Chronic wounds represent nowadays a major challenge for both clinicians and researchers in the regenerative setting. Obesity represents one of the major comorbidities in patients affected by chronic ulcers and therefore diverse studies aimed at assessing possible links between these two morbid conditions are currently ongoing. In particular, adipose tissue has recently been described as having metabolic and endocrine functions rather than serving as a mere fat storage deposit. In this setting, adipose-derived stem cells, a peculiar subset of mesenchymal stromal/stem cells (MSCs) located in adipose tissue, have been demonstrated to possess regenerative and immunological functions with a key role in regulating both adipocyte function and skin regeneration.
1. Introduction
At present, obesity affects 1.7 billion people worldwide
[1]. Notably, obesity often leads to a wide range of possible comorbidities, including cardiovascular disorders and diabetes (type 2 diabetes mellitus, T2DM, in particular)
[2]. Most of these obesity-related diseases carry an intrinsic risk of developing chronic ulcers and/or causing significant impairment in physiological wound healing. For example, venous insufficiency is the commonest cause of chronic ulcers and is often associated to—and worsened by—obesity. Venous stasis may also determine delayed wound healing because of altered capillary flow due to impaired hydrostatic pressure
[3][4][5][3,4,5]. Another frequent cause of chronic ulcers is peripheral artery disease (PAD), which is notably caused by atherosclerosis and therefore is, in turn, associated with obesity and/or T2DM. PAD determines a reduction in oxygen flow and nutrient supply, which are essential for tissue repair, and sometimes leads to the development of arterial ulcers through tissue ischemia. Neuropathy is another relatively common cause of cutaneous ulcers and represent a key driver for the development of chronic wounds in diabetic patients
[6].
Not only do obesity and chronic ulcers represent significant health-related issues from a clinical and economic point of view, but they also lead to social and psychological consequences related to body image
[7][8][7,8].
Obesity is by definition associated with an excess of adipose tissue. Adipose tissue is present in the human body in the form of brown and white adipose tissue (BAT and WAT), which are mainly responsible for thermogenesis and fat storage, respectively. However, brown adipocytes have been shown to possibly appear in WAT in response to specific thermogenic stimuli, therefore suggesting a more dynamic division between BAT and WAT and undermining previously consolidated notions of a static classification of adipose tissue subtypes
[9]. Moreover, current research is pointing to adipose tissue as a more complex system, involved in several other physiological and/or pathological processes, including hormone metabolism, inflammation and wound healing
[10][11][10,11].
Adipocyte dysfunction seems to play a role in obesity and its comorbidities, including chronic ulcers. For example, reduced adiponectin production by adipocytes has been described in obese patients and current evidence suggests a possible correlation with impaired wound healing
[12]. At the same time, adipocytes represent a possible source of leukotriene B4, which prevents macrophage M2 polarization, therefore affecting the remodeling phase of wound healing
[13]. Adipocyte lipolysis is also essential in wound repair
[14], but it is not clear whether significant impairment is present in obese patients. Furthermore, inflammatory mediators and insulin-related signaling proteins, such as leptin and resistin, have been postulated to contribute to delayed wound healing in the setting of obesity
[15].
Beyond adipocytes, other cell types are also present in the fibrous septa of the subcutaneous fat (e.g., endothelial cells, fibroblasts, inflammatory cells, etc.). Among these, ADSCs (adipose-derived mesenchymal stromal/stem cells) are of central importance for their role in wound healing. ADSCs are, in fact, adipose-tissue specific mesenchymal stromal/stem cells (MSCs) and are currently widely studied for their regenerative properties
[11][16][11,16]. ADSCs have recently been demonstrated to be able to replace the dermal compartment and to promote wound re-epithelization
[16][17][18][19][16,17,18,19]. ADSCs also seem to play a key role in the orchestration of the various phases of wound healing
[14]. ADSC function is regulated by the tissue microenvironment: obesity, hypoxia and inflammation affect ADSC cellular plasticity and alter their immunophenotypic profile and regenerative functions
[20]. In addition, obesity enhances their migratory potential and leads to their accumulation in visceral adipose tissue (VAT)
[21].
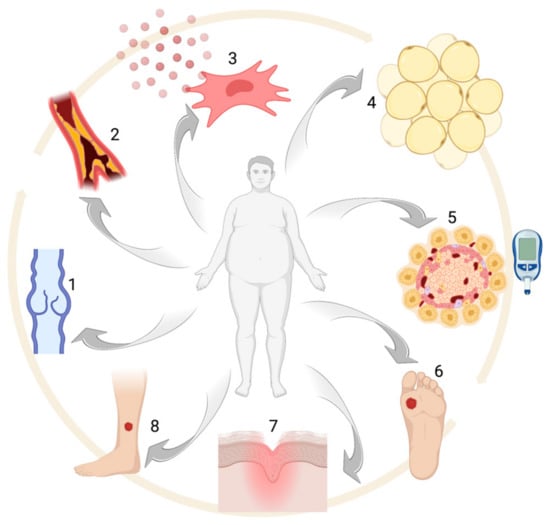
The most recent findings in the setting of wound healing and obesity, with a special focus on adipose tissue biology (see
Figure 1).
Figure 1. Obesity-associated alterations: (1) venous insufficiency, (2) atherosclerosis, (3) pro-inflammatory MSC phenotype, (4) adipocyte hypertrophy, (5) glucose intolerance and/or diabetes, (6–8) chronic ulcers.
2. Obesity: Epidemiology and Comorbidities
Obesity is defined by the presence of a body mass index (BMI) ≥ 30 kg/m
2, with normal range varying from 18.5 to 24.9 kg/m
2. A BMI between 25.0 to 29.9 kg/m
2 is considered overweight
[22]. Obesity and being overweight are almost invariably caused by excessive caloric intake compared to the necessary amount, which in turns determines fat storage increase and adipocyte hypertrophy
[23]. An unhealthy lifestyle is often the leading cause of obesity, but also genetic and epigenetic factors seem to play a very important role
[24]. About 30% of the adult population in the world is overweight or obese, with western countries showing the highest prevalence. The Organization for Economic Cooperation and Development (OECD) estimated a prevalence for obesity ranging from 3.7% in Japan up to 38.2% in the US
[25]. In the last two decades, the number of obese patients has tripled in Europe, where obesity is estimated to account for 7% of total healthcare costs
[26]. However, the prevalence of obesity is nowadays constantly rising, even in developing countries
[27][28][27,28], and rising childhood obesity rates portend worsening statistics
[29].
According to the UK National Audit Office, obesity-related disorders cause significant loss in terms of both working days and deaths, with subsequent direct and indirect costs being estimated at approximately £480,000,000 and £2,150,000,000 per year, respectively
[30]. In the U.S, the economic burden for obesity and its comorbidities was estimated to be around $147 billion in 2008 and $126 billion in 2016
[31][32][31,32].
Beyond these numbers, a large number of people is currently at risk of becoming obese, including children with familial history of obesity and/or metabolic syndrome, former smokers, lower social classes and older people
[33][34][35][36][33,34,35,36]. As just mentioned, obesity is usually framed in the broader context of metabolic syndrome (or syndrome X), where it is associated with hypertriglyceridemia, atherosclerosis, reduced HDL, hypertension and impaired glucose tolerance
[37]. In particular, metabolic syndrome is defined by the presence of three or more of the following criteria: (1) abdominal obesity (waist circumference ≥ 102 cm for men and ≥ 88 cm for women); (2) triglycerides ≥ 150 mg/dL; (3) high-density lipoprotein (HDL) cholesterol < 40 mg/dL for men and < 50 mg/dL for women; (4) systolic blood pressure ≥ 130 mmHg and/or diastolic blood pressure ≥ 85 mmHg and (5) fasting serum glucose ≥ 100 mg/dL
[38].
One of the main issues in obese subjects is T2D, which is notably associated with insulin resistance
[39][40][41][39,40,41]. Obese subjects also have an increased cardiovascular risk which, like other obesity-related comorbidities, can be explained by lipid accumulation in internal organs. Atherosclerosis, due to lipid accumulation in arterial walls, has got a pivotal role in coronary and cerebrovascular disease
[42]. However, obesity also leads to increased platelet activation, which is responsible for thrombosis and subsequent further inflammation, increasing the likelihood of developing ischemic complications
[40]. Triglyceride accumulation in the liver causes non-alcoholic fatty liver disease
[43]. Moreover, obesity is a well-established risk factor for cholelithiasis due to cholesterol gallstones
[44].
Obesity is also associated with a large variety of other possible comorbid conditions, including endocrine, oncological neurological, dermatological, respiratory and psychological disorders. Alterations in the hypothalamic–pituitary–gonadal (HPG) axis are often present in obese subjects
[44]. In particular, polycystic ovary syndrome (PCOS) is strictly connected with metabolic syndrome, and weight loss is often part of the therapeutic regimen
[45]. Obesity also carries a higher risk of developing several types of malignancies, such as colorectal, gastric, liver and gallbladder, endometrial and esophageal cancer
[46].
As for the neurological complications, obese patients are more likely to develop small fiber sensory neuropathy (SFSN)
[47] and recent studies suggest a higher risk of developing a form of cortical atrophy similar to Alzheimer’s disease (AD)
[48].
Ulcers, lymphedema, intertrigo, hidradenitis suppurativa, striae distensae, skin tags, acanthosis nigricans, psoriasis, acne, hirsutism and androgenetic alopecia are the main skin condition connected to obesity and metabolic syndrome
[49].
Biomechanical stress caused by a high body mass is responsible for numerous comorbidities of the musculoskeletal, respiratory, gastrointestinal and skin systems
[44].
From a respiratory point of view, obesity finally increases the risk of obstructive sleep apnea syndrome (OSAS), chronic obstructive pulmonary disease (COPD) and asthma
[50]. Even if not universally accepted, obesity is also associated with psychiatric/psychological conditions, including anxiety and depression
[51].
Most of the aforementioned comorbidities are associated with the low-grade inflammation which nearly invariably comes with obesity. In fact, adipocytes can produce proinflammatory cytokines, such as IL-6, therefore maintaining the pro-inflammatory state typical of obese subjects, as also confirmed by elevated levels of c-reactive protein (CRP) in these patients
[52][53][52,53]. This chronic inflammatory state is associated with hemodynamic and cardiac changes due to excessive body weight and contributes to the increased likelihood of having heart failure for obese subjects
[54].
3. Wound Healing
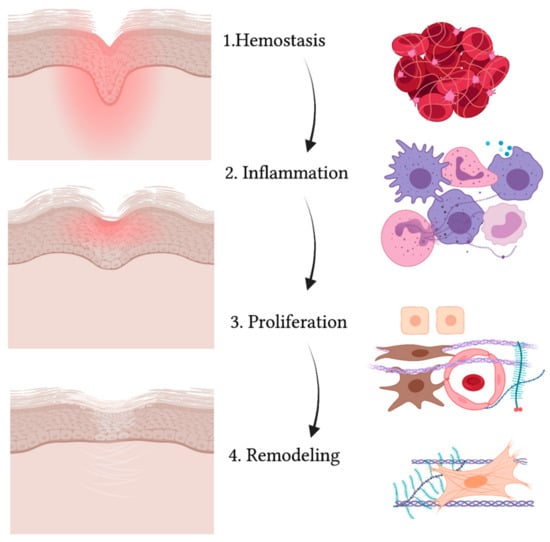
A wound is defined as a disruption in the normal continuity of the skin. When the skin is injured, a series of events takes place in order to close and heal the area where the barrier is compromised
[55]. Wound healing is an evolutionary-conserved process comprised of four sequential yet overlapping phases: hemostasis, inflammation, proliferation and remodeling (see
Figure 2)
[56]. These phases are strictly regulated through specific molecules that are expressed at different levels at each time interval
[57].
Figure 2. Schematic representation of the wound healing process. While the wound heals and the injured area (in red) reduces, gradually leading to a scar, the four phases take place (from upper to lower panel): hemostasis, inflammation, proliferation and remodeling. The main cell types are indicated on the right and include: red blood cells and platelets (1), leucocytes and professional phagocytes (2), keratinocytes (3), endothelial cells (3), MSCs (3) and fibroblasts (3,4).
The first process that takes place in the unwinding of wound healing consists of coagulation and hemostasis, aimed at stopping the bleeding while creating a temporary matrix for cells to infiltrate the site of injury
[58]. In fact, not only does vascular smooth muscle contraction reduce the diameter of injured vessels as a mechanism of reflex, but also activation of the coagulation cascade allows platelets to form a clot with fibronectin, fibrin, vitronectin and thrombospondin
[59]. Platelets also release several growth factors and cytokines when degranulating
[60]. Growth factors and cytokines such as PDGF (platelet-derived growth factor), TGFβ (transforming growth factor β) and EGF (epidermal growth factor) activate neutrophils, macrophages, endothelial cells, fibroblasts and keratinocytes
[61]. Then comes the inflammatory phase, where neutrophils, monocytes and macrophages flood to the site of injury
[62]. Neutrophils help in removing cell debris and microorganisms that may have slipped into the wound via phagocytosis
[63]. Macrophages also enter the site not only as professional phagocytes but also as regulatory cells secreting TGFα and TGFβ, HB-EGF (heparin-binding epidermal growth factor), FGFs (fibroblast growth factor) and collagenases
[64][65][64,65]. Partially overlapping with the inflammatory phase, the proliferation stage is characterized by the activation, expansion and migration of fibroblasts, keratinocytes and endothelial cells
[66]. Proliferation also involves the production of collagen, proteoglycans, hyaluronic acid and other ECM structural proteins that, along with fibroblast recruitment, give rise to granulation tissue
[67]. Moreover, endothelial cells aid in forming new vessels and thus epithelization takes place
[68]. Cytokines and growth factors activate keratinocytes so that they can migrate from the edges of the wound over the dermal matrix in order to close up the wound
[69]. The expression of specific keratins, such as K6 and K16, is observed in migrating keratinocytes
[70]. Lastly, collagenases, matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) are secreted by fibroblasts in the remodeling phase
[65].
4. Obesity and Wound Healing
Wound healing seems to be impaired in obese patients compared to subjects with a normal BMI (body mass index), despite the mechanism underlying such a difference not being totally understood
[71][94]. A multidisciplinary team is thus often essential for optimal wound care management in obese patients
[72][89]. Decreased vascularization can partially explain wound healing delay
[73][95]. In fact, the increase in the size of the adipocytes typical of obesity is generally not accompanied by an adequate rise in the number of vessels
[74][96]. This eventually leads to a fibrotic environment
[75][97], characterized by reduced elastin and increased collagen V and VI levels
[76][98].
Wound healing disorders seem to have a huge impact in obese patients, both form a clinical and a social point of view. Venous insufficiency, caused or worsened by an elevated intra-abdominal pressure due to fat accumulation in the abdominal area
[77][99], can cause leakage of proteinaceous-like material in the interstitial space, which can eventually occlude smaller vessels
[78][100]. The resulting reduction in oxygen tension not only affects the proliferative and remodeling phases but also increases the risk of wound infection through the impairment of leukocyte phagocytic properties
[12][79][12,101]. Arterial ulcers are associated to PAD, with atherosclerosis
[72][89] being a well-known comorbidity in obese patients affected by metabolic syndrome
[80][102] and/or T2D. Pressure ulcers are demonstrated to be common among obese patients staying in nursing homes
[81][103]. However, contrasting data regarding the effects of obesity on the risk of development of pressure ulcers have been published so far in other patient subpopulations
[82][83][104,105].
Peripheral neuropathy is another factor that can cause, aggravate or delay wound healing, and often represent the leading cause of cutaneous ulcers in diabetic patients
[84][85][106,107]. Microangiopathy often represents the principal cause of impaired wound healing in T2D patients, leading to reduced nerve vascularization, endothelial dysfunction and impaired microcirculation
[86][108]. On the other hand, macroangiopathy causes the production of prothrombotic factors and prevents the formation of an efficient network of collateral vessels, thus contributing to the pathogenesis of chronic wounds
[87][109]. Finally, Advanced Glycation End-products (AGEs) appear to play a role in delaying wound healing in diabetic patients, affecting both angiogenesis and
[88][110] extracellular matrix (ECM) production and remodeling
[89][111]. Recent studies have demonstrated insulin to promote cell migration, and insulin-based therapies have therefore been suggested to surmount the impact of insulin resistance on wound repairing
[89][90][111,112]. In addition, leptin, an anti-obesity hormone, improves wound repairing by accelerating angiogenesis and promoting the proliferation, differentiation and migration of keratinocytes
[91][113]. Leptin is physiologically produced by adipocytes and leads to a reduction in the caloric intake, regulating body weight. Nevertheless, a high-fat diet leads to a leptin-resistant condition over time, thus limiting the effects of this hormone
[92][114]. In obese patients, high leptin plasma levels are associated with peripheral receptor resistance
[93][115].