Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Christian Bailly | -- | 1971 | 2023-03-24 09:11:19 | | | |
| 2 | Jessie Wu | -4 word(s) | 1967 | 2023-03-24 09:30:06 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bailly, C. Mechanism of Action of the Plagiochilins. Encyclopedia. Available online: https://encyclopedia.pub/entry/42501 (accessed on 07 February 2026).
Bailly C. Mechanism of Action of the Plagiochilins. Encyclopedia. Available at: https://encyclopedia.pub/entry/42501. Accessed February 07, 2026.
Bailly, Christian. "Mechanism of Action of the Plagiochilins" Encyclopedia, https://encyclopedia.pub/entry/42501 (accessed February 07, 2026).
Bailly, C. (2023, March 24). Mechanism of Action of the Plagiochilins. In Encyclopedia. https://encyclopedia.pub/entry/42501
Bailly, Christian. "Mechanism of Action of the Plagiochilins." Encyclopedia. Web. 24 March, 2023.
Copy Citation
Plagiochilin A functions as an inhibitor of the termination phase of cytokinesis: the membrane abscission stage. This unique, innovative mechanism of action, coupled with its marked anticancer action, notably against prostate cancer cells, make plagiochilin A an interesting lead molecule for the development of novel anticancer agents. There are known options to increase its potency, as deduced from structure–activity relationships. The analysis shed light on this family of bryophyte species and the little-known group of bioactive terpenoid plagiochilins. Plagiochilin A and derivatives shall be further exploited for the design of novel anticancer targeting the cytokinesis pathway.
agents
bryophytes
cytokinesis
1. Introduction
Bryophytes are non-vascular plants which include thalloid and leafy liverworts, mosses and hornworts. These three lineages form a unique part of the vegetation. They are small-sized, structurally simple diversified plants able to adapt to most ecosystems on Earth [1][2]. Bryophytes and tracheophytes (non-vascular and vascular plants, respectively) derive from an ancestral land plant and diverged during the Cambrian, some 500 million years ago [3]. Bryophytes are collectively divided into three main groups: Bryophyta (mosses), Marchantiophyta (liverworts) and Anthocerotophyta (hornworts). They represent the second-largest group of land plants after angiosperms. Liverworts are particularly abundant, with some 7300 extant species [4]. The first representations of liverworts date from late antiquity [5].
Leafy (or scaly) liverworts are particularly abundant and diversified (order: Jungermanniales). They grow commonly on moist soil or damp rocks (such as thallose liverworts). In 2016, a worldwide checklist for liverworts and hornworts included 7486 species in 398 genera representing 92 families from the two phyla [6]. The genus Plagiochila (Plagiochilaceae) represents one of the largest groups of leafy liverworts, with more than 500 species distributed worldwide and a broad geographical amplitude, mostly in the humid tropics [7]. World Flora Online refers to 556 accepted names of Plagiochila species and more than 950 species including synonyms and unchecked species [8]. Another recent study refers to 1600 validly published Plagiochila names [9].
Despite their number and high adaptative capacities, the medicinal use of bryophytes remains relatively limited, probably because of their small size, lack of conspicuous organs such as colored fruits and flowers, and the difficulty of identification. There are, however, species with medicinal properties, such as Conocephalum conicum (L.) Dumort., Polytrichum commune and Marchantia chenopoda L. [10]. In the genus Plagiochila, a few species have also been used ethnomedicinally, such as P. beddomei Steph. used in the form of paste by tribe Melghat Region (India) for treating skin diseases [11][12] and P. disticha (Lehm. & Lindenb.) Lindenb used traditionally in Peru to treat rheumatism or to regulate menstruation [13].
Diverse bioactive products have been isolated from Plagiochila species, including antitumor agents [14], antifungal molecules [15][16], insecticidal compounds [17] and antimicrobial products [18]. Most of the isolated bioactive compounds are terpenoids such as the antifungal products plagicosins A-N, or alkaloids such as plagiochianins A-B from the Chinese liverworts P. fruticosa Mitt. and P. duthiana Steph., respectively [18][19]. However, the leading product isolated from Plagiochila species is without doubt the sesquiterpenoid plagiochilin A, first isolated from several Plagiochila species in the 1970s, together with its congeners plagiochilins B and C, and precursors plagiochilide and plagiochilal [20]. Over the past 43 years, different analogues have been isolated leading to a series of 24 derivatives, designated plagiochilins A-to-X, and related compounds (Figure 1). The present research deals the identification of these compounds and their pharmacological properties. Information about their mechanism of action is often very limited, but important observations have been made, leading to the identification of potential targets for some of these compounds, in particular for the leader product plagiochilin A (Figure 2).

Figure 1. History of plagiochilins discovery. The 24 plagiochilins (A–W) have been identified and structurally characterized aver a period of 30 years. They are produced by several Plagiochila species, such as those indicated (a non-exhaustive list).
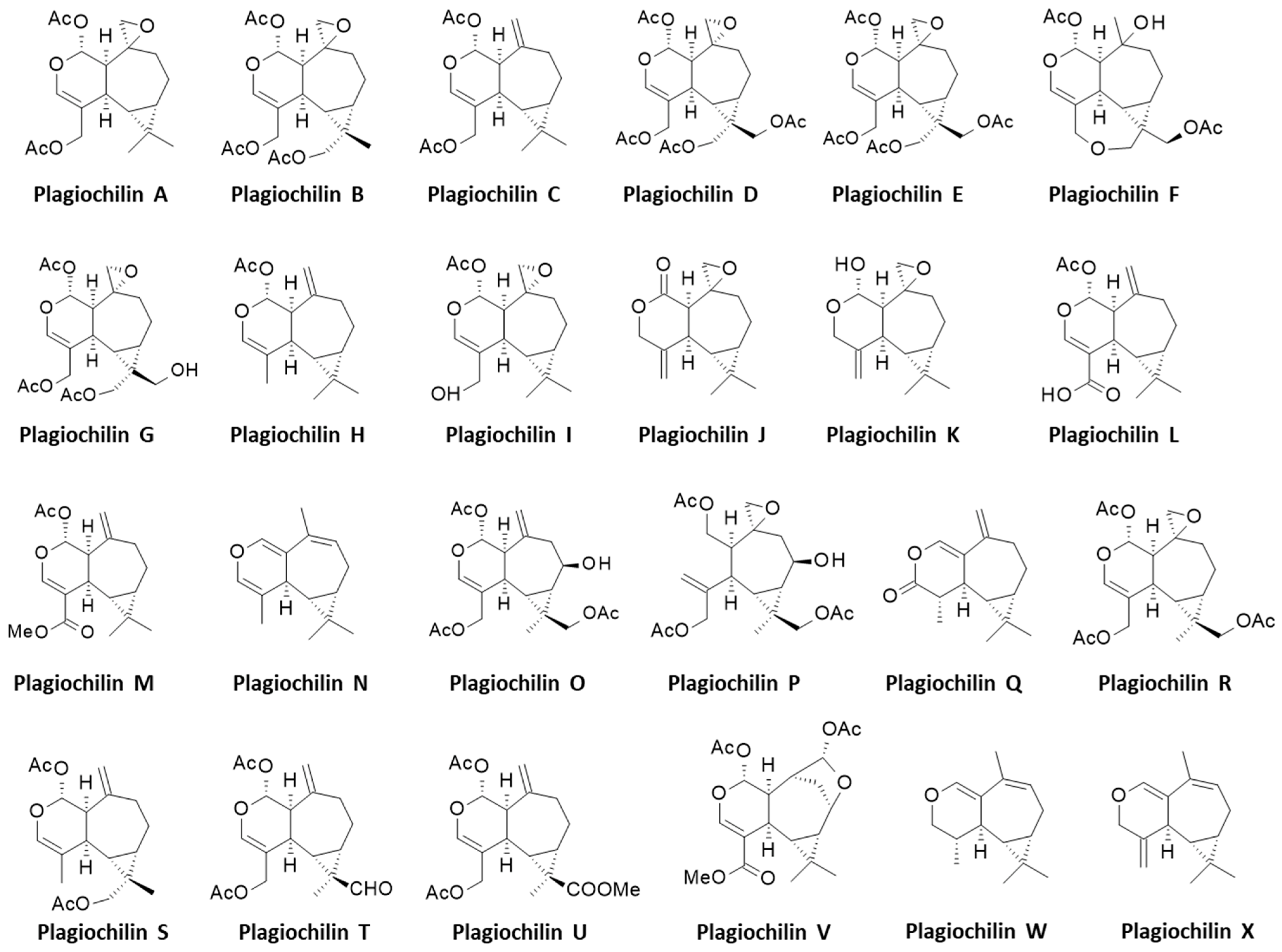
Figure 2. Structures of the 24 plagiochilins (A-to-W).
2. Pharmacological Properties of Plagiochilins A and C
The pharmacological effects of the plagiochilins have been rarely investigated. Nevertheless, a few types of bioactivities have been reported with plagiochilins A and C. The data in Table 1 illustrate the potential of the two compounds, but there is no systematic study comparing activities of all compounds, or activities of a given compound across multiple indications or pathologies. Initially, it was shown that plagiochilin A displays a modest antifeedant action against the armyworm Spodoptera exempta Walker (Lepidoptera: Noctuidae) which is an episodic migratory pest of cereal crops in sub-Saharan Africa. The level of activity is quite modest [21]. Another study has investigated the insecticidal activity of natural products isolated from P. diversifolia, but in that case the only plagiochilin tested was plagiochilin B and no activity was reported. A marked insecticidal action was observed with another epoxide-containing compound called fusicogigantone B, a fusicoccane-type diterpenoid [22]. Both fusicogigantones A and B, isolated from P. bursata and P. diversifolia, respectively, have been shown to inhibit the growth of another species of Lepidoptera (Spodoptera frugiperda) [17][22]. Plagiochilin A was shown to display a noticeable antiprotozoal activity, reducing the growth of the amastigote form of Leishmania amazonensis, with an IC50 value of 7.1 µM. However, the level of activity is quite modest compared to that of the control drug amphotericin B (IC50 = 0.13 µM). In the same study, no activity was observed against the fungus Mycobacterium tuberculosis [13].
Table 1. Bioactivities reported with plagiochilins.
| Compounds | Bioactivities | Tests/Species | End Points | Ref. |
|---|---|---|---|---|
| Plagiochilin A | Antifeedant | African armyworm Spodoptera exempta | Activity observed at 1–10 ng/cm2 | [21] |
| Plagiochilin A | Antiparasitic | Leishmania amazonensis axenic amastigotes | IC50 = 7.1 µM | [13] |
| Plagiochilin A | Antiparasitic | Trypanosoma cruzi trypomastigotes | MIC = 14.5 µM | [13] |
| Plagiochilin A | Anti- proliferative |
P-388 murine leukemia cells | IC50 = 3.0 µg/mL | [23] |
| Plagiochilin A | Anti- proliferative |
A172 glioblastoma cells | IC50 = 19.4 µM. | [24] |
| Plagiochilin-A-15-yl n-octanoate | Anti- proliferative |
P-388 murine leukemia cells | IC50 = 0.05 µg/mL | [23] |
| Plagiochilin C | Antiplatelet | Inhibition of arachidonate-induced rabbit platelet aggregation | 95% and 45% inhibition at 100 and 50 µg/mL, respectively. | [25] |
| Plagiochilin C | Anti- proliferative |
A172 glioblastoma cells | IC50 = 4.3 µM | [26] |
In a more interesting way, plagiochilin A was characterized as an antiproliferative agent, reducing the growth of different cultured cancer cell lines. Both plagiochilins A and C display marked antiproliferative activities and there are known options to further increase their anticancer potency. An option is to introduce a methoxy group at position C-3, as observed with the derivative methoxyplagiochilin A2 which has been shown to be more potent than plagiochilin C against H460 lung cancer cells (IC50 = 6.7 and 13.1 µM, respectively) [26]. Another option is to introduce a side chain at the C-14/C-15 position, either an octanoyl side chain or a dodecadienoate side chain, for example. In both cases, the resulting compounds were found to be 60 times more potent against P-388 leukemia cells than the parent compound plagiochilin A [23]. The extraordinary potency of plagiochilin A-15-yl n-octanoate raises questions (solubility, stability) and opens perspectives. The octanoate moiety may serve only as a “lipophilic carrier” (bioavailability enhancement), and may not be directly implicated in the target interaction. Novel C-12/C-13-substituted derivatives of plagiochilin A should be designed.
Plagiochilin A exhibits antiproliferative activities against different types of cancer cells. The growth inhibition GI50 values ranged from 1.4 to 6.8 µM with a range of tumor cell lines, including prostate (DU145), breast (MCF-7), lung (HT-29) and leukemia (K562) cells for example [13]. The level of activity against DU145 prostate cancer cell is interesting (GI50 = 1.4 µM) because it is superior to that observed with the reference anticancer drug fludarabine phosphate (GI50 = 3.0 µM). The sensitivity of prostate cancer cells toward plagiochilin A warranted further investigation. In 2018, Bates and coworkers analyzed the effect of plagiochilin A on the cell cycle progression of DU145 cells and their capacity to complete cytokinesis, the part of the cell division process during which the cytoplasm of a single eukaryotic cell divides into two daughter cells. Interestingly, it was observed that the compound (at 5 µM for 24–48 h) could block cell division by preventing completion of cytokinesis, and thereby inducing cell death [27]. The treated DU145 cells accumulated at the G2/M phase, notably cells still connected with intercellular bridges, corresponding to a late cytokinesis stage, the so-called membrane abscission stage (stained with an α-anti-tubulin antibody). The compound induced specific mitotic figures and reduced significantly the number and size of DU145 cell colonies. The failure of the cells to complete cytokinesis triggered apoptosis [27]. Altogether, these data indicated that plagiochilin A exerts an effect on the cytoskeleton, with a rearrangement of α-tubulin characteristic of cytokinetic membrane abscission, which is a spatially and temporally regulated process [27] (Figure 3).
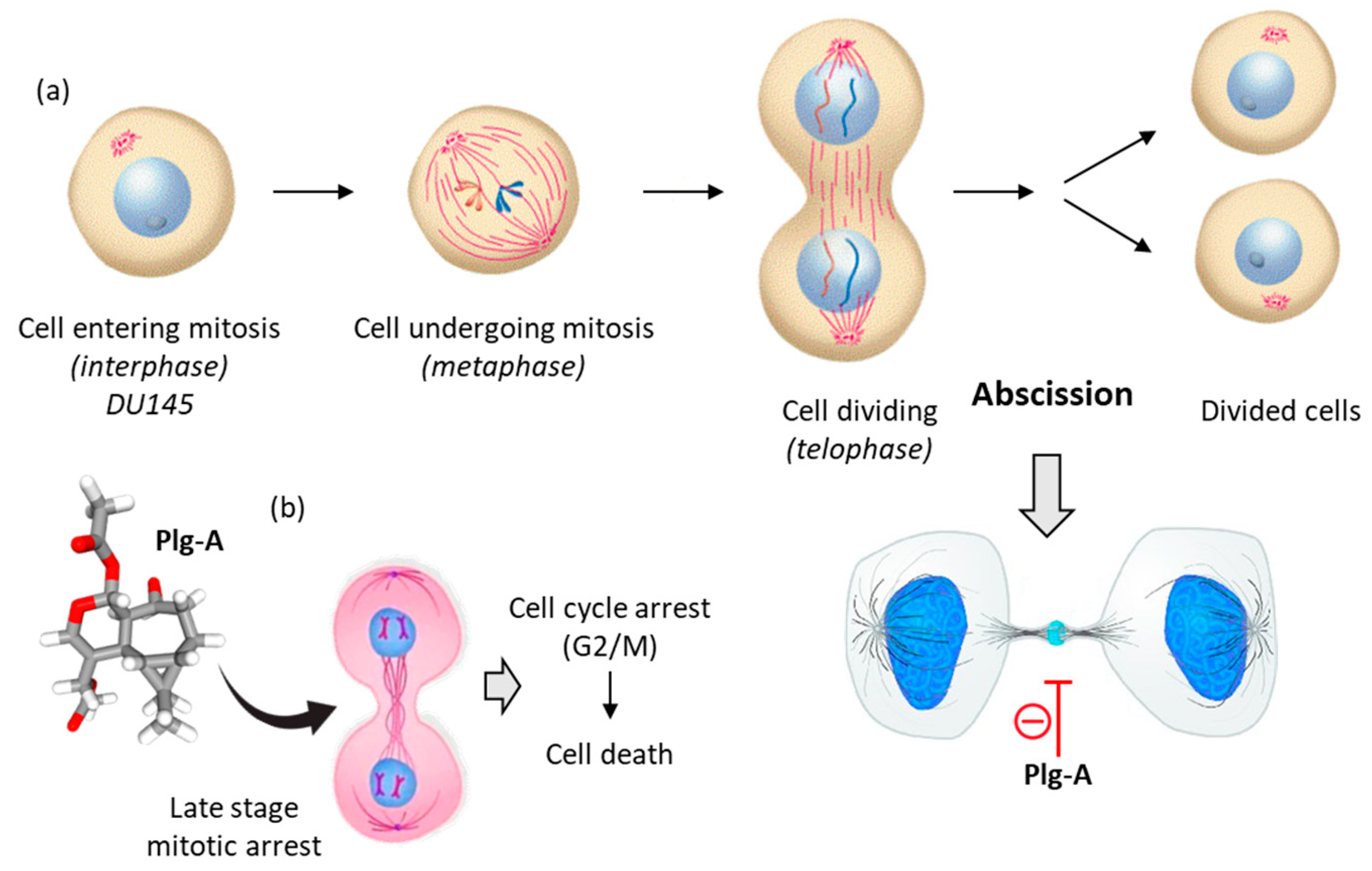
Figure 3. Mechanism of action of plagiochilin A (Plg-A). (a) The cell division process, to illustrate DU145 prostate cancer cells undergoing mitosis. Prior to cell division, at the telophase stage of mitosis, the two cells are connected by an intercellular bridge (with a central midbody). Plg-A inhibits cell division by preventing completion of cytokinesis, particularly at the final abscission stage. (b) Inhibition of the late stage of cytokinesis leads to cell cycle arrest (G2/M) and subsequently to inhibition of cell colony formation and induction of cell death [27].
3. Hypothesized Mechanism of Action of Plagiochilin A
The mechanics implicated in the regulation of abscission is relatively well-known. This process leads to the physical cut of the intercellular bridge which connects two daughter cells and concludes cell division. The process is tightly regulated in cells, with intervention of multiple protein effectors including different kinases (e.g., PLK4, Aurora B) and proteins containing microtubule-interacting and trafficking (MIT) domains [28][29][30]. The process opens perspectives to comprehend the mechanism of action of plagiochilin A. the compound may target MIT-containing proteins, or more directly it may associate with alpha-tubulin during mitosis, for example. There exist small molecules which induced cytokinesis failure at the point of abscission, such as a series of dynamin GTPase inhibitors called dynoles [31][32]. These products induce apoptosis following cytokinesis failure, as observed with plagiochilin A. Therefore, researchers can imagine that the natural product acts as an inhibitor of termination of cytokinesis (abscission) by blocking one or several proteins implicated in the process, or by directly altering the microtubule-organizing center which recruits α- and β-tubulins for microtubule nucleation. In this context, one of the potential mechanisms could be a direct binding of the compound to α-tubulin, in particular to the pironetin-binding site which is known to accommodate compounds bearing a dihydro-pyrone moiety [33][34]. This moiety can be found in plagiochilin Q, for example, and recently, researchers have shown that natural products with a 5,6-dihydro-α-pyrone unit (cryptoconcatones) can function as α-tubulin-binding agents [35]. Based on these considerations, researchers have initiated binding studies of plagiochilins to α-tubulin and the first information obtained by molecular docking look interesting. For the docking analysis, the high-resolution crystal structure of the reference product pironetin (a dihydropyrone derivative with an α,β-unsaturated lactone acting as a plant growth regulator) bound to α/β-tubulin dimer was used as a template (PDB: 5FNV) and the binding of plagiochilin A to the pironetin site was modeled. Apparently, plagiochilin A could form stable complexes with α-tubulin, via binding to the pironetin site, as represented in Figure 4. These are preliminary, but promising information. Researchers are now comparing various plagiochilins for their capacity to bind to α-tubulin, using molecular modeling. The mechanism whereby plagiochilin A specifically blocks abscission warrant further investigation. The compound is an atypical inhibitor of cytokinesis. The panoply of plagiochilins shall be further exploited to identify the best inhibitors and to delineate the structure–activity relationships in the series. The modeling analysis shall help also to delineate the mechanism of action of other aromadendrane derivatives, such as the related natural products hanegokedial, ovalifolienal, ovalifolienalone (from P. semidecurrens) and others [36][37].
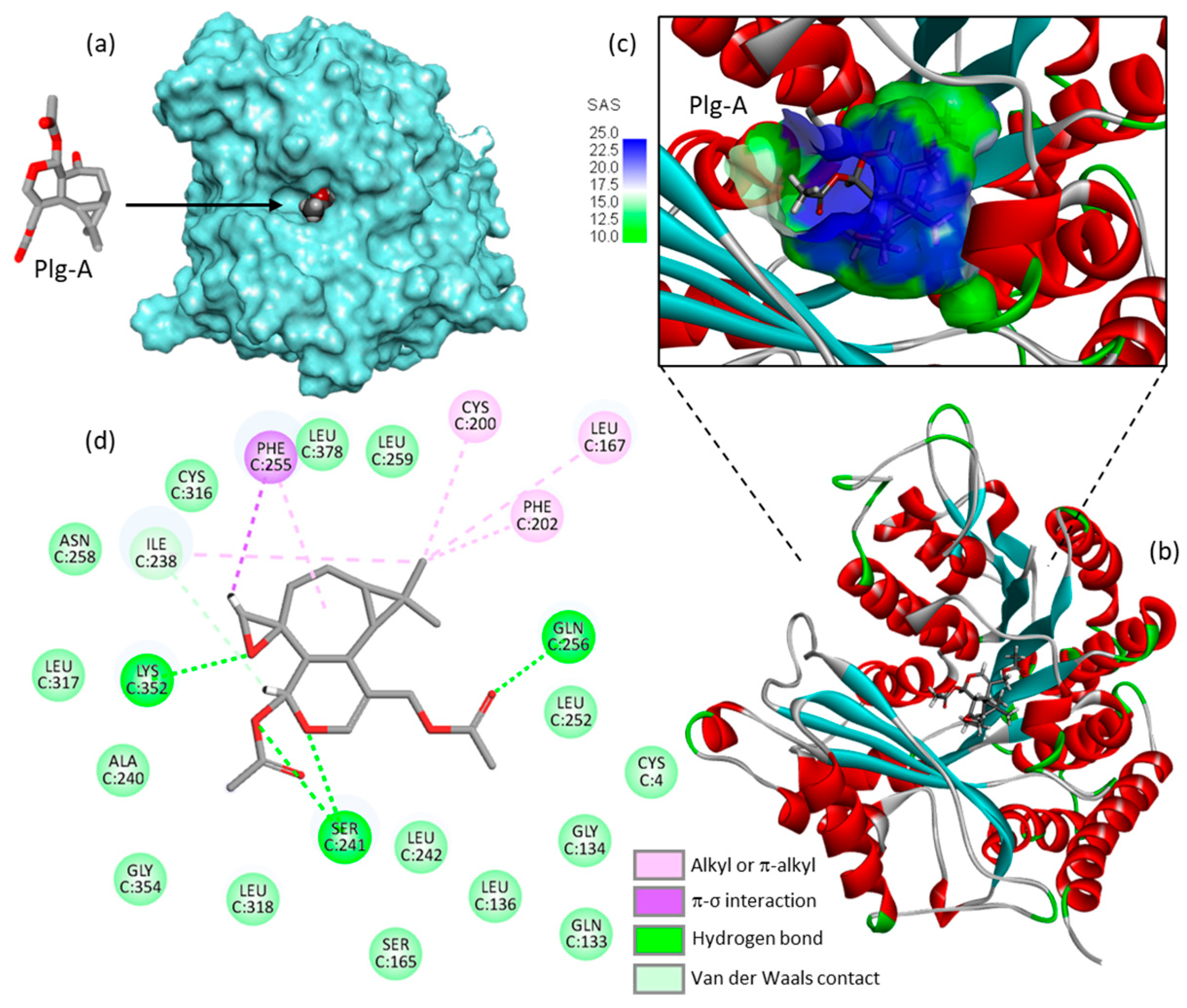
Figure 4. Molecular model of plagiochilin A (Plg-A) bound to the pironetin site of α-tubulin (PDB: 5FNV). (a) Plg-A fits into a central, deep cavity of the protein. (b) Ribbon model of α-tubulin with bound Plg-A, with α-helices (in red) and β-sheets (in cyan). (c) A close-up view of Plg-A inserted into the binding cavity, with the solvent-accessible surface (SAS) surrounding the drug binding zone (color code indicated). (d) Binding map contacts for Plg-A bound to α-tubulin (color code indicated). The docking model was kindly provided by Prof. Gérard Vergoten (University of Lille, France). The docking analysis was performed as recently described [33].
References
- Wang, Q.H.; Zhang, J.; Liu, Y.; Jia, Y.; Jiao, Y.N.; Xu, B.; Chen, Z.D. Diversity, phylogeny, and adaptation of bryophytes: Insights from genomic and transcriptomic data. J. Exp. Bot. 2022, 73, 4306–4322.
- Kulshrestha, S.; Jibran, R.; van Klink, J.W.; Zhou, Y.; Brummell, D.A.; Albert, N.W.; Schwinn, K.E.; Chagné, D.; Landi, M.; Bowman, J.L.; et al. Stress, senescence, and specialized metabolites in bryophytes. J. Exp. Bot. 2022, 73, 4396–4411.
- Harris, B.J.; Clark, J.W.; Schrempf, D.; Szöllősi, G.J.; Donoghue, P.C.J.; Hetherington, A.M.; Williams, T.A. Divergent evolutionary trajectories of bryophytes and tracheophytes from a complex common ancestor of land plants. Nat. Ecol. Evol. 2022, 6, 1634–1643.
- Dong, S.; Yu, J.; Zhang, L.; Goffinet, B.; Liu, Y. Phylotranscriptomics of liverworts: Revisiting the backbone phylogeny and ancestral gene duplications. Ann. Bot. 2022, 130, 951–964.
- Bowman, J.L. A Brief History of Marchantia from Greece to Genomics. Plant Cell Physiol. 2016, 57, 210–229.
- Söderström, L.; Hagborg, A.; von Konrat, M.; Bartholomew-Began, S.; Bell, D.; Briscoe, L.; Brown, E.; Cargill, D.C.; Costa, D.P.; Crandall-Stotler, B.J.; et al. World checklist of hornworts and liverworts. PhytoKeys 2016, 59, 1–828.
- Heinrichs, J.; Hentschel, J.; Feldberg, K.; Bombosch, A.; Schneider, H. Phylogenetic biogeography and taxonomy of disjunctly distributed bryophytes. J. Syst. Evol. 2009, 47, 497–508.
- The World Flora Online (WFO). Available online: http://www.worldfloraonline.org (accessed on 13 January 2023).
- Renner, M.A.M. The typification of Australasian Plagiochila species (Plagiochilaceae: Jungermanniidae): A review with Recommendations. N. Z. J. Bot. 2021, 59, 323–375.
- Drobnik, J.; Stebel, A. Four Centuries of Medicinal Mosses and Liverworts in European Ethnopharmacy and Scientific Pharmacy: A Review. Plants 2021, 10, 1296.
- Manoj, G.S.; Murugan, K. Wound healing activity of methanolic and aqueous extracts of Plagiochila beddomei Steph. thallus in rat model. Indian J. Exp. Biol. 2012, 50, 551–558.
- Manoj, G.S.; Murugan, K. Phenolic profiles, antimicrobial and antioxidant potentiality of methanolic extract of a liverwort, Plagiochila beddomei Steph. Indian J. Nat. Prod. Resour. 2012, 3, 173–183.
- Aponte, J.C.; Yang, H.; Vaisberg, A.J.; Castillo, D.; Málaga, E.; Verástegui, M.; Casson, L.K.; Stivers, N.; Bates, P.J.; Rojas, R.; et al. Cytotoxic and anti-infective sesquiterpenes present in Plagiochila disticha (Plagiochilaceae) and Ambrosia peruviana (Asteraceae). Planta Med. 2010, 76, 705–707.
- Morita, H.; Tomizawa, Y.; Tsuchiya, T.; Hirasawa, Y.; Hashimoto, T.; Asakawa, Y. Antimitotic activity of two macrocyclic bis(bibenzyls), isoplagiochins A and B from the Liverwort Plagiochila fruticosa. Bioorg. Med. Chem. Lett. 2009, 19, 493–496.
- Lorimer, S.D.; Perry, N.B.; Tangney, R.S. An antifungal bibenzyl from the New Zealand liverwort, Plagiochila stephensoniana. Bioactivity-directed isolation, synthesis, and analysis. J. Nat. Prod. 1993, 56, 1444–1450.
- Lorimer, S.D.; Perry, N.B. Antifungal hydroxy-acetophenones from the New Zealand liverwort, Plagiochila fasciculata. Planta Med. 1994, 60, 386–387.
- Ramírez, M.; Kamiya, N.; Popich, S.; Asakawa, Y.; Bardón, A. Insecticidal constituents from the argentine liverwort Plagiochila bursata. Chem. Biodivers. 2010, 7, 1855–1861.
- Qiao, Y.N.; Jin, X.Y.; Zhou, J.C.; Zhang, J.Z.; Chang, W.Q.; Li, Y.; Chen, W.; Ren, Z.J.; Zhang, C.Y.; Yuan, S.Z.; et al. Terpenoids from the Liverwort Plagiochila fruticosa and Their Antivirulence Activity against Candida albicans. J. Nat. Prod. 2020, 83, 1766–1777.
- Han, J.J.; Zhang, J.Z.; Zhu, R.X.; Li, Y.; Qiao, Y.N.; Gao, Y.; Jin, X.Y.; Chen, W.; Zhou, J.C.; Lou, H.X. Plagiochianins A and B, Two ent-2,3-seco-Aromadendrane Derivatives from the Liverwort Plagiochila duthiana. Org. Lett. 2018, 20, 6550–6553.
- Asakawa, Y.; Toyota, M.; Takemoto, T. Plagiochilide et plagiochilin a, secoaromadendrane-type sesquiterpenes de la mousse, plagiochila yokogurensis (plagiochilaceae). Tetrahedron Lett. 1978, 19, 1553–1556.
- Asakawa, Y.; Toyota, M.; Takemoto, T.; Kubo, I.; Nakanishi, K. Insect antifeedant secoaromadendrane-type sesquiterpenes from Plagiochila species. Phytochemistry 1980, 19, 2147–2154.
- Ramírez, M.; Kamiya, N.; Popich, S.; Asakawa, Y.; Bardón, A. Constituents of the Argentine Liverwort Plagiochila diversifolia and Their Insecticidal Activities. Chem. Biodivers. 2017, 14, e1700229.
- Toyota, M.; Tanimura, K.; Asakawa, Y. Cytotoxic 2,3-secoaromadendrane-type sesquiterpenoids from the liverwort Plagiochila ovalifolia. Planta Med. 1998, 64, 462–464.
- Asakawa, Y.; Ludwiczuk, A.; Nagashima, F. Phytochemical and biological studies of bryophytes. Phytochemistry 2013, 91, 52–80.
- Lin, S.J.; Wu, C.L. Isoplagiochilide from the liverwort Plagiochila elegans. Phytochemistry 1996, 41, 1439–1440.
- Wang, S.; Liu, S.S.; Lin, Z.M.; Li, R.J.; Wang, X.N.; Zhou, J.C.; Lou, H.X. Terpenoids from the Chinese liverwort Plagiochila pulcherrima and their cytotoxic effects. J. Asian Nat. Prod. Res. 2013, 15, 473–481.
- Stivers, N.S.; Islam, A.; Reyes-Reyes, E.M.; Casson, L.K.; Aponte, J.C.; Vaisberg, A.J.; Hammond, G.B.; Bates, P.J. Plagiochilin A Inhibits Cytokinetic Abscission and Induces Cell Death. Molecules 2018, 23, 1418.
- Andrade, V.; Echard, A. Mechanics and regulation of cytokinetic abscission. Front. Cell Dev. Biol. 2022, 10, 1046617.
- Sechi, S.; Piergentili, R.; Giansanti, M.G. Minor Kinases with Major Roles in Cytokinesis Regulation. Cells 2022, 11, 3639.
- Wenzel, D.M.; Mackay, D.R.; Skalicky, J.J.; Paine, E.L.; Miller, M.S.; Ullman, K.S.; Sundquist, W.I. Comprehensive analysis of the human ESCRT-III-MIT domain interactome reveals new cofactors for cytokinetic abscission. Elife 2022, 11, e77779.
- Chircop, M.; Perera, S.; Mariana, A.; Lau, H.; Ma, M.P.; Gilbert, J.; Jones, N.C.; Gordon, C.P.; Young, K.A.; Morokoff, A.; et al. Inhibition of dynamin by dynole 34-2 induces cell death following cytokinesis failure in cancer cells. Mol. Cancer Ther. 2011, 10, 1553–1562.
- Tremblay, C.S.; Chiu, S.K.; Saw, J.; McCalmont, H.; Litalien, V.; Boyle, J.; Sonderegger, S.E.; Chau, N.; Evans, K.; Cerruti, L.; et al. Small molecule inhibition of Dynamin-dependent endocytosis targets multiple niche signals and impairs leukemia stem cells. Nat. Commun. 2020, 11, 6211.
- Huang, D.S.; Wong, H.L.; Georg, G.I. Synthesis and Cytotoxicity Evaluation of C4- and C5-Modified Analogues of the α,β-Unsaturated Lactone of Pironetin. ChemMedChem 2017, 12, 520–528.
- Coulup, S.K.; Georg, G.I. Revisiting microtubule targeting agents: α-Tubulin and the pironetin binding site as unexplored targets for cancer therapeutics. Bioorg. Med. Chem. Lett. 2019, 29, 1865–1873.
- Vergoten, G.; Bailly, C. Molecular Docking of Cryptoconcatones to α-Tubulin and Related Pironetin Analogues. Plants 2023, 12, 296.
- Matsuo, A.; Atsumi, K.; Nakayama, M. Structures of ent-2,3-Secoalloaromadendrane Sesquiterpenoids, which have plant growth inhibitory Activity, from Plagiochila semidecurrens (Liverwort). J. Chem. Soc. Perkin Trans. 1981, 1, 2816–2824.
- Durán-Peña, M.J.; Botubol Ares, J.M.; Hanson, J.R.; Collado, I.G.; Hernández-Galán, R. Biological activity of natural sesquiterpenoids containing a gem-dimethylcyclopropane unit. Nat. Prod. Rep. 2015, 32, 1236–1248.
More
Information
Subjects:
Biochemistry & Molecular Biology; Oncology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
899
Revisions:
2 times
(View History)
Update Date:
24 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




