
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alessia Paganelli | -- | 2351 | 2023-03-24 07:39:14 | | | |
| 2 | Rita Xu | Meta information modification | 2351 | 2023-03-24 07:58:45 | | |
Video Upload Options
Chronic wounds represent nowadays a major challenge for both clinicians and researchers in the regenerative setting. Obesity represents one of the major comorbidities in patients affected by chronic ulcers and therefore diverse studies aimed at assessing possible links between these two morbid conditions are currently ongoing. In particular, adipose tissue has recently been described as having metabolic and endocrine functions rather than serving as a mere fat storage deposit. In this setting, adipose-derived stem cells, a peculiar subset of mesenchymal stromal/stem cells (MSCs) located in adipose tissue, have been demonstrated to possess regenerative and immunological functions with a key role in regulating both adipocyte function and skin regeneration.
1. Introduction
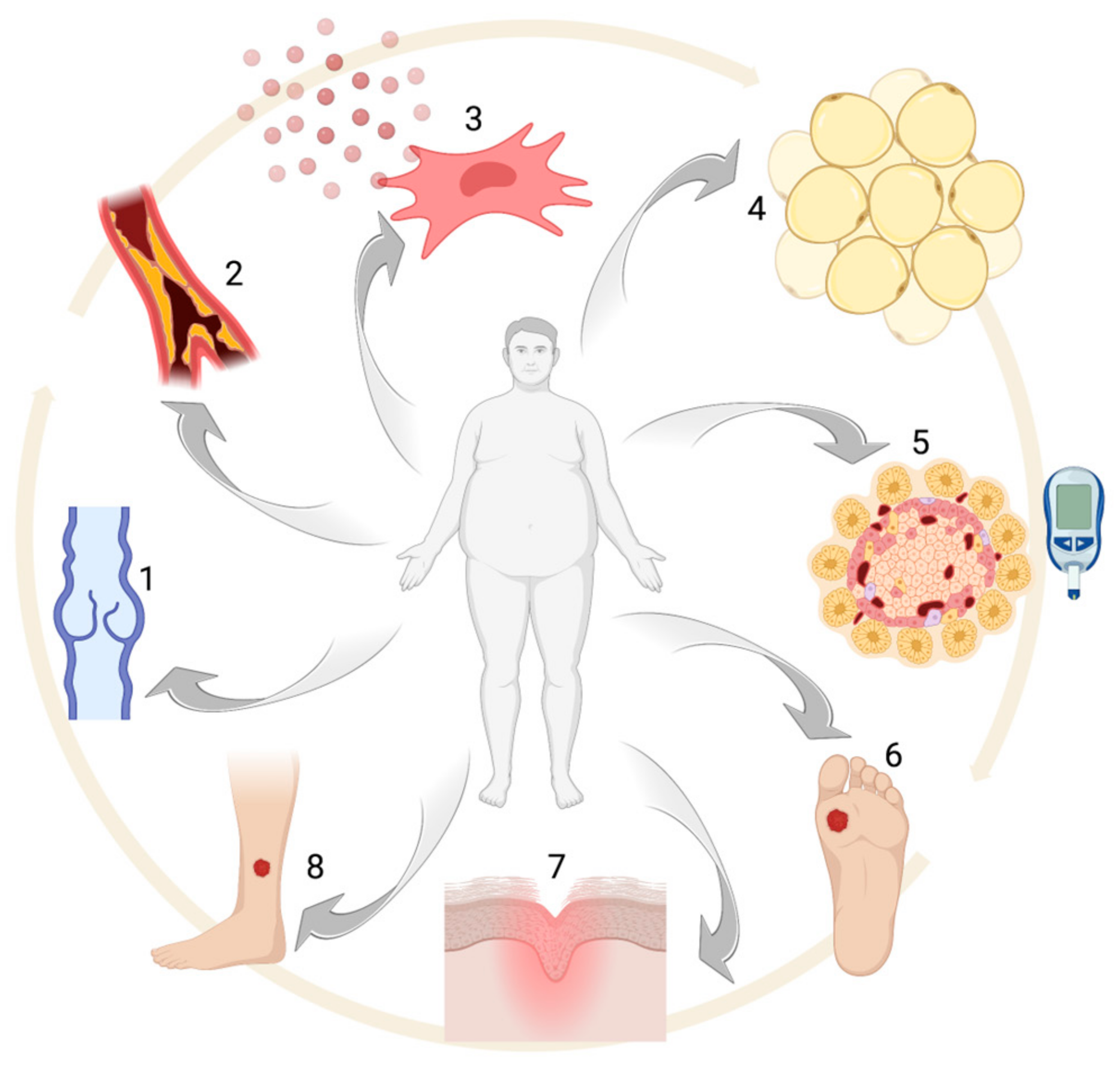
2. Obesity: Epidemiology and Comorbidities
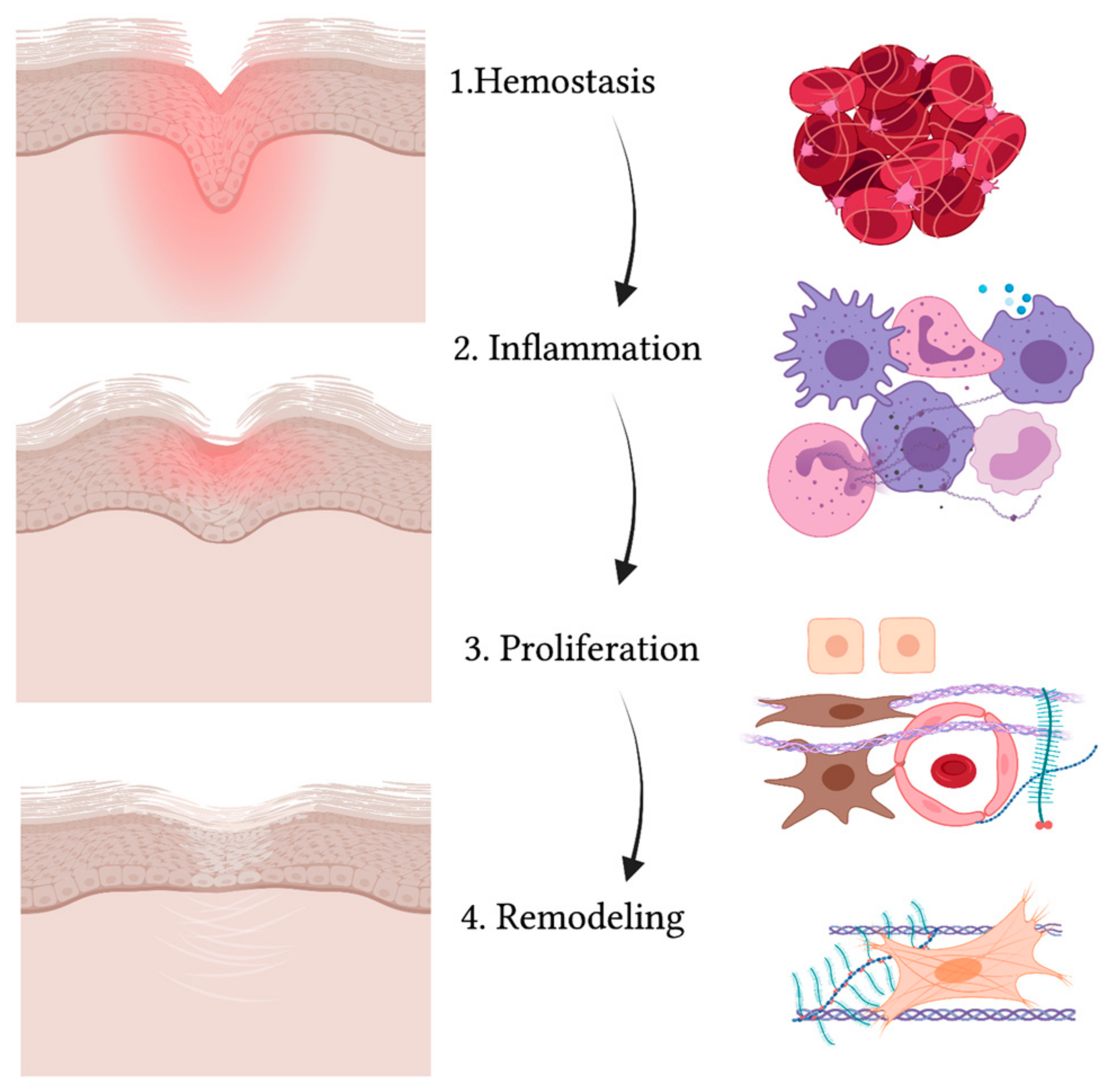
3. Wound Healing
4. Obesity and Wound Healing
References
- Deitel, M. Overweight and Obesity Worldwide Now Estimated to Involve 1.7 Billion People. Obes. Surg. 2003, 13, 329–330.
- Baghbani-Naghadehi, F.; Armijo-Olivo, S.; Prado, C.M.; Woodhouse, L.J. Obesity, Comorbidities, and the Associated Risk among Patients Who Underwent Total Knee Arthroplasty in Alberta. J. Knee Surg. 2022, s-0042-1742646.
- Ligi, D.; Croce, L.; Mosti, G.; Raffetto, J.D.; Mannello, F. Chronic Venous Insufficiency: Transforming Growth Factor-β Isoforms and Soluble Endoglin Concentration in Different States of Wound Healing. Int. J. Mol. Sci. 2017, 18, 2206.
- Moss, J.-L.; Pugliese, M.; Richards, T. Ultrasound Patterns of Venous Disease in Patients with Venous Leg Ulcers and Morbid Obesity. Phlebology 2022, 37, 732–738.
- Costa, D.; Andreucci, M.; Ielapi, N.; Serraino, G.F.; Mastroroberto, P.; Bracale, U.M.; Serra, R. Molecular Determinants of Chronic Venous Disease: A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 1928.
- Dayya, D.; O’Neill, O.J.; Huedo-Medina, T.B.; Habib, N.; Moore, J.; Iyer, K. Debridement of Diabetic Foot Ulcers. Adv. Wound Care 2022, 11, 666–686.
- Luo, R.; Ji, Y.; Liu, Y.-H.; Sun, H.; Tang, S.; Li, X. Relationships among Social Support, Coping Style, Self-Stigma, and Quality of Life in Patients with Diabetic Foot Ulcer: A Multicentre, Cross-Sectional Study. Int. Wound J. 2023, 20, 716–724.
- Jung, F.U.C.E.; Riedel-Heller, S.G.; Luck-Sikorski, C. The Relationship between Weight History and Psychological Health-Differences Related to Gender and Weight Loss Patterns. PLoS ONE 2023, 18, e0281776.
- Giralt, M.; Villarroya, F. White, Brown, Beige/Brite: Different Adipose Cells for Different Functions? Endocrinology 2013, 154, 2992–3000.
- Li, J.; Xu, R. Obesity-Associated ECM Remodeling in Cancer Progression. Cancers 2022, 14, 5684.
- Esteve Ràfols, M. Adipose Tissue: Cell Heterogeneity and Functional Diversity. Endocrinol. Nutr. 2014, 61, 100–112.
- Pierpont, Y.N.; Dinh, T.P.; Salas, R.E.; Johnson, E.L.; Wright, T.G.; Robson, M.C.; Payne, W.G. Obesity and Surgical Wound Healing: A Current Review. ISRN Obes. 2014, 2014, 638936.
- Friedman, A.; Siewe, N. Mathematical Model of Chronic Dermal Wounds in Diabetes and Obesity. Bull. Math. Biol. 2020, 82, 137.
- Shook, B.A.; Wasko, R.R.; Mano, O.; Rutenberg-Schoenberg, M.; Rudolph, M.C.; Zirak, B.; Rivera-Gonzalez, G.C.; López-Giráldez, F.; Zarini, S.; Rezza, A.; et al. Dermal Adipocyte Lipolysis and Myofibroblast Conversion Are Required for Efficient Skin Repair. Cell Stem Cell 2020, 26, 880–895.e6.
- Rawal, K.; Patel, T.P.; Purohit, K.M.; Israni, K.; Kataria, V.; Bhatt, H.; Gupta, S. Influence of Obese Phenotype on Metabolic Profile, Inflammatory Mediators and Stemness of HADSC in Adipose Tissue. Clin. Nutr. 2020, 39, 3829–3835.
- Paganelli, A.; Benassi, L.; Rossi, E.; Tarentini, E.; Magnoni, C. Mesenchymal Stromal Cells Promote the Proliferation of Basal Stem Cells and Efficient Epithelization in Organotypic Models of Wound Healing. Microsc. Res. Tech. 2022, 85, 2752–2756.
- García-Gómez, I.; Elvira, G.; Zapata, A.G.; Lamana, M.L.; Ramírez, M.; Castro, J.G.; Arranz, M.G.; Vicente, A.; Bueren, J.; García-Olmo, D. Mesenchymal Stem Cells: Biological Properties and Clinical Applications. Expert Opin. Biol. Ther. 2010, 10, 1453–1468.
- Villagrasa, A.; Posada-González, M.; García-Arranz, M.; Zapata, A.G.; Vorwald, P.; Olmedillas-López, S.; Vega-Clemente, L.; García-Olmo, D. Implicación de las células madre derivadas del tejido adiposo en la cicatrización de heridas de pacientes obesos y pacientes oncológicos. CIRU 2022, 90, 6528.
- Paganelli, A.; Benassi, L.; Pastar, I.; Pellegrini, M.; Azzoni, P.; Vaschieri, C.; Pisciotta, A.; Carnevale, G.; Pellacani, G.; Magnoni, C. In Vitro Engineering of a Skin Substitute Based on Adipose-Derived Stem Cells. Cells Tissues Organs 2019, 207, 46–57.
- Pachón-Peña, G.; Serena, C.; Ejarque, M.; Petriz, J.; Duran, X.; Oliva-Olivera, W.; Simó, R.; Tinahones, F.J.; Fernández-Veledo, S.; Vendrell, J. Obesity Determines the Immunophenotypic Profile and Functional Characteristics of Human Mesenchymal Stem Cells From Adipose Tissue. Stem Cells Transl. Med. 2016, 5, 464–475.
- De Assis-Ferreira, A.; Saldanha-Gama, R.; de Brito, N.M.; Renovato-Martins, M.; Simões, R.L.; Barja-Fidalgo, C.; Vargas da Silva, S. Obesity Enhances the Recruitment of Mesenchymal Stem Cells to Visceral Adipose Tissue. J. Mol. Endocrinol. 2021, 67, 15–26.
- Pluta, W.; Dudzińska, W.; Lubkowska, A. Metabolic Obesity in People with Normal Body Weight (MONW)-Review of Diagnostic Criteria. Int. J. Environ. Res. Public Health 2022, 19, 624.
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose Tissue and Insulin Resistance in Obese. Biomed. Pharmacother. 2021, 137, 111315.
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358.
- Nam, G.E.; Park, H.S. Perspective on Diagnostic Criteria for Obesity and Abdominal Obesity in Korean Adults. JOMES 2018, 27, 134–142.
- Hyde, R. Europe Battles with Obesity. Lancet 2008, 371, 2160–2161.
- Żukiewicz-Sobczak, W.; Wróblewska, P.; Zwoliński, J.; Chmielewska-Badora, J.; Adamczuk, P.; Krasowska, E.; Zagórski, J.; Oniszczuk, A.; Piątek, J.; Silny, W. Obesity and Poverty Paradox in Developed Countries. Ann. Agric. Environ. Med. 2014, 21, 590–594.
- Héraïef, E. The contribution of epidemiology to the definition of obesity and its risk factors. Ther. Umsch. 1989, 46, 275–280.
- Levine, J.A. Poverty and Obesity in the U.S. Diabetes 2011, 60, 2667–2668.
- Avenell, A.; Broom, J.; Brown, T.J.; Poobalan, A.; Aucott, L.; Stearns, S.C.; Smith, W.C.S.; Jung, R.T.; Campbell, M.K.; Grant, A.M. Systematic Review of the Long-Term Effects and Economic Consequences of Treatments for Obesity and Implications for Health Improvement. Health Technol. Assess. 2004, 8, iii–iv, 1–182.
- Finkelstein, E.A.; Trogdon, J.G.; Cohen, J.W.; Dietz, W. Annual Medical Spending Attributable to Obesity: Payer-and Service-Specific Estimates. Health Aff. 2009, 28, w822–w831.
- Van den Broek-Altenburg, E.; Atherly, A.; Holladay, E. Changes in Healthcare Spending Attributable to Obesity and Overweight: Payer- and Service-Specific Estimates. BMC Public Health 2022, 22, 962.
- Guillaume, M.; Lapidus, L.; Beckers, F.; Lambert, A.; Björntorp, P. Familial Trends of Obesity through Three Generations: The Belgian-Luxembourg Child Study. Int. J. Obes. Relat. Metab. Disord. 1995, 19, S5–S9.
- Grio, R.; Porpiglia, M. Obesity: Internal Medicine, Obstetric and Gynecological Problems Related to Overweight. Panminerva Med. 1994, 36, 138–141.
- Flegal, K.M.; Troiano, R.P.; Pamuk, E.R.; Kuczmarski, R.J.; Campbell, S.M. The Influence of Smoking Cessation on the Prevalence of Overweight in the United States. N. Engl. J. Med. 1995, 333, 1165–1170.
- Parsons, T.J.; Power, C.; Logan, S.; Summerbell, C.D. Childhood Predictors of Adult Obesity: A Systematic Review. Int. J. Obes. Relat. Metab. Disord. 1999, 23, S1–S107.
- Mauras, N.; Delgiorno, C.; Kollman, C.; Bird, K.; Morgan, M.; Sweeten, S.; Balagopal, P.; Damaso, L. Obesity without Established Comorbidities of the Metabolic Syndrome Is Associated with a Proinflammatory and Prothrombotic State, Even before the Onset of Puberty in Children. J. Clin. Endocrinol. Metab. 2010, 95, 1060–1068.
- Ziogas, I.A.; Zapsalis, K.; Giannis, D.; Tsoulfas, G. Metabolic Syndrome and Liver Disease in the Era of Bariatric Surgery: What You Need to Know! World J. Hepatol. 2020, 12, 709–721.
- Schienkiewitz, A.; Schulze, M.B.; Hoffmann, K.; Kroke, A.; Boeing, H. Body Mass Index History and Risk of Type 2 Diabetes: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam Study. Am. J. Clin. Nutr. 2006, 84, 427–433.
- Samad, F.; Ruf, W. Inflammation, Obesity, and Thrombosis. Blood 2013, 122, 3415–3422.
- Reaven, G.M. Insulin Resistance: The Link Between Obesity and Cardiovascular Disease. Med. Clin. N. Am. 2011, 95, 875–892.
- Rocha, V.Z.; Libby, P. Obesity, Inflammation, and Atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 399–409.
- Zaman, C.F.; Sultana, J.; Dey, P.; Dutta, J.; Mustarin, S.; Tamanna, N.; Roy, A.; Bhowmick, N.; Khanam, M.; Sultana, S.; et al. A Multidisciplinary Approach and Current Perspective of Nonalcoholic Fatty Liver Disease: A Systematic Review. Cureus 2022, 14, e29657.
- Kyrou, I.; Randeva, H.S.; Tsigos, C.; Kaltsas, G.; Weickert, M.O. Clinical Problems Caused by Obesity. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Hofland, J., Dungan, K., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000.
- Yue, W.; Huang, X.; Zhang, W.; Li, S.; Liu, X.; Zhao, Y.; Shu, J.; Liu, T.; Li, W.; Liu, S. Metabolic Surgery on Patients With Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 848947.
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. International Agency for Research on Cancer Handbook Working Group Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798.
- Zhou, L.; Li, J.; Ontaneda, D.; Sperling, J. Metabolic Syndrome in Small Fiber Sensory Neuropathy. J. Clin. Neuromuscul. Dis. 2011, 12, 235–243.
- For the Alzheimer’s Disease Neuroimaging Initiative; Morys, F.; Potvin, O.; Zeighami, Y.; Vogel, J.; Lamontagne-Caron, R.; Duchesne, S.; Dagher, A. Obesity-Associated Neurodegeneration Pattern Mimics Alzheimer’s Disease in an Observational Cohort Study. J. Alzheimers Dis. 2023, 91, 1059–1071.
- Tobin, A.-M.; Ahern, T.; Rogers, S.; Collins, P.; O’Shea, D.; Kirby, B. The Dermatological Consequences of Obesity: Dermatological Consequences of Obesity. Int. J. Dermatol. 2013, 52, 927–932.
- Sebastian, J.C. Respiratory Physiology and Pulmonary Complications in Obesity. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 157–161.
- Kivimäki, M.; Batty, G.D.; Singh-Manoux, A.; Nabi, H.; Sabia, S.; Tabak, A.G.; Akbaraly, T.N.; Vahtera, J.; Marmot, M.G.; Jokela, M. Association between Common Mental Disorder and Obesity over the Adult Life Course. Br. J. Psychiatry 2009, 195, 149–155.
- Mohamed-Ali, V.; Goodrick, S.; Rawesh, A.; Katz, D.R.; Miles, J.M.; Yudkin, J.S.; Klein, S.; Coppack, S.W. Subcutaneous Adipose Tissue Releases Interleukin-6, but Not Tumor Necrosis Factor-Alpha, in Vivo. J. Clin. Endocrinol. Metab. 1997, 82, 4196–4200.
- Bastard, J.P.; Jardel, C.; Delattre, J.; Hainque, B.; Bruckert, E.; Oberlin, F. Evidence for a Link between Adipose Tissue Interleukin-6 Content and Serum C-Reactive Protein Concentrations in Obese Subjects. Circulation 1999, 99, 2221–2222.
- Bozkurt, B.; Aguilar, D.; Deswal, A.; Dunbar, S.B.; Francis, G.S.; Horwich, T.; Jessup, M.; Kosiborod, M.; Pritchett, A.M.; Ramasubbu, K.; et al. Contributory Risk and Management of Comorbidities of Hypertension, Obesity, Diabetes Mellitus, Hyperlipidemia, and Metabolic Syndrome in Chronic Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e535–e578.
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464.
- Broszczak, D.A.; Sydes, E.R.; Wallace, D.; Parker, T.J. Molecular Aspects of Wound Healing and the Rise of Venous Leg Ulceration: Omics Approaches to Enhance Knowledge and Aid Diagnostic Discovery. Clin. Biochem. Rev. 2017, 38, 35–55.
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound Repair and Regeneration: Mechanisms, Signaling, and Translation. Sci. Transl. Med. 2014, 6, 265sr6.
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542.
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321.
- Żurawska-Płaksej, E.; Kuliczkowski, W.; Karolko, B.; Cielecka-Prynda, M.; Dębski, J.; Kaaz, K.; Mysiak, A.; Wróbel, T.; Podolak-Dawidziak, M.; Usnarska-Zubkiewicz, L. Platelet Polyphosphate Level Is Elevated in Patients with Chronic Primary Thrombocytopenia: A Preliminary Study. Adv. Clin. Exp. Med. 2020, 29, 1051–1056.
- Werner, S.; Grose, R. Regulation of Wound Healing by Growth Factors and Cytokines. Physiol. Rev. 2003, 83, 835–870.
- Zhao, G.; Usui, M.L.; Lippman, S.I.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Biofilms and Inflammation in Chronic Wounds. Adv. Wound Care 2013, 2, 389–399.
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525.
- Qiang, L.; Yang, S.; Cui, Y.-H.; He, Y.-Y. Keratinocyte Autophagy Enables the Activation of Keratinocytes and Fibroblastsand Facilitates Wound Healing. Autophagy 2021, 17, 2128–2143.
- Stricklin, G.P.; Li, L.; Jancic, V.; Wenczak, B.A.; Nanney, L.B. Localization of MRNAs Representing Collagenase and TIMP in Sections of Healing Human Burn Wounds. Am. J. Pathol. 1993, 143, 1657–1666.
- Harding, K.G.; Morris, H.L.; Patel, G.K. Science, Medicine and the Future: Healing Chronic Wounds. BMJ 2002, 324, 160–163.
- Zheng, Y.; Xu, P.; Pan, C.; Wang, Y.; Liu, Z.; Chen, Y.; Chen, C.; Fu, S.; Xue, K.; Zhou, Q.; et al. Production and Biological Effects of Extracellular Vesicles from Adipose-Derived Stem Cells Were Markedly Increased by Low-Intensity Ultrasound Stimulation for Promoting Diabetic Wound Healing. Stem Cell Rev. Rep. 2022, 1–23.
- Zwaginga, J.J.; Doevendans, P. Stem Cell-Derived Angiogenic/Vasculogenic Cells: Possible Therapies for Tissue Repair and Tissue Engineering. Clin. Exp. Pharmacol. Physiol. 2003, 30, 900–908.
- Pilcher, B.K.; Wang, M.; Qin, X.-J.; Parks, W.C.; Senior, R.M.; Welgus, H.G. Role of Matrix Metalloproteinases and Their Inhibition in Cutaneous Wound Healing and Allergic Contact Hypersensitivity. Ann. N. Y. Acad. Sci. 1999, 878, 12–24.
- Stojadinovic, O.; Brem, H.; Vouthounis, C.; Lee, B.; Fallon, J.; Stallcup, M.; Merchant, A.; Galiano, R.D.; Tomic-Canic, M. Molecular Pathogenesis of Chronic Wounds: The Role of Beta-Catenin and c-Myc in the Inhibition of Epithelialization and Wound Healing. Am. J. Pathol. 2005, 167, 59–69.
- Gallagher Camden, S.; Gates, J. Obesity: Changing the Face of Geriatric Care. Ostomy Wound Manag. 2006, 52, 36–38, 40–44.
- Schneider, C.; Stratman, S.; Kirsner, R.S. Lower Extremity Ulcers. Med. Clin. N. Am. 2021, 105, 663–679.
- Lazar, M.; Ershadi, S.; Bolton, L.; Phillips, T. Patient-Centered Outcomes for Individuals with a Venous Leg Ulcer: A Scoping Review. Adv. Ski. Wound Care 2023, 36, 10–17.
- Corvera, S.; Solivan-Rivera, J.; Yang Loureiro, Z. Angiogenesis in Adipose Tissue and Obesity. Angiogenesis 2022, 25, 439–453.
- Khan, T.; Muise, E.S.; Iyengar, P.; Wang, Z.V.; Chandalia, M.; Abate, N.; Zhang, B.B.; Bonaldo, P.; Chua, S.; Scherer, P.E. Metabolic Dysregulation and Adipose Tissue Fibrosis: Role of Collagen VI. Mol. Cell. Biol. 2009, 29, 1575–1591.
- Spencer, M.; Unal, R.; Zhu, B.; Rasouli, N.; McGehee, R.E.; Peterson, C.A.; Kern, P.A. Adipose Tissue Extracellular Matrix and Vascular Abnormalities in Obesity and Insulin Resistance. J. Clin. Endocrinol. Metab. 2011, 96, E1990–E1998.
- Stadelmann, W.K.; Digenis, A.G.; Tobin, G.R. Impediments to Wound Healing. Am. J. Surg. 1998, 176, 39S–47S.
- Yosipovitch, G.; DeVore, A.; Dawn, A. Obesity and the Skin: Skin Physiology and Skin Manifestations of Obesity. J. Am. Acad. Dermatol. 2007, 56, 901–916.
- Robson, M.C. Wound infection. Surg. Clin. N. Am. 1997, 77, 637–650.
- Yu, M.; Zhang, S.; Wang, L.; Wu, J.; Li, X.; Yuan, J. Metabolically Healthy Obesity and Carotid Plaque among Steelworkers in North China: The Role of Inflammation. Nutrients 2022, 14, 5123.
- Cai, S.; Rahman, M.; Intrator, O. Obesity and Pressure Ulcers among Nursing Home Residents. Med. Care 2013, 51, 478–486.
- Alipoor, E.; Mehrdadi, P.; Yaseri, M.; Hosseinzadeh-Attar, M.J. Association of Overweight and Obesity with the Prevalence and Incidence of Pressure Ulcers: A Systematic Review and Meta-Analysis. Clin. Nutr. 2021, 40, 5089–5098.
- Großschädl, F.; Bauer, S. The Relationship between Obesity and Nursing Care Problems in Intensive Care Patients in Austria. Nurs. Crit. Care 2022, 27, 512–518.
- Boulton, A.J.M.; Whitehouse, R.W. The Diabetic Foot. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000.
- Andrews, K.L.; Dyck, P.J.; Kavros, S.J.; Vella, A.; Kazamel, M.; Clark, V.; Litchy, W.J.; Dyck, P.J.B.; Lodermeier, K.A.; Davies, J.L.; et al. Plantar Ulcers and Neuropathic Arthropathies: Associated Diseases, Polyneuropathy Correlates, and Risk Covariates. Adv. Ski. Wound Care 2019, 32, 168–175.
- Costa, D.; Ielapi, N.; Caprino, F.; Giannotta, N.; Sisinni, A.; Abramo, A.; Ssempijja, L.; Andreucci, M.; Bracale, U.M.; Serra, R. Social Aspects of Diabetic Foot: A Scoping Review. Soc. Sci. 2022, 11, 149.
- Ackermann, P.W.; Hart, D.A. Influence of Comorbidities: Neuropathy, Vasculopathy, and Diabetes on Healing Response Quality. Adv. Wound Care 2013, 2, 410–421.
- Peppa, M.; Raptis, S.A. Glycoxidation and Wound Healing in Diabetes: An Interesting Relationship. Curr. Diabetes Rev. 2011, 7, 416–425.
- Strollo, F.; Gentile, S.; Pipicelli, A.M.V.; Mambro, A.; Monici, M.; Magni, P. Space Flight-Promoted Insulin Resistance as a Possible Disruptor of Wound Healing. Front. Bioeng. Biotechnol. 2022, 10, 868999.
- Yang, P.; Pei, Q.; Yu, T.; Chang, Q.; Wang, D.; Gao, M.; Zhang, X.; Liu, Y. Compromised Wound Healing in Ischemic Type 2 Diabetic Rats. PLoS ONE 2016, 11, e0152068.
- Tadokoro, S.; Ide, S.; Tokuyama, R.; Umeki, H.; Tatehara, S.; Kataoka, S.; Satomura, K. Leptin Promotes Wound Healing in the Skin. PLoS ONE 2015, 10, e0121242.
- Fam, B.C.; Morris, M.J.; Hansen, M.J.; Kebede, M.; Andrikopoulos, S.; Proietto, J.; Thorburn, A.W. Modulation of Central Leptin Sensitivity and Energy Balance in a Rat Model of Diet-Induced Obesity. Diabetes Obes. Metab. 2007, 9, 840–852.
- Dopytalska, K.; Baranowska-Bik, A.; Roszkiewicz, M.; Bik, W.; Walecka, I. The Role of Leptin in Selected Skin Diseases. Lipids Health Dis. 2020, 19, 215.




