulgarin, C. Principal parameters affecting virus inactivation by the solar photo-Fenton process at neutral pH and concentrations of H2O2 and Fe2+. Appl. Catal. B Environ. 2015, 174–175, 395–402.
- Barwal, A.; Chaudhary, R. Effectiveness of solar photo—Fenton process for simultaneous detoxification of heavy metals and disinfection in municipal wastewater by using response surface method. Environ. Prog. Sustain. Energy 2016, 36, 448–459.
- Rodriguez, L.P.; Oller, I.; Klamerth, N.; Aguera, A.; Rodriguez, E.M.; Malato, S. Application of AOPs and ozonation for elimination of micropollutants in municipal wastewater treatment plant effluents. Water Res. Nano Energy 2013, 47, 1521–1528.
- Garcia, P.D.; Buitron, G. Improvement of the robustness of solar photo-Fenton processes using chemometric techniques for the decolorization of azo dye mixtures. J. Environ. Manag. 2013, 131, 66–73.
- Xu, M.; Wang, Q.; Hao, Y. Removal of organic carbon from wastewater pulp effluent by lab-scale solar photo-Fenton process. J. Hazard. Mater. 2007, 148, 103–109.
- Sheik, M.A.; Kumar, A.; Paliwal, M.; Ameta, R.; Khandelwal, R.C. Degradation of organic effluents containing wastewater by photo-Fenton oxidation process. Indian J. Chem. 2008, 4, 1681–1684.
- Karimi, S.; Abdolkhani, A.; Karimi, A. Discoloration of Soda Pulping Effluent by Advanced Oxidation Processes. Eng. e-Trans. 2011, 6, 20–25, ISSN 1823-6379.
- Lucas, M.S.; Peres, J.A.; Amor, C.; Rodriguez, L.P.; Maldonado, M.I.; Malato, S. Tertiary treatment of pulp mill wastewater by solar photo—Fenton. J. Hazard. Mater. 2012, 226, 173–181.
- Bernabeu, A.; Palacios, S.; Vicente, R.; Vercher, R.F.; Malato, S.; Arques, A.; Amat, A.M. Solar photo-Fenton at mild conditions to treat a mixture of six emerging pollutants. Chem. Eng. J. 2012, 198–199, 65–72.
- Trovo, A.G.; Nogueira, R.F.P.; Aguera, A.; Alba, A.R.F.; Malato, S. Paracetamol degradation intermediates and toxicity during photo-Fenton treatment using different iron species. Water Res. 2012, 46, 5374–5380.
- Turbay, E.Y.; Jaen, E.; Graells, M.; Moya, M.P. Enhanced photo-Fenton process for tetracycline degradation using efficient hydrogen peroxide dosage. J. Photochem. Photobiol. A Chem. 2013, 267, 11–16.
- Rocha, O.R.S.; Dantas, R.F.; Duarte, M.M.M.B.; Duarte, M.M.L.; da Silva, V.L. Solar photo-Fenton treatment of petroleum extraction wastewater. Desal. Water Treat. 2014, 51, 5785–5791.
- Rodrıguez, M.J.H.; Rodrıguez, C.F.; Rodrıguez, J.M.D.; Dıaz, O.M.G.; Zerbani, D.; Pena, J.P. Treatment of effluents from wool dyeing process by photo-Fenton at solar pilot plant. J. Environ. Chem. Eng. 2014, 2, 163–171.
- Velegraki, T.; Mantzavinos, D. Solar photo-Fenton treatment of winery effluents in a pilot photocatalytic reactor. Catal. Today 2015, 240, 153–159.
- Chueca, J.R.; Lopez, M.I.P.; Mosteo, R.; Ormad, M.R.; Ibanez, P.F. Disinfection of real and simulated urban wastewater effluents using a mild solar photo-Fenton. Appl. Catal. B Environ. 2015, 150–151, 619–629.
- Soares, P.A.; Batalha, M.; Souza, S.M.A.G.U.; Boaventura, R.A.R.; Vilar, V.J.P. Enhancemnet of a solar photo-Fenton reaction with ferric-organic ligands for the treatment of acrylic-textile dyeing wastewater. J. Environ. Manag. 2015, 152, 120–131.
- Guzman, J.; Mosteo, R.; Sarasa, J.; Alba, J.A.; Ovelleiro, J.L. Evaluation of solar photo-Fenton and ozone-based processes as citrus wastewater pre-treatments. Sep. Purif. Technol. 2016, 164, 155–162.
- Benitez, H.Z.N.; Penuela, G.A. Application of solar photo-Fenton for benzophenone-type UV filters removal. J. Environ. Manag. 2018, 217, 929–938.
- Costa, N.M.; Silva, G.D.; Marson, E.O.; Richter, E.M.; Machado, A.E.H.; Trovo, A.G. Enhanced treatment of a biodiesel effluent using ferrioxalate in a photo-Fenton process based on the use of solar radiation. Fuel 2018, 221, 110–115.
- García, A.B.E.; Szymanski, K.; Mozia, S.; Perez, J.A.S. Treatment of laundry wastewater by solar photo-Fenton process at pilot plant scale. Environ. Sci. Pollut. Res. 2021, 28, 8576–8584.
- Arka, A.; Asaithambi, P.; Debela, S.K. Development of Solar Photo-Fenton Process for the Removal of Color, COD and Turbidity from Institutional Wastewater. J. Energy Environ. Chem. Eng. 2022, 7, 26–35.
- Lumbaque, E.C.; Cardoso, R.M.; de Araújo Gomes, A.; Malato, S.; Sanchez Perez, J.A.; Sirtori, C. Removal of pharmaceuticals in hospital wastewater by solar photo-Fenton with Fe3+-EDDS using a pilot raceway pond reactor: Transformation productsand in silico toxicity assessment. Microchem. J. 2021, 164, 106014.
- Lin, H.H.H.; Lin, A.Y.C. Solar photo-Fenton oxiadation of cytostatic drugs via FeIII-EDDS at circumneutral pHin an aqueous environment. J. Water Process Eng. 2021, 41, 102066.
- Gualda-Alonso, E.; Soriano-Molina, P.; Lopez, J.L.C.; Sanchez, J.L.G.; Plaza-Bolaños, P.; Agüera, A.; Perez, J.A.S. Large scale raceway pond reactor for CEC removal from municipal WWTP effluents by solar photo-Fenton. Appl. Catal. B Environ. 2022, 319, 121908.
- Pandey, Y.; Verma, A.; Toor, A.P. Abatement of paraquat contaminated water using solar assisted heterogeneous photo-Fenton like treatment with iron-containing industrial wastes as catalysts. J. Environ. Manag. 2023, 325, 116550.
- Antunes, C.S.A.; Bietti, M.; Salamone, M.; Scione, N. Early stages in the TiO2-photocatalyzed degradation of simple phenolic and non-phenolic lignin model compounds. J. Photochem. Photobiol. A Chem. 2004, 163, 453–462.
- Fotiou, T.; Triantis, T.; Kaloudis, T.; Hiskia, A. Photocatalytic degradation of cylindrospermopsin under UV-A, solar and visible light using TiO2. Mineralization and intermediate products. Chemosphere 2015, 119, 589–594.
- Rashid, J.; Barakat, M.A. Ag3PO4 enhanced TiO2 for visible light photocatalysis of 2-chlorophenol in wastewater. Int. J. Environ. Eng. 2015, 2, 93–97.
- Shinde, S.S.; Bhosale, C.H.; Rajpure, K.Y. Photodegradation of organic pollutants using N-titanium oxide catalyst. J. Photochem. Photobiol. B Biol. 2014, 141, 186–191.
- Portjanskaja, E.; Stepnova, K.; Klauson, D.; Preis, S. The influence of titanium dioxide modifications on photocatalytic oxidation of lignin and humic acids. Catal. Today 2009, 144, 26–30.
- Amat, A.M.; Arques, A.; Lopez, F.; Miranda, M.A. Solar photocatalysis to remove paper mill wastewater pollutants. Sol. Energy 2005, 79, 393–401.
- Fernandez, I.G.; Calderero, I.F.; Lopez, M.I.P.; Ibanez, P.F. Disinfection of urban effluents using solar TiO2 photocatalysis: A study of significance of dissolved oxygen, temperature, type of microorganism and water matrix. Catal. Today 2015, 240, 30–38.
- Karat, I. Advanced Oxidation Processes for Removal of COD from Pulp and Paper Mill Effluents A Technical, Economical and Environmental Evaluation, Royal Institute of Technology. Stockholm 2013, 6, 1–104.
- Singh, C.; Chaudhary, R.; Gandhi, K. Preliminary study on optimization of pH, oxidant and catalyst dose for high COD content: Solar parabolic trough collector. Iran. J. Environ. Health Sci. Eng. 2013, 10, 1–10.
- Adishkumar, S.; Kanmani, S. Treatment of phenolics wastewater in single baffle reactor by solar/TiO2/H2O2 process. Desal. Water Treat. 2010, 24, 67–73.
- Kumar, P.; Kumar, S.; Bhardwaj, N.K.; Choudhary, A.K. Advanced Oxidation of Pulp and Paper Industry Effluent. Int. Conf. Environ. Agric. Eng. Singap. 2011, 15, 170–178.
- Dimitrakopoulou, D.; Rethemiotaki, I.; Frontistis, Z.; Xekuokuolotakis, N.P.; Veniere, D.; Mantzavinos, D. Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO2 photocatalysis. J. Environ. Manag. 2012, 98, 168–174.
- Nagpure, H.; Banakar, V.; Dhanda, R.; Wani, K.S. Degradation of Paper Mill Wastewater using Batch (Photo catalytic) Reactor. Int. J. Green Chem. Bioprocess 2013, 3, 24–29.
- Ruiz, O.S.; Zanella, R.; Prado, B. Photocatalytic degradation of trimethoprim by pulp metallic nano particles TiO2 P25. J. Hazard. Mater. 2013, 263, 28–35.
- Shao, C.; Zhou, G.; Li, Z.; Wu, Y.; Xu, D.; Sun, B. Fabrication of large-diameter tube-like mesoporous TiO2 via homogeneous precipitation and photocatalytic decomposition of papermaking wastewater. Chem. Eng. J. 2013, 230, 227–235.
- Antonopoulou, M.; Konstantinou, I. Photocatalytic treatment of metribuzin herbicide over TiO2 aqueous suspensions: Removal efficiency, identification of transformation products, reaction pathways and ecotoxicity evaluation. J. Photochem. Photobiol. A Chem. 2014, 294, 110–120.
- Thomas, R.T.; Rasheed, P.A.; Sandhyarani, N. Synthesis of nanotitania decorated few-layer graphene for enhanced visible light driven photocatalysis. J. Colloid Interface Sci. 2014, 428, 214–221.
- Murgolo, S.; Petronella, F.; Ciannarella, R.; Comparelli, R.; Agostiano, A.; Curri, M.L.; Mascolo, G. UV and solar-based photocatalytic degradation of organic pollutants by nano-sized TiO2 grown on carbon nanotubes. Catal. Today 2015, 240, 114–124.
- Solano, R.; Cerri, G.; Herrera, A.; Vargas, X. Cr6+ and Zn2+ removal for heterogeneous photocatalysis with TiO2 in synthetic wastewater. Int. J. Chem. Tech. Res. 2018, 11, 312–320.
- Yang, H.; Yang, J. Photocatalytic degradation of rhodamine B catalysed by TiO2 films on a capillary column. RSC Adv. 2018, 8, 11921–11929.
- Aljouboury, D.A.D.A.; Shaik, F. Optimization of the petroleum wastewater treatment process using TiO2/Zn photocatalyst. S. Afr. J. Chem. Eng. 2021, 38, 61–69.
- Kader, S.; Al-Mamun, M.R.; Suhan, M.B.K.; Shuchi, S.B.; Islam, M.S. Enhanced photodegradation of methyl orange dye under UV irradiation using MoO3and Ag doped TiO2 photocatalysts. Environ. Technol. Innov. 2022, 27, 102476.
- Abusallout, I.; Hua, G. Solar photocatalytic degradation of total organic halogen in water using TiO2 catalyst. Chemopshere 2022, 308, 136206.
- Martins, R.C.; Sacras, A.; Jovanovic, S.; Alves, P.; Ferreira, P.; Gomes, J. Solar energy for liquid wastewater treatment with novel TiO2 supported catalysts. Energy Rep. 2022, 8, 489–494.
- Behera, A.K.; Shadangi, K.P.; Sarangi, P.K. Synthesis of dye-sensitized TiO2/Ag doped nano-composites using UV photoreduction process for phenol degradation: A comparative study. Environ. Pollut. 2022, 312, 120019.
- Rueda-Marquez, J.J.; Palacios-Villarreal, C.; Manzano, M.; Blanco, E.; del Solar, M.R.; Levchuk, I. Photocatalytic degradation of pharmaceutically active compounds (PhACs) in urban wastewater treatment plants effluents under controlled and natural solar irradiation using immobilized TiO2. Sol. Energy 2020, 208, 480–492.
- Gai, H.; Wang, H.; Liu, L.; Feng, B.; Xiao, M.; Tang, Y.; Qu, X.; Song, H.; Huang, T. Potassium and iodide codoped mesoporous titanium dioxide for enhancing photocatalytic degradation of phenolic compounds. Chem. Phys. Lett. 2021, 767, 138367.
- Giang, N.T.H.; Tan, N.N.; Huang, L.M.; Hai, N.D.; Thinh, N.T.; Phuc, N.T.; Dat, N.M.; Phong, M.T.; Hieu, N.H. Photocatalytic degradation of crystal violet on titanium dioxide/grapheme aerogel doped sulfur. J. Mol. Struct. 2023, 1271, 134031.
- Rapti, I.; Kosma, C.; Albanis, T.; Konstantinou, I. Solar photocatalytic degradation of inherent pharmaceutical residues in real hospital WWTP effluents using titanium dioxide on a CPC pilot scale reactor. Catal. Today, 2022; in press.
- Cunha, D.L.; Kuznetzov, A.; Achete, C.A.; Machado, A.E.d.H.; Marques, M. Immobilised TiO2 on glass spheres applied to heterogeneous photocatalysis: Photoactivity, leaching and regeneration. PeerJ 2018, 6, e4464.
More
Information
Subjects:
Engineering, Environmental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.7K
Revisions:
2 times
(View History)
Update Date:
28 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





