Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Jeyakumar Rajesh Banu.
Advanced oxidation processes (AOPs) involves the generation of powerful oxidizing radical groups, such as hydroxyl radicals, which function as oxidizing agents and mineralize organic chemical substances into CO2 and H2O. AOPs such as photocatalysis and photo-Fenton have been widely considered to be very effective in removing persistent organic pollutants.
- advanced oxidation processes
- solar photo-Fenton
- solar photocatalysis
- biological process
1. Introduction
One of the essential needs of all living things, including humans, is safe and uncontaminated drinking water. However, these days, finding it has become a big issue. The water used by humans for most activities produces wastewater. Wastewater accounts for over 80% of the water provided for domestic use [1]. About 5–10 billion tons of industrial waste are generated each year and discharged untreated into the environment [2]. If released directly into the environment without treatment, the wastewater generated from various industries can cause significant environmental problems [3]. Therefore, removing emerging pollutants is essential to making the water safe for drinking, and the release of wastewater should not cause any adverse effect on the environment. Moreover, recycling and reusing treated wastewater is necessary to reduce freshwater consumption. There are physical, chemical, and biological treatment methods; these are the most commonly employed wastewater treatment technologies. In the case of physical treatment, the removal of organics from the wastewater is challenging. The existing biological and chemical treatment methods include ozonation, electrochemical treatment, photocatalysis, aerobic treatment, activated sludge, anaerobic treatment, coagulation–flocculation treatment, etc. [4]. Aerobic biological activities require a lot of energy and generate a significant amount of biomass. Anaerobic biological processes are susceptible to shock loading, which requires further treatment of the wastewater produced before the final release. Although biological processes are efficient and cost-effective, they primarily need a vast area and have a significant energy demand (for aeration) and a substantial quantity of produced sludge [5]. Chemical treatments can quickly oxidize and totally breakdown the organic contaminants, making them an effective wastewater treatment approach [6]. As a result, the development of advanced oxidation processes (AOPs), which have an excellent potential for ultimately destroying a variety of resistant pollutants, has shown to be a promising alternative [7].
2. Mechanism of Advanced Oxidation Processes
There are two types of AOPs: homogeneous processes and heterogeneous processes. The homogeneous processes in the AOP are defined by the chemical alterations resulting solely from the interactions between the chemical reagents and the target substances [17][8]. In heterogeneous processes, the reactants and products adsorb and desorb on the catalyst’s active sites. As the reaction occurs, the desorption of products and the absorption of new species takes place on the active sites; thereby, the efficiency is affected by the surface characteristics and the pore structure of the catalyst [18][9]. The different methods for the production of hydroxyl radicals take place via several combinations, such as the Fenton process (Fe2+/H2O2), the solar photo-Fenton process (solar/Fe2+/H2O2), solar photocatalysis (solar/TiO2/H2O2), peroxone (Ozone/H2O2), the combination of peroxone with ultraviolet (Ozone/H2O2), Ozone/UV, and photolysis (H2O2/UV). The oxidation by-product formation mechanisms in the various AOPs are discussed.2.1. Solar Photo-Fenton Process
Among the AOPs, the solar photo-Fenton process is particularly interesting for treating a large variety of hazardous pollutants and is one of the most environmentally benign treatment systems [19][10]. Due to the potential utilization of sunlight, this technique has drawn more attention. As a result, it may be considered to be an affordable AOP as sunlight is always available in a tropical country such as India [20,21][11][12]. Figure 1 shows the schematic diagram of the SPF.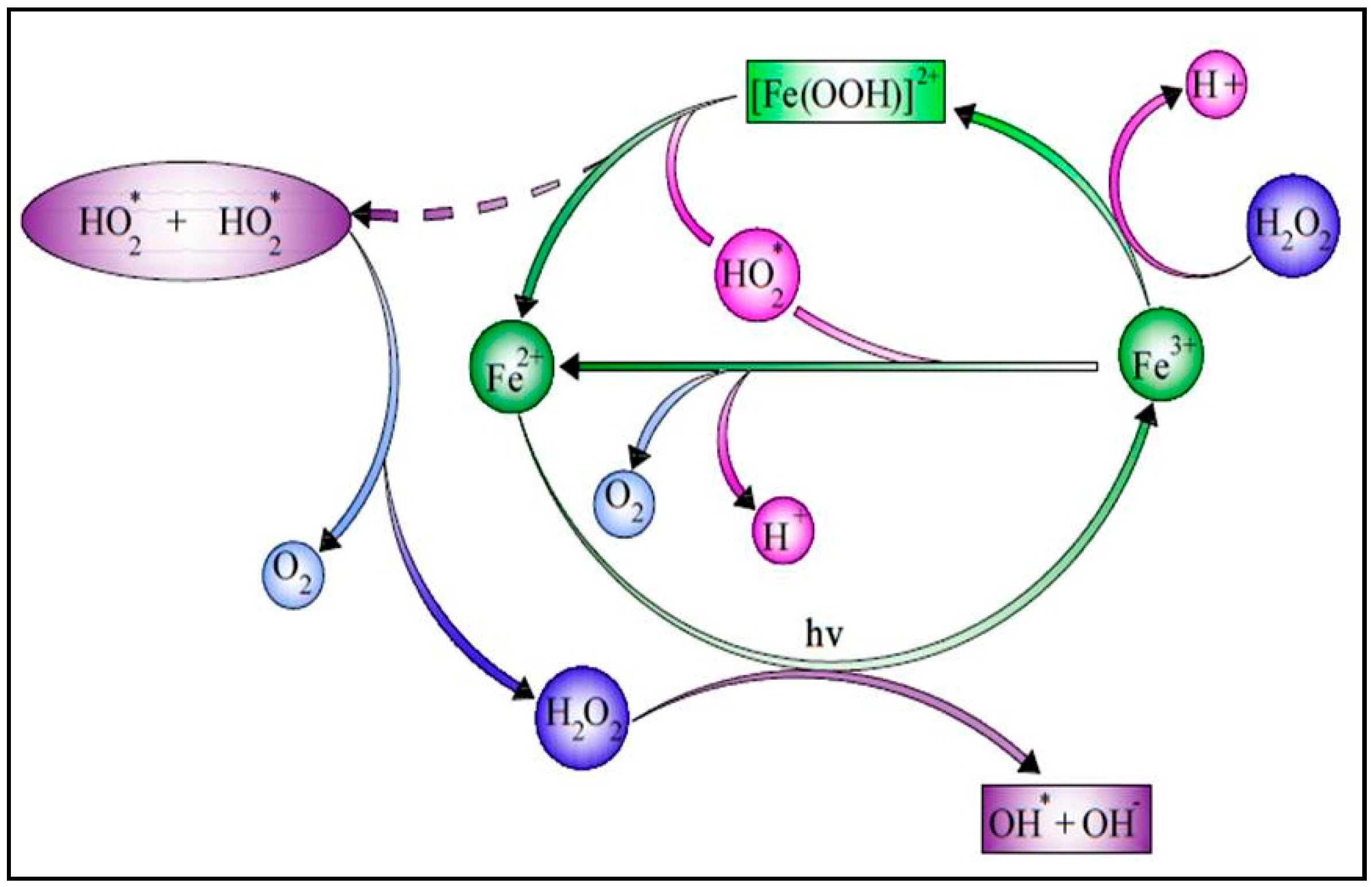
Figure 1.
Schematic diagram of a solar photo-Fenton process.
Fe
2+
+ H
2
O
2
→ Fe
3+
+ OH
● + HO
+ HO
[Fe(OH)]
2+
+ hν → Fe
2+
+ OH
●
+ H
+
Fe(OOCR)
2+
+ hν → Fe
2+
+ CO
2
+ R
•
Table 1.
Treatment of wastewaters by various solar photo-Fenton processes.
| S. No. | Wastewater | Process | Operational Conditions | Degradation Efficiency | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Pulp and paper mill | Solar photo-Fenton | Fe(II) concentration from 31 to 310 mg L−1 (initial pH 3.0, 30 °C), initial H2O2 concentration from 0.5 to 3 Dth | TOC—82% Time—120 min |
Xu et al., 2007 [23][14] | ||||||
| 2 | p-nitroaniline | Solar photo-Fenton | Sheik et al. (2008) [ | ||||||||
| 2 | Amoxicillin | Solar photocatalytic (TiO2) | TiO2 catalysts 100 to 750 mg/L | 24 | ][15] | ||||||
| 93% mineralization | Dimitrakopoulou et al. (2012) | [ | 55 | ] | [46] | 3 | Soda pulping effluent | Fenton and photo-Fenton | FeSO | ||
| 3 | 4 | —1 mM | H2O2—570 μL |
Color—65% | Karimi et al. (2011) [25][16] | ||||||
| Synthetic wastewater | Photocatalytic oxidation | pH values 2, 4, 6, 8, 10 | COD—86% | Singh et al. (2013) | [52][43] | 4 | Pulp and paper mill | Solar photo-Fenton | 5 mg/L of Fe2+ and H2O2 of 50 mM | 90% of DOC mineralization | Lucas et al. (2012) [26][17 |
| 4 | ] | ||||||||||
| Trimethoprim | Photocatalytic oxidation | - | 50% mineralization | Ruiz et al. (2013) | [57][48] | 5 | Azo dye, Acid Blue 161 | Solar photo-Fenton | H2O2/Fe2+ = 12, pH = 2.5 to 4.0 | Degradation 40 % | Trovo et al., 2016 [ |
| 5 | Pulp and paper mill | 28 | Photocatalytic oxidation | - | COD—73%, chroma percent—95%][19] | ||||||
| Shao et al. (2013) | [ | 58 | ] | [ | 49 | 6 | Sulfonated azo dyes | Solar photo-Fenton | Fe2+ = 5 to 38 mg/L, H2O2 = 98 to 828 mg/L | Color—97% | Garcia and Buitron (2013) [22][13] |
| ] | |||||||||||
| 6 | Metribuzin | Photocatalytic oxidation | TiO2—100 mg/L and I = 750 W/m2 |
80% mineralization Reaction time—300 min |
Antonopoulou et al. (2014) [59][50] | 7 | Petroleum extraction | Photo-Fenton | 485.3 mmol/L of H2O2 | Hydrocarbons and aromaticity of about 92.7% and 96.2% | |
| 7 | Organic pollutants | Nanosized TiO2 supported on single-wall carbon nanotubes | Rocha et al. (2014) | [ | 30][21] | ||||||
| - | UV—9 to 87%, simulated solar light—9 to 96% | Murgolo et al. (2015) | [ | 61 | ][52] | 8 | Winery effluent | Photo-Fenton | (Fe2+ = 5 mg/L) | - | Velegraki and Mantzavinos (2015) [ |
| 8 | Hexavalent chromium | 32 | ] | [ | 23] | ||||||
| Solar photocatalytic (TiO | 2 | ) | pH = 2, 6 and 10, TiO2—2.0, 2.5, and 3.0 g/L | Complete removal for 5 mg/L Cr6+ | Solano et al. (2018) [62][53] | 9 | Citrus wastewater | Photo-Fenton | H2 | ||
| 9 | Rhodamine B | Solar photocatalytic (TiO2) | O2 = 1017 mg/L, pH = 7 | Film thickness = 361 nm Specific surface area of 47.72 m2COD—77% | g−1Guzman et al., 2016 | 98.33% in 30 min[35][26] | |||||
| 10 | Biodiesel effluent | Solar photo-Fenton | 1 mmol L−1 of ferrioxalate | 72% of COD and 76% of BOD | Costa et al. (2018) [37][28] | ||||||
| 11 | Laundry effluent | Solar photo-Fenton | H2O2 (50–400 mg/L), Fe2+ (2.75–10 mg/L) | SDS removal—96% | Garcia et al. (2021) [38][29] | ||||||
| 12 | Industrial effluent | Solar photo-Fenton | (H2O2)—0.25 to 1.25 g/L, Fe2+—0.005 to 0.12 g/L, pH (2 to 10), time (30 to 180 min) | color (91%), turbidity (90%), and COD—86%) | Arka et al. (2021) [39][30] | ||||||
| 13 | Hospital wastewater | Solar photo-Fenton | Fe3+:EDDS = 1:2, H2O2 (230 mg L−1) |
Degradation = 77% | Lumbaque et al., 2021 [40][31] | ||||||
| 14 | Cytostatic drugs | Solar photo-Fenton | pH 3.0–8.5, 0.1 mM Fe(III)-EDDS, 1 mM H2O2 | Lin and Lin, 2021 [41][32] | |||||||
| 15 | Emerging contaminants | Solar photo-Fenton | 0.1 mM FeSO4 and 1.47 mM H2O2 at an acidic pH and 1 h of hydraulic residence time | >85% | Gualda-Alonso et al., 2022 [42][33] | ||||||
| 16 | Paraquat (PQ)-contaminated water | Photo-Fenton | Fe-containing industrial waste | COD—82% | Pandey et al., 2023 [43][34] |
2.2. Solar Photocatalytic Process
Solar photocatalysis has been widely used to degrade organic compounds. According to numerous studies, many organic contaminants have been totally oxidized in irradiated semiconductor solutions [44][35]. Photocatalysts remove pollutants from wastewater primarily through hydroxyl radical (OH●) attacks by transforming them into harmless compounds such as water and CO2 [45][36]. The photocatalyst that has been researched the most is TiO2. However, its usage is restricted to ultraviolet light because of the wide energy band gap (3.2 eV) [46][37]. When exposed to ultraviolet light, a semiconductor catalyst such as TiO2 or another transition metal oxide is initiated by the photon absorption with enough energy to be equal to or greater than the catalyst’s bandgap energy, stimulating an electron to move from the valence band to the conduction band, creating a hole in the valence band while using just 4% of the solar radiation [47,48,49][38][39][40]. TiO2 photocatalysis uses UV-A radiation (λ < 387 nm) in the presence of oxygen and water to produce hydroxyl radicals for the excitation of the photocatalyst [50][41]. The overall rates of pollutant degradation are significantly influenced by the surface area of the TiO2 catalysts that contains active sites. Thus, increasing the TiO2-specific surface area effectively increases the pollutant adsorption capacity on the catalyst. As a result, a larger surface area with a more significant number of active sites will lead to a faster and more extensive reaction [51][42]. The activation Equation (4) can be written asTiO
2
+ hν → h
+
+ e
−
h
+
+ OH
−
→ -OH
●
e
−
+ O
2ads−
→ O
2ads−
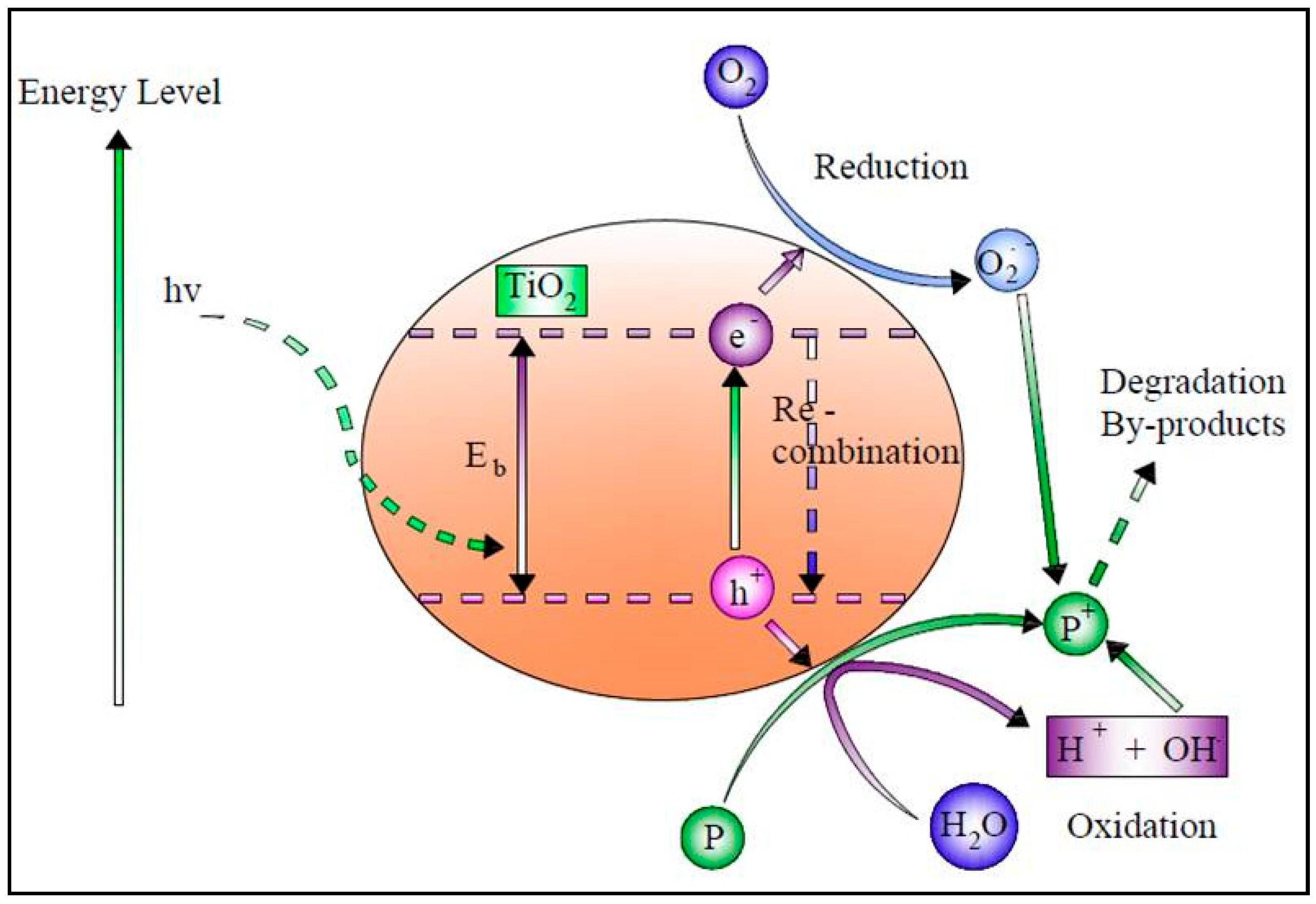
Figure 2.
Schematic diagram of the solar photocatalytic process.
Table 2.
Treatment of wastewater by various solar photocatalytic processes.
| S. No. | Wastewater | Process | Operational Conditions | Degradation Efficiency | Reference |
|---|---|---|---|---|---|
| 1 | Pulp and paper mill | Solar photocatalytic (TiO2) | - | COD—57.9%, BOD—42.9%, color—89.2% | Kumar et al. (2011) [54][45] |
| Yang and Yang (2018) | |||||
| [ | |||||
| 63 | |||||
| ] | |||||
| [ | |||||
| 54 | |||||
| ] | |||||
| 10 | Urban wastewater | Immobilized TiO2 | High removal (>40%) | Rueda-Marquez et al., 2020 [69][60] | |
| 11 | Petroleum wastewater | TiO2/ZnO/Fenton/Solar and TiO2/ZnO/Air/Solar | ZnO dosage of 54 g/L and TiO2 dosage 50 g/L | COD—74%, TOC—99% | Aljouboury et al., 2021 [64][55] |
| 12 | Organic pollutant | Solar TiO2 photocatalysis | Perfect acid and alkali resistance | Gai et al., 2021 [70][61] | |
| 13 | Methyl orange dye | TiO2-based photocatalysts | pH—7.0, reaction time—5.5 h of UV irradiation, photocatalyst (0.12 g) | Degradation—75.8% | Kader et al. (2022) [65][56] |
| 14 | Dehalogenation disinfection by-products | Solar TiO2 photocatalysis | pH 9, TiO2—100 mg/L | Effective removal of TOX | Abusallout and Hua (2022) [66][57] |
| 15 | Pharmaceutical residues | Solar TiO2 photocatalysis | TiO2—200 and 300 mg L−1 | Higher than 56% | Rapti et al. (2022) [72][63] |
| 16 | Crystal violet | Titanium dioxide/graphene aerogel-doped sulfur | Enhanced photo-degradation | Giang et al., 2023 [71][62] |
References
- Renuka, R.; Mohan, M.S.; Sowmiya, B.; Raj, A.S. Performance evaluation of panelled anaerobic baffle-cum-filter reactor in treating municipal wastewater. Ecol. Eng. 2016, 97, 1–12.
- Nandkumar, P. Studies on the effluent generated during the pulping process in paper industry. Curr. World Environ. 2008, 3, 189–193.
- Subramonian, W.; Wu, T.Y.; Chai, S.P. A comprehensive study on coagulant performance and floc characterization of natural Cassia obtusifolio seed gum in treatment of raw pulp and paper mill effluent. Ind. Crops Prod. 2014, 61, 317–324.
- Assalin, M.R.; Almeida, E.S.; Duran, N. Combined System of Activated Sludge and Ozonation for the Treatment of Kraft E1 Effluent. Int. J. Environ. Res. Public Health 2009, 6, 1145–1154.
- Davarnejad, R.; Nasiri, S. Slaughterhouse wastewater treatment using an advanced oxidation process: Optimization study. Environ. Pollut. 2016, 223, 1–10.
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104.
- Barcelo, M.A.; Lopez, M.I.P.; Lucena, F.; Jofre, J.; Ibanez, P.F. Solar Advanced Oxidation Processes as disinfection tertiary treatments for real wastewater: Implications for water reclamation. Appl. Catal. B Environ. 2013, 136–137, 341–350.
- Aramyan, S.M.; Moussavi, M. Advances in Fenton and Fenton based Oxidation processes for industrial effluent contaminants control—A review. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 555594.
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51.
- Gomez, E.; Martin, M.M.B.; Carratala, A.P.; Ibanez, F.; Pérez, J.A.S.; Pulgarin, C. Principal parameters affecting virus inactivation by the solar photo-Fenton process at neutral pH and concentrations of H2O2 and Fe2+. Appl. Catal. B Environ. 2015, 174–175, 395–402.
- Barwal, A.; Chaudhary, R. Effectiveness of solar photo—Fenton process for simultaneous detoxification of heavy metals and disinfection in municipal wastewater by using response surface method. Environ. Prog. Sustain. Energy 2016, 36, 448–459.
- Rodriguez, L.P.; Oller, I.; Klamerth, N.; Aguera, A.; Rodriguez, E.M.; Malato, S. Application of AOPs and ozonation for elimination of micropollutants in municipal wastewater treatment plant effluents. Water Res. Nano Energy 2013, 47, 1521–1528.
- Garcia, P.D.; Buitron, G. Improvement of the robustness of solar photo-Fenton processes using chemometric techniques for the decolorization of azo dye mixtures. J. Environ. Manag. 2013, 131, 66–73.
- Xu, M.; Wang, Q.; Hao, Y. Removal of organic carbon from wastewater pulp effluent by lab-scale solar photo-Fenton process. J. Hazard. Mater. 2007, 148, 103–109.
- Sheik, M.A.; Kumar, A.; Paliwal, M.; Ameta, R.; Khandelwal, R.C. Degradation of organic effluents containing wastewater by photo-Fenton oxidation process. Indian J. Chem. 2008, 4, 1681–1684.
- Karimi, S.; Abdolkhani, A.; Karimi, A. Discoloration of Soda Pulping Effluent by Advanced Oxidation Processes. Eng. e-Trans. 2011, 6, 20–25, ISSN 1823-6379.
- Lucas, M.S.; Peres, J.A.; Amor, C.; Rodriguez, L.P.; Maldonado, M.I.; Malato, S. Tertiary treatment of pulp mill wastewater by solar photo—Fenton. J. Hazard. Mater. 2012, 226, 173–181.
- Bernabeu, A.; Palacios, S.; Vicente, R.; Vercher, R.F.; Malato, S.; Arques, A.; Amat, A.M. Solar photo-Fenton at mild conditions to treat a mixture of six emerging pollutants. Chem. Eng. J. 2012, 198–199, 65–72.
- Trovo, A.G.; Nogueira, R.F.P.; Aguera, A.; Alba, A.R.F.; Malato, S. Paracetamol degradation intermediates and toxicity during photo-Fenton treatment using different iron species. Water Res. 2012, 46, 5374–5380.
- Turbay, E.Y.; Jaen, E.; Graells, M.; Moya, M.P. Enhanced photo-Fenton process for tetracycline degradation using efficient hydrogen peroxide dosage. J. Photochem. Photobiol. A Chem. 2013, 267, 11–16.
- Rocha, O.R.S.; Dantas, R.F.; Duarte, M.M.M.B.; Duarte, M.M.L.; da Silva, V.L. Solar photo-Fenton treatment of petroleum extraction wastewater. Desal. Water Treat. 2014, 51, 5785–5791.
- Rodrıguez, M.J.H.; Rodrıguez, C.F.; Rodrıguez, J.M.D.; Dıaz, O.M.G.; Zerbani, D.; Pena, J.P. Treatment of effluents from wool dyeing process by photo-Fenton at solar pilot plant. J. Environ. Chem. Eng. 2014, 2, 163–171.
- Velegraki, T.; Mantzavinos, D. Solar photo-Fenton treatment of winery effluents in a pilot photocatalytic reactor. Catal. Today 2015, 240, 153–159.
- Chueca, J.R.; Lopez, M.I.P.; Mosteo, R.; Ormad, M.R.; Ibanez, P.F. Disinfection of real and simulated urban wastewater effluents using a mild solar photo-Fenton. Appl. Catal. B Environ. 2015, 150–151, 619–629.
- Soares, P.A.; Batalha, M.; Souza, S.M.A.G.U.; Boaventura, R.A.R.; Vilar, V.J.P. Enhancemnet of a solar photo-Fenton reaction with ferric-organic ligands for the treatment of acrylic-textile dyeing wastewater. J. Environ. Manag. 2015, 152, 120–131.
- Guzman, J.; Mosteo, R.; Sarasa, J.; Alba, J.A.; Ovelleiro, J.L. Evaluation of solar photo-Fenton and ozone-based processes as citrus wastewater pre-treatments. Sep. Purif. Technol. 2016, 164, 155–162.
- Benitez, H.Z.N.; Penuela, G.A. Application of solar photo-Fenton for benzophenone-type UV filters removal. J. Environ. Manag. 2018, 217, 929–938.
- Costa, N.M.; Silva, G.D.; Marson, E.O.; Richter, E.M.; Machado, A.E.H.; Trovo, A.G. Enhanced treatment of a biodiesel effluent using ferrioxalate in a photo-Fenton process based on the use of solar radiation. Fuel 2018, 221, 110–115.
- García, A.B.E.; Szymanski, K.; Mozia, S.; Perez, J.A.S. Treatment of laundry wastewater by solar photo-Fenton process at pilot plant scale. Environ. Sci. Pollut. Res. 2021, 28, 8576–8584.
- Arka, A.; Asaithambi, P.; Debela, S.K. Development of Solar Photo-Fenton Process for the Removal of Color, COD and Turbidity from Institutional Wastewater. J. Energy Environ. Chem. Eng. 2022, 7, 26–35.
- Lumbaque, E.C.; Cardoso, R.M.; de Araújo Gomes, A.; Malato, S.; Sanchez Perez, J.A.; Sirtori, C. Removal of pharmaceuticals in hospital wastewater by solar photo-Fenton with Fe3+-EDDS using a pilot raceway pond reactor: Transformation productsand in silico toxicity assessment. Microchem. J. 2021, 164, 106014.
- Lin, H.H.H.; Lin, A.Y.C. Solar photo-Fenton oxiadation of cytostatic drugs via FeIII-EDDS at circumneutral pHin an aqueous environment. J. Water Process Eng. 2021, 41, 102066.
- Gualda-Alonso, E.; Soriano-Molina, P.; Lopez, J.L.C.; Sanchez, J.L.G.; Plaza-Bolaños, P.; Agüera, A.; Perez, J.A.S. Large scale raceway pond reactor for CEC removal from municipal WWTP effluents by solar photo-Fenton. Appl. Catal. B Environ. 2022, 319, 121908.
- Pandey, Y.; Verma, A.; Toor, A.P. Abatement of paraquat contaminated water using solar assisted heterogeneous photo-Fenton like treatment with iron-containing industrial wastes as catalysts. J. Environ. Manag. 2023, 325, 116550.
- Antunes, C.S.A.; Bietti, M.; Salamone, M.; Scione, N. Early stages in the TiO2-photocatalyzed degradation of simple phenolic and non-phenolic lignin model compounds. J. Photochem. Photobiol. A Chem. 2004, 163, 453–462.
- Fotiou, T.; Triantis, T.; Kaloudis, T.; Hiskia, A. Photocatalytic degradation of cylindrospermopsin under UV-A, solar and visible light using TiO2. Mineralization and intermediate products. Chemosphere 2015, 119, 589–594.
- Rashid, J.; Barakat, M.A. Ag3PO4 enhanced TiO2 for visible light photocatalysis of 2-chlorophenol in wastewater. Int. J. Environ. Eng. 2015, 2, 93–97.
- Shinde, S.S.; Bhosale, C.H.; Rajpure, K.Y. Photodegradation of organic pollutants using N-titanium oxide catalyst. J. Photochem. Photobiol. B Biol. 2014, 141, 186–191.
- Portjanskaja, E.; Stepnova, K.; Klauson, D.; Preis, S. The influence of titanium dioxide modifications on photocatalytic oxidation of lignin and humic acids. Catal. Today 2009, 144, 26–30.
- Amat, A.M.; Arques, A.; Lopez, F.; Miranda, M.A. Solar photocatalysis to remove paper mill wastewater pollutants. Sol. Energy 2005, 79, 393–401.
- Fernandez, I.G.; Calderero, I.F.; Lopez, M.I.P.; Ibanez, P.F. Disinfection of urban effluents using solar TiO2 photocatalysis: A study of significance of dissolved oxygen, temperature, type of microorganism and water matrix. Catal. Today 2015, 240, 30–38.
- Karat, I. Advanced Oxidation Processes for Removal of COD from Pulp and Paper Mill Effluents A Technical, Economical and Environmental Evaluation, Royal Institute of Technology. Stockholm 2013, 6, 1–104.
- Singh, C.; Chaudhary, R.; Gandhi, K. Preliminary study on optimization of pH, oxidant and catalyst dose for high COD content: Solar parabolic trough collector. Iran. J. Environ. Health Sci. Eng. 2013, 10, 1–10.
- Adishkumar, S.; Kanmani, S. Treatment of phenolics wastewater in single baffle reactor by solar/TiO2/H2O2 process. Desal. Water Treat. 2010, 24, 67–73.
- Kumar, P.; Kumar, S.; Bhardwaj, N.K.; Choudhary, A.K. Advanced Oxidation of Pulp and Paper Industry Effluent. Int. Conf. Environ. Agric. Eng. Singap. 2011, 15, 170–178.
- Dimitrakopoulou, D.; Rethemiotaki, I.; Frontistis, Z.; Xekuokuolotakis, N.P.; Veniere, D.; Mantzavinos, D. Degradation, mineralization and antibiotic inactivation of amoxicillin by UV-A/TiO2 photocatalysis. J. Environ. Manag. 2012, 98, 168–174.
- Nagpure, H.; Banakar, V.; Dhanda, R.; Wani, K.S. Degradation of Paper Mill Wastewater using Batch (Photo catalytic) Reactor. Int. J. Green Chem. Bioprocess 2013, 3, 24–29.
- Ruiz, O.S.; Zanella, R.; Prado, B. Photocatalytic degradation of trimethoprim by pulp metallic nano particles TiO2 P25. J. Hazard. Mater. 2013, 263, 28–35.
- Shao, C.; Zhou, G.; Li, Z.; Wu, Y.; Xu, D.; Sun, B. Fabrication of large-diameter tube-like mesoporous TiO2 via homogeneous precipitation and photocatalytic decomposition of papermaking wastewater. Chem. Eng. J. 2013, 230, 227–235.
- Antonopoulou, M.; Konstantinou, I. Photocatalytic treatment of metribuzin herbicide over TiO2 aqueous suspensions: Removal efficiency, identification of transformation products, reaction pathways and ecotoxicity evaluation. J. Photochem. Photobiol. A Chem. 2014, 294, 110–120.
- Thomas, R.T.; Rasheed, P.A.; Sandhyarani, N. Synthesis of nanotitania decorated few-layer graphene for enhanced visible light driven photocatalysis. J. Colloid Interface Sci. 2014, 428, 214–221.
- Murgolo, S.; Petronella, F.; Ciannarella, R.; Comparelli, R.; Agostiano, A.; Curri, M.L.; Mascolo, G. UV and solar-based photocatalytic degradation of organic pollutants by nano-sized TiO2 grown on carbon nanotubes. Catal. Today 2015, 240, 114–124.
- Solano, R.; Cerri, G.; Herrera, A.; Vargas, X. Cr6+ and Zn2+ removal for heterogeneous photocatalysis with TiO2 in synthetic wastewater. Int. J. Chem. Tech. Res. 2018, 11, 312–320.
- Yang, H.; Yang, J. Photocatalytic degradation of rhodamine B catalysed by TiO2 films on a capillary column. RSC Adv. 2018, 8, 11921–11929.
- Aljouboury, D.A.D.A.; Shaik, F. Optimization of the petroleum wastewater treatment process using TiO2/Zn photocatalyst. S. Afr. J. Chem. Eng. 2021, 38, 61–69.
- Kader, S.; Al-Mamun, M.R.; Suhan, M.B.K.; Shuchi, S.B.; Islam, M.S. Enhanced photodegradation of methyl orange dye under UV irradiation using MoO3and Ag doped TiO2 photocatalysts. Environ. Technol. Innov. 2022, 27, 102476.
- Abusallout, I.; Hua, G. Solar photocatalytic degradation of total organic halogen in water using TiO2 catalyst. Chemopshere 2022, 308, 136206.
- Martins, R.C.; Sacras, A.; Jovanovic, S.; Alves, P.; Ferreira, P.; Gomes, J. Solar energy for liquid wastewater treatment with novel TiO2 supported catalysts. Energy Rep. 2022, 8, 489–494.
- Behera, A.K.; Shadangi, K.P.; Sarangi, P.K. Synthesis of dye-sensitized TiO2/Ag doped nano-composites using UV photoreduction process for phenol degradation: A comparative study. Environ. Pollut. 2022, 312, 120019.
- Rueda-Marquez, J.J.; Palacios-Villarreal, C.; Manzano, M.; Blanco, E.; del Solar, M.R.; Levchuk, I. Photocatalytic degradation of pharmaceutically active compounds (PhACs) in urban wastewater treatment plants effluents under controlled and natural solar irradiation using immobilized TiO2. Sol. Energy 2020, 208, 480–492.
- Gai, H.; Wang, H.; Liu, L.; Feng, B.; Xiao, M.; Tang, Y.; Qu, X.; Song, H.; Huang, T. Potassium and iodide codoped mesoporous titanium dioxide for enhancing photocatalytic degradation of phenolic compounds. Chem. Phys. Lett. 2021, 767, 138367.
- Giang, N.T.H.; Tan, N.N.; Huang, L.M.; Hai, N.D.; Thinh, N.T.; Phuc, N.T.; Dat, N.M.; Phong, M.T.; Hieu, N.H. Photocatalytic degradation of crystal violet on titanium dioxide/grapheme aerogel doped sulfur. J. Mol. Struct. 2023, 1271, 134031.
- Rapti, I.; Kosma, C.; Albanis, T.; Konstantinou, I. Solar photocatalytic degradation of inherent pharmaceutical residues in real hospital WWTP effluents using titanium dioxide on a CPC pilot scale reactor. Catal. Today, 2022; in press.
- Cunha, D.L.; Kuznetzov, A.; Achete, C.A.; Machado, A.E.d.H.; Marques, M. Immobilised TiO2 on glass spheres applied to heterogeneous photocatalysis: Photoactivity, leaching and regeneration. PeerJ 2018, 6, e4464.
More
