
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Prof Iannuzzi | -- | 3902 | 2023-03-23 20:42:44 | | | |
| 2 | Conner Chen | -1 word(s) | 3901 | 2023-03-27 04:00:21 | | | | |
| 3 | Conner Chen | + 4 word(s) | 3905 | 2023-03-27 04:03:09 | | |
Video Upload Options
Molecular cytogenetics, and particularly the fluorescence in situ hybridization (FISH) technique, allows a deeper investigation on the chromosomes of domestic animals to perform various results such as (a) the physical map of DNA-sequences of specific chromosome regions; (b) the use of molecular markes to confirm or to correctly identify the chromosomes involved in chromosome abnormalities, (c) the detailed comparison between related and unrelated species by both FISH-mapping or Zoo-FISH; (d) the study of meiotic segregation, especially by sperm-FISH, in some chromosome abnormalities; (e) the establishment of genetic losses or gains occurring during the chromosome abnormalities by using the aCGH (array Comparative Genomic Hybridization).
1. The Fluorescence In Situ Hybridization (FISH) Technique
2. FISH and Chromosome Abnormalities
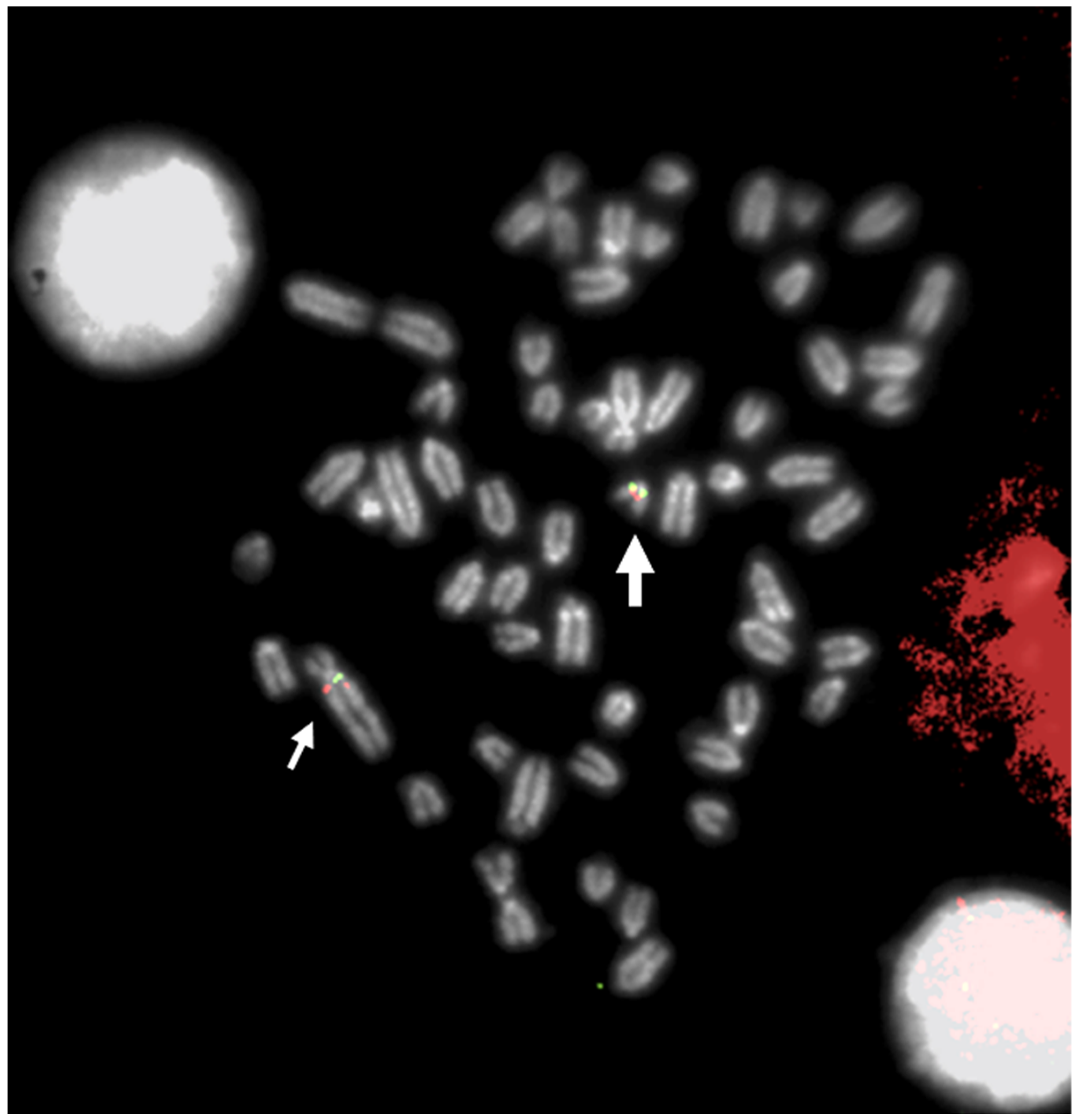
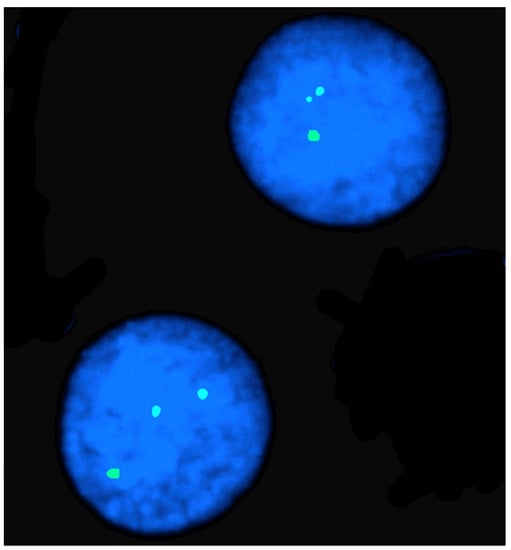
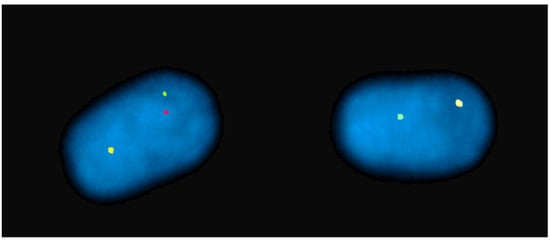
3. FISH in Physical Mapping
| Gene/Genes/Marker | Species | Reference |
|---|---|---|
| Lysozyme gene cluster | BBU | [31] |
| Uridine monophosphate synthase | BTA | [32] |
| Uridine monophosphate synthase | BBU | [33] |
| BTA1 to 7 | BTA | [34] |
| Microsatellites | BTA | [35] |
| Microsatellites | BTA | [36] |
| Beta-defensin genes | BTA; OAR | [37] |
| Alpha-S2 casein | BTA; BBU | [38] |
| Fas/APO-1 | BTA | [39] |
| Interferon gamma | OAR | [40] |
| Interleukin-2 receptor gamma | BTA | [41] |
| Beta-lactoglobulin pseudogene | BTA, OAR, CHI | [42] |
| Bone morphogenetic protein 1 | BTA | [43] |
| TSPY | BTA, OAR, CHI | [44] |
| VIL | OAR, CHI, BBU | [45] |
| Type I markers | BTA | [46] |
| Prion protein gene | BTA, OAR, CHI, BBU | [47] |
| IL2RA, VIM, THBD, PLC-II, CSNK2A1, TOP1 | BTA | [48] |
| NF1, CRYB1, CHRNB1, TP53, P4HB, GH1 | OAR, BBU | [49] |
| PAX8 | BTA, OAR, CHI | [50] |
| Type I markers | BTA | [19] |
| PREF1 | BTA | [51] |
| PRKCI | BTA | [52] |
| MHC | BTA | [53] |
| Type I markers | OAR, CHI | [22] |
| CACNA2D1 | BTA | [54] |
| SLC26a2 | BTA | [55] |
| SMN | BTA, OAR, CHI, BBU | [56] |
| Type I markers | BBU | [57] |
| Type I and II markers | OAR | [58] |
| PRPH | BTA | [59] |
| CYP11b/CYHR1 | BTA | [60] |
| SRY, ANT3, CSF2RA | BTA | [61] |
| Autosomal loci (11) | BTA, OAR, CHI, BBU | [62] |
| Autosomal loci (88) | OAR | [63] |
| Autosomal loci (68) | BBU | [64] |
| BMPR1B, BMP15, GDF9 | BTA, OAR, CHI, BBU | [65] |
4. Comparative FISH Mapping
| Author/s | Results |
|---|---|
| [29] | Mapping omega and trophoblast interferon genes in cattle and river buffalo |
| [84] | Mapping of lactoperoxidase, retinoblastoma, and alpha-lactalbumin genes in cattle, sheep, and goats |
| [30] | Mapping omega and trophoblast interferon genes in sheep and goats |
| [85] | Mapping LGB and IGHML in cattle, sheep, and goats |
| [86] | Mapping CASAS2 gene to the cattle, sheep, and goat chromosome 4 |
| [87] | Mapping MHC-complex in cattle and river buffalo |
| [88] | Mapping inhibin-alpha (INHA) to OAR2 and BTA2 |
| [89] | Mapping inhibin subunit beta b to OAR2 and BTA2 |
| [42] | Mapping beta-lactoglobulin pseudogene in sheep, goats, and cattle |
| [90] | Mapping ZNF164, ZNF146, GGTA1, SOX2, PRLR, and EEF2 in bovids |
| [38] | Mapping of the alpha-S2 casein gene on river buffalo and cattle |
| [37] | Mapping of beta-defensin genes to river buffalo and sheep chromosomes suggest a chromosome discrepancy in cattle standard karyotypes |
| [91] | Mapping STAT5A gene maps to BTA19, CHI19, and ORA11 |
| [92] | Mapping in Y chromosomes of cattle and zebu by microdissected painting probes |
| [45] | Mapping of villin (VIL) gene in river buffalo, sheep, and goats |
| [47] | Mapping prion protein gene (PRNP) on cattle, river buffalo, sheep, and goats |
| [93] | Mapping BCAT2 gene to cattle, sheep, and goats |
| [94] | Comparative mapping in X chromosomes of bovids |
| [95] | Comparative mapping between BTA-X and CHI-X |
| [96] | Survey of chromosome rearrangements between ruminants and humans |
| [97] | Comparative mapping between cattle and pig chromosomes using pig painting probes |
| [98] | Extensive conservation of human chromosome regions in euchromatic regions of river buffalo chromosomes |
| [49] | Mapping of six expressed gene loci (NF1, CRYB1, CHRNB1, TP53, P4HB, and GH1) to river buffalo and sheep chromosomes |
| [99] | Comparison of human and sheep chromosomes using human chromosome painting probes |
| [100] | Mapping four HSA2 type I loci in river buffalo chromosomes 2q and 12 |
| [101] | Mapping BCAT1 in cattle, sheep, and goats |
| [102] | Comparative mapping in bovid X chromosomes reveals homologies and divergences between the subfamilies Bovinae and Caprinae |
| [103] | Mapping 16 type I loci in river buffalo and sheep |
| [104] | Mapping 13 type I loci from HSA4q, HSA6p, HSA7q, and HSA12q on in river buffalo |
| [105] | Mapping forty autosomal type I loci in river buffalo and sheep chromosomes and assignment from sixteen human chromosomes |
| [106] | Mapping eight genes from HSA11 to bovine chromosomes 15 and 29 |
| [20] | International chromosome nomenclature in domestic bovids based on Q-, G-, and R-banding and FISH with 31 specific Texas marker chromosomes |
| [107] | Mapping 28 loci in river buffalo and sheep chromosomes |
| [108] | Sheep/human comparative map in a chromosome region involved in scrapie incubation time shows multiple breakpoints between human chromosomes 14 and 15 and sheep chromosomes 7 and 18 |
| [56] | Physical map of the survival of motor neuron gene (SMN) in domestic bovids |
| [22] | Assignment of the 31 type I Texas bovine markers in sheep and goat chromosomes by comparative FISH mapping and R-banding |
| [109] | Mapping 195 genes in cattle and updated comparative map with humans, mice, rats, and pigs |
| [110] | Mapping of F9, HPRT, and XIST in BTAX and HSAX clarifies breakpoints between the two species |
| [111] | 15 gene loci were mapped in the telomeric region of BTA18q and HSA19q |
| [112] | Comparative G- and Q-banding of saola and cattle chromosomes as well as FISH mapping of 32 type I Texas markers |
| [113] | Mapping of fragile histidine triad (FHIT) gene in bovids |
| [114] | Chromosome evolution and improved cytogenetic maps of the Y chromosome in cattle, zebu, river buffalo, sheep, and goats |
| [115] | Physical map of mucin 1, transmembrane (MUC1) among cattle, river buffalo, sheep, and goat chromosomes and comparison with HSA1 |
| [116] | Mapping of LEP and SLC26A2 in bovidae chrom. 4 (BTA4/OAR4/CHI4) and HSA7 |
| [62] | Mapping 11 genes to BTA2, BBU2q, OAR2q, and CHI2, and comparison with HSA2q |
| [117] | Mapping among humans, cattle, and mice suggests a role for repeat sequences in mammalian genome evolution |
| [118] | Mapping sheep and goat BAC clones identifies the transcriptional orientation of T cell receptor gamma genes on chromosome 4 in bovids |
| [119] | Mapping of twelve loci in river buffalo and sheep chromosomes: comparison with HSA8p and HSA4q |
| [120] | Mapping 25 new loci in BTA27 and comparison with both human and mouse chromosomes |
| [63] | An advanced sheep cytogenetic map and assignment of 88 new autosomal loci |
| [121] | Cross-species FISH with cattle whole-chromosome paints and satellite DNA I probes was used to identify the chromosomes involved in the translocations of some tribe Bovinae species |
| [64] | Extended river buffalo cytogenetic map, assignment of 68 autosomal loci and comparison with human chromosomes |
| [122] | FISH with 28S and telomeric probes in 17 bovid species. NORs are an important and frequently overlooked source of additional phylogenetic information within the Bovidae |
| [123] | Mapping DMRT1 genes to BTA8 and HSA9 |
| [124] | Comparative DM domain genes between cattle and pigs |
| [125] | Assignments of new loci to BBU7 and OAR6 and comparison with HSA4 |
| [126] | Mapping 22 ovine BAC clones in sheep, cattle, and human X chromosome |
| [127] | Mapping and genomic annotation of bovine oncosuppressor gene in domestic bovids |
| [128] | Cytogenetic map in sheep as anchor of genomic maps also using different genomic resources from other species |
| [129] | Molecular cytogenetics in goats and comparative mapping with human maps |
| [130] | Mapping of 6 loci containing genes involved in the dioxin metabolism of domestic bovids |
| [131] | Extended cytogenetic maps of sheep chromosome 1 and their cattle and river buffalo homologues: comparison with the OAR1 RH-map and HSA2, 3, 21, and 1q |
| [132] | Mapping between BTA5 and some Antilopinae species using Sat-I and SAT-II sequence and BTA-painting probes |
| [133] | Comparison of centromeric repeats between cattle and other Bovidae species |
| [134] | Advanced comparative map in X chromosome of Bovidae |
| [65] | Physical map of BMPR1B, BMP15, and GDF9 fecundity genes on cattle, river buffalo, sheep, and goat chromosomes |
| [74] | Physical mapping of 20 unmapped fragments in Btau 4.0 Genome Assembly in cattle, sheep, and river buffalo |
| [135] | Physical map of LCA5L gene in cattle, sheep, and goats |
| [136] | New cryptic difference between cattle and goat karyotypes |
| [137] | Small evolutionary rearrangement between BTA21 and homologous OAR18 |
| [138] | Assignment of 23 endogenous retrovirus to both sheep and homologous chromosomes regions of river buffalo |
5. Fiber-FISH
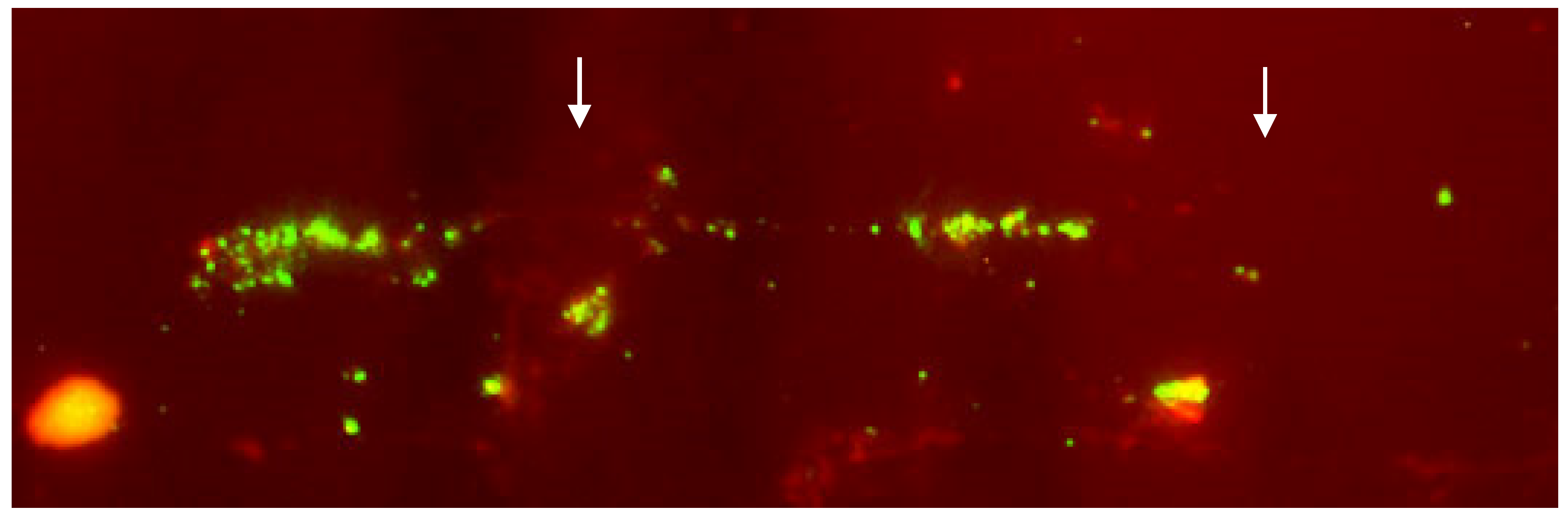
| Species | Author/s | Results |
|---|---|---|
| Cattle | [152] | Genomic organization of the bovine aromatase |
| [153] | Molecular characterization of STAT5A- and STAT5B-encoding genes | |
| [56] | Demonstration of survival of motor neuron gene (SMN) duplication in a calf affected by arthrogryposis | |
| [154] | Demonstration of multiple TSPY copies on the Y chromosome | |
| Sheep | [155] | DNA fiber barcodes indicated a chromosomal deletion |
6. CGH Arrays
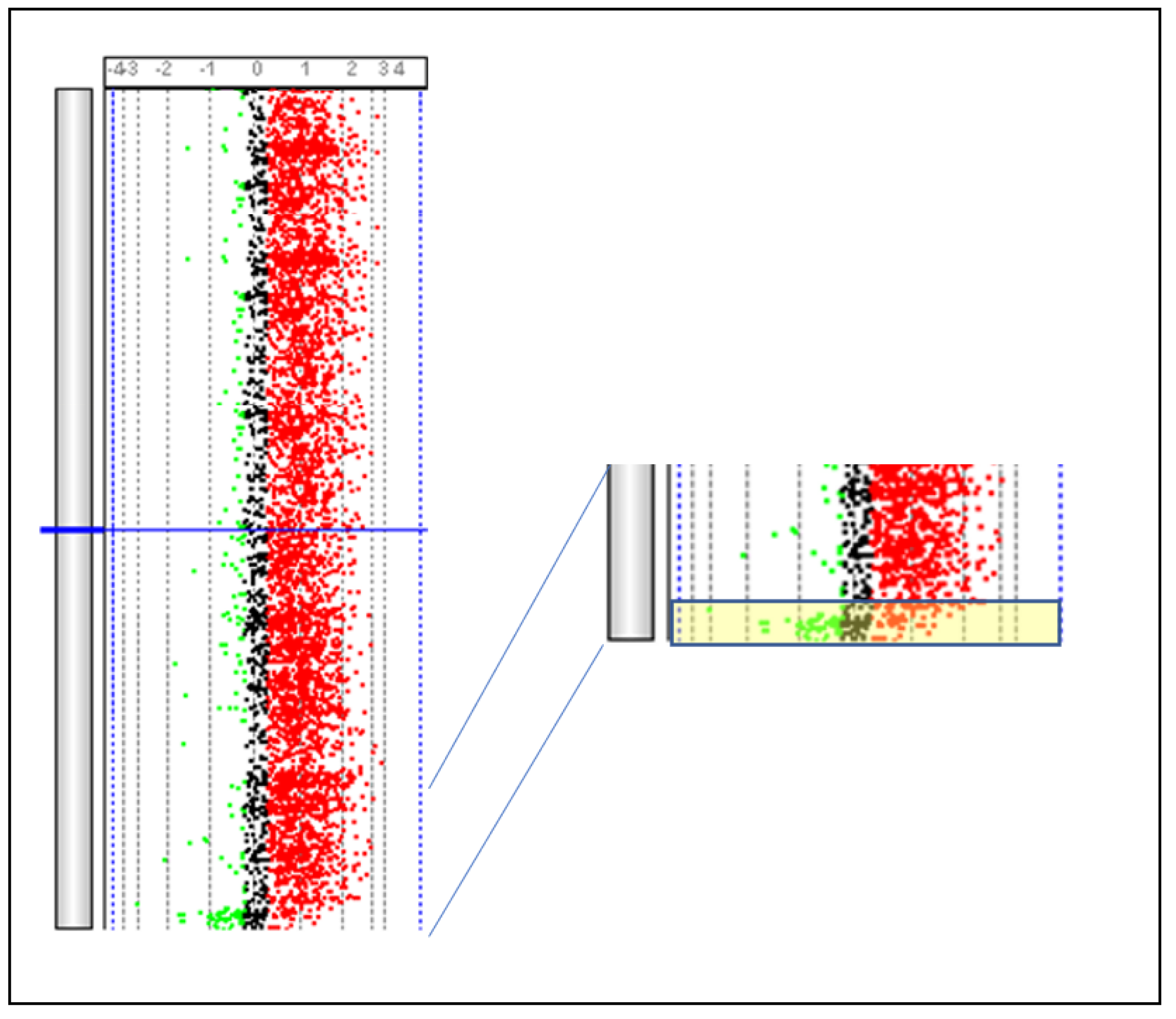
| Specie | Reference | Note |
|---|---|---|
| Cattle | [166] | 3 Holstein bulls |
| Cattle | [167] | 90 animals: 11 Bos taurus breeds, 3 Bos indicus breeds, and 3 composite breeds for beef, dairy, or dual purpose |
| Cattle | [168] | 20 animals: 14 Holsteins, 3 Simmental 2 Red Danish and 1 Hereford |
| Cattle | [169] | 47 Holstein bulls |
| Cattle | [170] | 24 animals from Chianese breeds |
| Cattle | [171] | 3 Angus, 6 Brahman, and 1 composite animal |
| Sheep | [172] | 36 animals |
| Sheep | [173] | 12 animals |
| Goat | [174] | 10 animals |
References
- Pinkel, D.; Gray, J.W.; Trask, B.; Van Den Engh, G.; Fuscoe, J.; Van Dekken, H. Cytogenetic analysis by in situ hybridization with fluorescently labeled nucleic acid probes. Cold Spring Harb. Symp. Quant. Biol. 1986, 51, 151–157.
- Trask, B.; Pinkel, D. Fluorescence in situ hybridization with DNA probes. Methods Cell Biol. 1990, 33, 383–400.
- Gallagher, D.S.; Basrur, P.K.; Womack, J.E. Identification of an autosome to X chromosome translocation in the domestic cow. J. Hered. 1992, 83, 451–453.
- De Lorenzi, L.; Genualdo, V.; Gimelli, S.; Rossi, E.; Perucatti, A.; Iannuzzi, A.; Zannotti, M.; Malagutti, L.; Molteni, L.; Iannuzzi, L.; et al. Genomic analysis of cattle rob(1;29). Chromosome Res. 2012, 20, 815–823.
- Iannuzzi, A.; Parma, P.; Iannuzzi, L. Chromosome Abnormalities and Fertility in Domestic Bovids: A Review. Animals 2021, 11, 802.
- Iannuzzi, L.; Di Meo, G.P.; Leifsson, P.S.; Eggen, A.; Christensen, K. A case of trisomy 28 in cattle revealed by both banding and FISH-mapping techniques. Hereditas 2001, 134, 147–151.
- Christensen, K.; Juul, L. A case of trisomy 22 in a live hereford calf. Acta Vet. Scand. 1999, 40, 85–88.
- De Giovanni, A.; Molteni, L.; Succi, G.; Galliani, C.; Bocher, J.; Popescu, C.P. A new type of Robertsonian translocation in cattle. In Proceedings of the 8th European Colloquium on Cytogenetics of Domestic Animals, Bristol, UK, 19–22 July 1988; pp. 53–59.
- De Giovanni, A.; Succi, G.; Molteni, L.; Castiglioni, M. A new autosomal translocation in “Alpine grey cattle”. Ann. Genet. Sel. Anim. 1979, 11, 115–120.
- Di Meo, G.P.; Molteni, L.; Perucatti, A.; De Giovanni, A.; Incarnato, D.; Succi, G.; Schibler, L.; Cribiu, E.P.; Iannuzzi, L. Chromosomal characterization of three centric fusion translocations in cattle using G-, R- and C-banding and FISH technique. Caryologia 2000, 53, 213–218.
- Switoński, M.; Stranzinger, G. Studies of synaptonemal complexes in farm mammals- a review. J. Hered. 1998, 89, 473–480.
- Hart, E.J.; Pinton, A.; Powell, A.; Wall, R.; King, W.A. Meiotic recombination in normal and clone bulls and their offspring. Cytogenet. Genome Res. 2008, 120, 97–101.
- Sebestova, H.; Vozdova, M.; Kubickova, S.; Cernohorska, H.; Kotrba, R.; Rubes, J. Effect of species-specific differences in chromosome morphology on chromatin compaction and the frequency and distribution of RAD51 and MLH1 foci in two bovid species: Cattle (Bos taurus) and the common eland (Taurotragus oryx). Chromosoma 2016, 125, 137–149.
- Villagómez, D.A.; Pinton, A. Chromosomal abnormalities, meiotic behaviour and fertility in domestic animals. Cytogenet. Genome Res. 2008, 120, 69–80.
- Riggs, P.K.; Rønne, M. Fragile sites in domestic animal chromosomes: Molecular insights and challenges. Cytogenet. Genome Res. 2009, 126, 97–109.
- Ford, C.E.; Pollock, D.L.; Gustavsson, I. Proceedings of the First International Conference for the Standardisation of Banded Karyotypes of Domestic Animals. University of Reading, Reading, England. 2nd–6th August 1976. Hereditas 1980, 92, 145–162.
- Di Berardino, D.; Hayes, H.; Fries, R.; Long, S. ISCNDA1989: International System for Cytogenetic Nomenclature of Domestic Animals. Cytogenet. Cell Genet. 1990, 53, 65–79.
- Popescu, C.P.; Long, S.; Riggs, P.; Womack, J.; Schmutz, S.; Fries, R.; Gallagher, D.S. Standardization of cattle karyotype nomenclature: Report of the committee for the standardization of the cattle karyotype. Cytogenet. Cell Genet. 1996, 74, 259–261.
- Hayes, H.; Di Meo, G.P.; Gautier, M.; Laurent, P.; Eggen, A.; Iannuzzi, L. Localization by FISH of the 31 Texas nomenclature type I markers to both Q- and R-banded bovine chromosomes. Cytogenet. Cell Genet. 2000, 90, 315–320.
- Cribiu, E.P.; Di Berardino, D.; Di Meo, G.P.; Gallagher, D.S.; Hayes, H.; Iannuzzi, L.; Popescu, C.P.; Rubes, J.; Schmutz, S.; Stranzinger, G.; et al. International System for Chromosome Nomenclature of Domestic Bovids (ISCNDB 2000). Cytogenet. Cell Genet. 2001, 92, 283–299.
- Iannuzzi, L.; Di Meo, G.P.; Hayes, H.; Perucatti, A.; Incarnato, D.; Gautier, M.; Eggen, A. FISH-mapping of 31 type I loci (Texas markers) to river buffalo chromosomes. Chromosome Res. 2001, 9, 339–342.
- Di Meo, G.P.; Perucatti, A.; Gautier, M.; Hayes, H.; Incarnato, D.; Eggen, A.; Iannuzzi, L. Chromosome localization of the 31 type I Texas bovine markers in sheep and goat chromosomes by comparative FISH-mapping and R-banding. Anim. Genet. 2003, 34, 294–296.
- Watson, J.D.; Crick, F.H. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature 1953, 171, 737–738.
- Pardue, M.L.; Gall, J.G. Molecular hybridization of radioactive DNA to the DNA of cytological preparations. Proc. Natl. Acad. Sci. USA 1969, 64, 600–604.
- Fries, R.; Hediger, R.; Stranzinger, G. Tentative chromosomal localization of the bovine major histocompatibility complex by in situ hybridization. Anim. Genet. 1986, 17, 287–294.
- Yerle, M.; Gellin, J.; Echard, G.; Lefevre, F.; Gillois, M. Chromosomal localization of leukocyte interferon gene in the pig (Sus scrofa domestica L.) by in situ hybridization. Cytogenet. Cell Genet. 1986, 42, 129–132.
- Rudkin, G.T.; Stollar, B.D. High resolution detection of DNA-RNA hybrids in situ by indirect immunofluorescence. Nature 1977, 265, 472–473.
- Ryan, A.M.; Gallagher, D.S.; Womack, J.E. Syntenic mapping and chromosomal localization of bovine alpha and beta interferon genes. Mamm. Genome 1992, 3, 575–578.
- Iannuzzi, L.; Gallagher, D.S.; Ryan, A.M.; Di Meo, G.P.; Womack, J.E. Chromosomal localization of omega and trophoblast interferon genes in cattle and river buffalo by sequential R-banding and fluorescent in situ hybridization. Cytogenet. Cell Genet. 1993, 62, 224–227.
- Iannuzzi, L.; Di Meo, G.P.; Gallagher, D.S.; Ryan, A.M.; Ferrara, L.; Womack, J.E. Chromosomal localization of omega and trophoblast interferon genes in goat and sheep by fluorescent in situ hybridization. J. Hered. 1993, 84, 301–304.
- Iannuzzi, L.; Gallagher, D.S.; Di Meo, G.P.; Ryan, A.M.; Perucatti, A.; Ferrara, L.; Irwin, D.M.; Womack, J.E. Chromosomal localization of the lysozyme gene cluster in river buffalo (Bubalus bubalis L.). Chromosome Res. 1993, 1, 253–255.
- Friedl, R.; Rottmann, O.J. Assignment of the bovine uridine monophosphate synthase gene to the bovine chromosome region 1q34-36 by FISH. Mamm. Genome 1994, 5, 38–40.
- Iannuzzi, L.; Di Meo, G.P.; Ryan, A.M.; Gallagher, D.S.; Ferrara, L.; Womack, J.E. Localization of uridine monophosphate synthase (UMPS) gene to river buffalo chromosomes by FISH. Chromosome Res. 1994, 2, 255–256.
- Solinas-Toldo, S.; Mezzelani, A.; Hawkins, G.A.; Bishop, M.D.; Olsaker, I.; Mackinlay, A.; Ferretti, L.; Fries, R. Combined Q-banding and fluorescence in situ hybridization for the identification of bovine chromosomes 1 to 7. Cytogenet. Cell Genet. 1995, 69, 1–6.
- Hawkins, G.A.; Toldo, S.S.; Bishop, M.D.; Kappes, S.M.; Fries, R.; Beattie, C.W. Physical and linkage mapping of the bovine genome with cosmids. Mamm Genome 1995, 6, 249–254.
- Mezzelani, A.; Zhang, Y.; Redaelli, L.; Castiglioni, B.; Leone, P.; Williams, J.L.; Toldo, S.S.; Wigger, G.; Fries, R.; Ferretti, L. Chromosomal localization and molecular characterization of 53 cosmid-derived bovine microsatellites. Mamm. Genome 1995, 6, 629–635.
- Iannuzzi, L.; Gallagher, D.S.; Di Meo, G.P.; Diamond, G.; Bevins, C.L.; Womack, J.E. High-resolution FISH mapping of beta-defensin genes to river buffalo and sheep chromosomes suggests a chromosome discrepancy in ffttle standard karyotypes. Cytogenet. Cell Genet. 1996, 75, 10–13.
- Iannuzzi, L.; Gallagher, D.S.; Womack, J.E.; Meo, G.P.; Shelling, C.P.; Groenen, M.A. FISH mapping of the alpha-S2 casein gene on river buffalo and cattle chromosomes identifies a nomenclature discrepancy in the bovine karyotype. Chromosome Res. 1996, 4, 159–162.
- Yoo, J.; Stone, R.T.; Kappes, S.M.; Toldo, S.S.; Fries, R.; Beattie, C.W. Genomic organization and chromosomal mapping of the bovine Fas/APO-1 gene. DNA Cell Biol. 1996, 15, 377–385.
- Goldammer, T.; Brunner, R.M.; Schmidt, P.; Schwerin, M. Mapping of the interferon gamma gene (IFNG) to chromosomes 3 in sheep and 5 in goat by FISH. Mamm. Genome 1996, 7, 470–471.
- Yoo, J.; Stone, R.T.; Solinas-Toldo, S.; Fries, R.; Beattie, C.W. Cloning and chromosomal mapping of bovine interleukin-2 receptor gamma gene. DNA Cell Biol. 1996, 15, 453–459.
- Folch, J.M.; Coll, A.; Hayes, H.C.; Sànchez, A. Characterization of a caprine beta-lactoglobulin pseudogene, identification and chromosomal localization by in situ hybridization in goat, sheep and cow. Gene 1996, 177, 87–91.
- Martín-Burriel, I.; Goldammer, T.; Elduque, C.; Lundin, M.; Barendse, W.; Zaragoza, P.; Olsaker, I. Physical and linkage mapping of the bovine bone morphogenetic protein 1 on the evolutionary break region of BTA 8. Cytogenet. Cell Genet. 1997, 79, 179–183.
- Vogel, T.; Borgmann, S.; Dechend, F.; Hecht, W.; Schmidtke, J. Conserved Y-chromosomal location of TSPY in Bovidae. Chromosome Res. 1997, 5, 182–185.
- Iannuzzi, L.; Skow, L.; Di Meo, G.P.; Gallagher, D.S.; Womack, J.E. Comparative FISH-mapping of villin (VIL) gene in river buffalo, sheep and goat chromosomes. Chromosome Res. 1997, 5, 199–202.
- Gallagher, D.S.; Yang, Y.P.; Burzlaff, J.D.; Womack, J.E.; Stelly, D.M.; Davis, S.K.; Taylor, J.F. Physical assignment of six type I anchor loci to bovine chromosome 19 by fluorescence in situ hybridization. Anim. Genet. 1998, 29, 130–134.
- Iannuzzi, L.; Palomba, R.; Di Meo, G.P.; Perucatti, A.; Ferrara, L. Comparative FISH-mapping of the prion protein gene (PRNP) on cattle, river buffalo, sheep and goat chromosomes. Cytogenet. Cell Genet. 1998, 81, 202–204.
- Gallagher, D.S.; Schläpfer, J.; Burzlaff, J.D.; Womack, J.E.; Stelly, D.M.; Davis, S.K.; Taylor, J.F. Cytogenetic alignment of the bovine chromosome 13 genome map by fluorescence in-situ hybridization of human chromosome 10 and 20 comparative markers. Chromosome Res. 1999, 7, 115–119.
- Iannuzzi, L.; Gallagher, D.S.; Di Meo, G.P.; Yang, Y.; Womack, J.E.; Davis, S.K.; Taylor, J.F. Comparative FISH-mapping of six expressed gene loci to river buffalo and sheep. Cytogenet. Cell Genet. 1999, 84, 161–163.
- Lòpez-Corrales, N.L.; Sonstegard, T.S.; Smith, T.P. Physical mapping of the bovine, caprine and ovine homologues of the paired box gene PAX8. Cytogenet. Cell Genet. 1999, 84, 179–181.
- Minoshima, Y.; Taniguchi, Y.; Tanaka, K.; Yamada, T.; Sasaki, Y. Molecular cloning, expression analysis, promoter characterization, and chromosomal localization of the bovine PREF1 gene. Anim. Genet. 2001, 32, 333–339.
- De Donato, M.; Gallagher, D.S.; Davis, S.K.; Stelly, D.M.; Taylor, J.F. The assignment of PRKCI to bovine chromosome 1q34-->q36 by FISH suggests a new assignment to human chromosome 3. Cytogenet. Cell Genet. 2001, 95, 79–81.
- McShane, R.D.; Gallagher, D.S., Jr.; Newkirk, H.; Taylor, J.F.; Burzlaff, J.D.; Davis, S.K.; Skow, L.C. Physical localization and order of genes in the class I region of the bovine MHC. Anim. Genet. 2001, 32, 235–239.
- Buitkamp, J.; Ewald, D.; Masabanda, J.; Bishop, M.D.; Fries, R. FISH and RH mapping of the bovine alpha (2)/delta calcium channel subunit gene (CACNA2D1). Anim. Genet. 2003, 34, 309–310.
- Brenig, B.; Baumgartner, B.G.; Kriegesmann, B.; Habermann, F.; Fries, R.; Swalve, H.H. Molecular cloning, mapping, and functional analysis of the bovine sulfate transporter SLC26a2 gene. Gene 2003, 319, 161–166.
- Iannuzzi, L.; Di Meo, G.P.; Perucatti, A.; Rullo, R.; Incarnato, D.; Longeri, M.; Bongioni, G.; Molteni, L.; Galli, A.; Zanotti, M.; et al. Comparative FISH-mapping of the survival of motor neuron gene (SMN) in domestic bovids. Cytogenet. Genome Res. 2003, 102, 39–41.
- Iannuzzi, L.; Di Meo, G.P.; Perucatti, A.; Schibler, L.; Incarnato, D.; Gallagher, D.; Eggen, A.; Ferretti, L.; Cribiu, E.P.; Womack, J. The river buffalo (Bubalus bubalis, 2n = 50) cytogenetic map: Assignment of 64 loci by fluorescence in situ hybridization and R-banding. Cytogenet. Genome Res. 2003, 102, 65–75.
- Iannuzzi, L.; Perucatti, A.; Di Meo, G.P.; Schibler, L.; Incarnato, D.; Cribiu, E.P. Chromosomal localization of sixty autosomal loci in sheep (Ovis aries, 2n = 54) by fluorescence in situ hybridization and R-banding. Cytogenet. Genome Res. 2003, 103, 135–138.
- Mömke, S.; Kuiper, H.; Spötter, A.; Drögemüller, C.; Williams, J.L.; Distl, O. Assignment of the PRPH gene to bovine chromosome 5q1.4 by FISH and confirmation by RH mapping. Anim. Genet. 2004, 35, 477–478.
- Kaupe, B.; Kollers, S.; Fries, R.; Erhardt, G. Mapping of CYP11B and a putative CYHR1 paralogous gene to bovine chromosome 14 by FISH. Anim. Genet. 2004, 35, 478–479.
- Liu, W.S.; de León, F.A. Assignment of SRY, ANT3, and CSF2RA to the bovine Y chromosome by FISH and RH mapping. Anim. Biotechnol. 2004, 15, 103–109.
- Di Meo, G.P.; Gallagher, D.; Perucatti, A.; Wu, X.; Incarnato, D.; Mohammadi, G.; Taylor, J.F.; Iannuzzi, L. Mapping of 11 genes by FISH to BTA2, BBU2q, OAR2q and CHI2, and comparison with HSA2q. Anim. Genet. 2006, 37, 299–300.
- Di Meo, G.P.; Perucatti, A.; Floriot, S.; Hayes, H.; Schibler, L.; Rullo, R.; Incarnato, D.; Ferretti, L.; Cockett, N.; Cribiu, E.; et al. An advanced sheep (Ovis aries, 2n = 54) cytogenetic map and assignment of 88 new autosomal loci by fluorescence in situ hybridization and R-banding. Anim. Genet. 2007, 38, 233–240.
- Di Meo, G.P.; Perucatti, A.; Floriot, S.; Hayes, H.; Schibler, L.; Incarnato, D.; Di Berardino, D.; Williams, J.; Cribiu, E.; Eggen, A.; et al. An extended river buffalo (Bubalus bubalis, 2n = 50) cytogenetic map: Assignment of 68 autosomal loci by FISH-mapping and R-banding and comparison with human chromosomes. Chromosome Res. 2008, 16, 827–837.
- Farhadi, A.; Genualdo, V.; Perucatti, A.; Hafezian, S.H.; Rahimi-Mianji, G.; De Lorenzi, L.; Parma, P.; Iannuzzi, L.; Iannuzzi, A. Comparative FISH mapping of BMPR1B, BMP15 and GDF9 fecundity genes on cattle, river buffalo, sheep and goat chromosomes. J. Genet. 2013, 92, 595–597.
- Eggen, A.; Gautier, M.; Billaut, A.; Petit, E.; Hayes, H.; Laurent, P.; Urban, C.; Pfister-Genskow, M.; Eilertsen, K.; Bishop, M.D. Construction and characterization of a bovine BAC library with four genome-equivalent coverage. Genetics, selection, evolution. Genet. Sel. Evol. 2001, 33, 543–548.
- Bovine Genome Sequencing and Analysis Consortium; Elsik, C.G.; Tellam, R.L.; Worley, K.C.; Gibbs, R.A.; Muzny, D.M.; Weinstock, G.M.; Adelson, D.L.; Eichler, E.E.; Elnitski, L.; et al. The genome sequence of taurine cattle: A window to ruminant biology and evolution. Science 2009, 324, 522–528.
- Zimin, A.V.; Delcher, A.L.; Florea, L.; Kelley, D.R.; Schatz, M.C.; Puiu, D.; Hanrahan, F.; Pertea, G.; Van Tassell, C.P.; Sonstegard, T.S.; et al. A whole-genome assembly of the domestic cow, Bos taurus. Genome Biol. 2009, 10, R42.
- Dong, Y.; Xie, M.; Jiang, Y.; Xiao, N.; Du, X.; Zhang, W.; Tosser-Klopp, G.; Wang, J.; Yang, S.; Liang, J.; et al. Sequencing and automated whole-genome optical mapping of the genome of a domestic goat (Capra hircus). Nat. Biotechnol. 2013, 31, 135–141.
- International Sheep Genomics Consortium; Archibald, A.L.; Cockett, N.E.; Dalrymple, B.P.; Faraut, T.; Kijas, J.W.; Maddox, J.F.; McEwan, J.C.; Hutton Oddy, V.; Raadsma, H.W.; et al. The sheep genome reference sequence: A work in progress. Anim. Genet. 2010, 41, 449–453.
- Mintoo, A.A.; Zhang, H.; Chen, C.; Moniruzzaman, M.; Deng, T.; Anam, M.; Emdadul Huque, Q.M.; Guang, X.; Wang, P.; Zhong, Z.; et al. Draft genome of the river water buffalo. Ecol. Evol. 2019, 9, 3378–3388.
- De Lorenzi, L.; Molteni, L.; Parma, P. FISH mapping in cattle (Bos taurus L.) is not yet out of fashion. J. Appl. Genet. 2010, 51, 497–499.
- Partipilo, G.; D’Addabbo, P.; Lacalandra, G.M.; Liu, G.E.; Rocchi, M. Refinement of Bos taurus sequence assembly based on BAC-FISH experiments. BMC Genom. 2011, 12, 639.
- De Lorenzi, L.; Genualdo, V.; Perucatti, A.; Iannuzzi, A.; Iannuzzi, L.; Parma, P. Physical mapping of 20 unmapped fragments of the btau_4.0 genome assembly in cattle, sheep and river buffalo. Cytogenet. Genome Res. 2013, 140, 29–35.
- Toldo, S.S.; Fries, R.; Steffen, P.; Neibergs, H.L.; Barendse, W.; Womack, J.E.; Hetzel, D.J.; Stranzinger, G. Physically mapped, cosmid-derived microsatellite markers as anchor loci on bovine chromosomes. Mamm. Genome 1993, 4, 720–727.
- Vaiman, D.; Mercier, D.; Eggen, A.; Bahri-Darwich, I.; Grohs, C.; Cribiu, E.P.; Dolf, G.; Oustry, A.; Guérin, G.; Levéziel, H. A genetic and physical map of bovine chromosome 11. Mamm. Genome 1994, 5, 553–556.
- Drögemüller, C.; Bader, A.; Wöhlke, A.; Kuiper, H.; Leeb, T.; Distl, O. A high-resolution comparative RH map of the proximal part of bovine chromosome 1. Anim. Genet. 2002, 33, 271–279.
- Smith, T.P.; Lopez-Corrales, N.; Grosz, M.D.; Beattie, C.W.; Kappes, S.M. Anchoring of bovine chromosomes 4, 6, 7, 10, and 14 linkage group telomeric ends via FISH analysis of lambda clones. Mamm. Genome 1997, 8, 333–336.
- Scherthan, H.; Cremer, T.; Arnason, U.; Weier, H.U.; Lima-de-Faria, A.; Frönicke, L. Comparative chromosome painting discloses homologous segments in distantly related mammals. Nat. Genet. 1994, 6, 342–347.
- Jauch, A.; Wienberg, J.; Stanyon, R.; Arnold, N.; Tofanelli, S.; Ishida, T.; Cremer, T. Reconstruction of genomic rearrangements in great apes and gibbons by chromosome painting. Proc. Natl. Acad. Sci. USA 1992, 89, 8611–8615.
- Lengauer, C.; Wienberg, J.; Cremer, T.; Lüdecke, H.J.; Horstehmke, B. Comparative chromosome band mapping in primates by in situ suppression hybridization of band specific DNA microlibraries. Hum. Evol. 1991, 6, 67–71.
- Wienberg, J.; Stanyon, R.; Jauch, A.; Cremer, T. Homologies in human and Macaca fuscata chromosomes revealed by in situ suppression hybridization with human chromosome specific DNA libraries. Chromosoma 1992, 101, 265–270.
- Chowdhary, B.P.; Raudsepp, T.; Frönicke, L.; Scherthan, H. Emerging patterns of comparative genome organization in some mammalian species as revealed by Zoo-FISH. Genome Res. 1998, 8, 577–589.
- Hayes, H.C.; Popescu, P.; Dutrillaux, B. Comparative gene mapping of lactoperoxidase, retinoblastoma, and alpha-lactalbumin genes in cattle, sheep, and goats. Mamm. Genome 1993, 4, 593–597.
- Hayes, H.C.; Petit, E.J. Mapping of the beta-lactoglobulin gene and of an immunoglobulin M heavy chain-like sequence to homoeologous cattle, sheep, and goat chromosomes. Mamm. Genome 1993, 4, 207–210.
- Hayes, H.; Petit, E.; Bouniol, C.; Popescu, P. Localization of the alpha-S2-casein gene (CASAS2) to the homoeologous cattle, sheep, and goat chromosomes 4 by in situ hybridization. Cytogenet. Cell Genet. 1993, 64, 281–285.
- Iannuzzi, L.; Gallagher, D.S.; Womack, J.E.; Di Meo, G.P.; Skow, L.C.; Ferrara, L. Chromosomal localization of the major histocompatibility complex in cattle and river buffalo by fluorescent in situ hybridization. Hereditas 1993, 118, 187–190.
- Brunner, R.M.; Goldammer, T.; Hiendleder, S.; Jäger, C.; Schwerin, M. Comparative mapping of the gene coding for inhibin-alpha (INHA) to chromosome 2 in sheep and cattle. Mamm. Genome 1995, 6, 309.
- Goldammer, T.; Brunner, R.M.; Hiendleder, S.; Schwerin, M. Comparative mapping of sheep inhibin subunit beta b to chromosome 2 in sheep and cattle by fluorescence in situ hybridization. Anim. Genet. 1995, 26, 199–200.
- Hayes, H.; Le Chalony, C.; Goubin, G.; Mercier, D.; Payen, E.; Bignon, C.; Kohno, K. Localization of ZNF164, ZNF146, GGTA1, SOX2, PRLR and EEF2 on homoeologous cattle, sheep and goat chromosomes by fluorescent in situ hybridization and comparison with the human gene map. Cytogenet. Cell Genet. 1996, 72, 342–346.
- Goldammer, T.; Meyer, L.; Seyfert, H.M.; Brunner, R.M.; Schwerin, M. STAT5A encoding gene maps to chromosome 19 in cattle and goat and to chromosome 11 in sheep. Mamm. Genome 1997, 8, 705–706.
- Goldammer, T.; Brunner, R.M.; Schwerin, M. Comparative analysis of Y chromosome structure in Bos taurus and B. indicus by FISH using region-specific, microdissected, and locus-specific DNA probes. Cytogenet. Cell Genet. 1997, 77, 238–241.
- Faure, M.; Hayes, H.; Bledsoe, R.K.; Hutson, S.M.; Papet, I. Assignment of the gene of mitochondrial branched chain aminotransferase (BCAT2) to sheep chromosome band 14q24 and to cattle and goat chromosome bands 18q24 by in situ hybridization. Cytogenet. Cell Genet. 1998, 83, 96–97.
- Robinson, T.J.; Harrison, W.R.; Ponce de León, F.A.; Davis, S.K.; Elder, F.F. A molecular cytogenetic analysis of X-chromosome repatterning in the Bovidae: Transpositions, inversions, and phylogenetic inference. Cytogenet. Cell Genet. 1998, 80, 179–184.
- Piumi, F.; Schibler, L.; Vaiman, D.; Oustry, A.; Cribiu, E.P. Comparative cytogenetic mapping reveals chromosome rearrangements between the X chromosomes of two closely related mammalian species (cattle and goats). Cytogenet. Cell Genet. 1998, 81, 36–41.
- Schibler, L.; Vaiman, D.; Oustry, A.; Giraud-Delville, C.; Cribiu, E.P. Comparative gene mapping: A fine-scale survey of chromosome rearrangements between ruminants and humans. Genome Res. 1998, 8, 901–915.
- Schmitz, A.; Oustry, A.; Vaiman, D.; Chaput, B.; Frelat, G.; Cribiu, E.P. Comparative karyotype of pig and cattle using whole chromosome painting probes. Hereditas 1998, 128, 257–263.
- Iannuzzi, L.; Di Meo, G.P.; Perucatti, A.; Bardaro, T. ZOO-FISH and R-banding reveal extensive conservation of human chromosome regions in euchromatic regions of river buffalo chromosomes. Cytogenet. Cell Genet. 1998, 82, 210–214.
- Iannuzzi, L.; Di Meo, G.P.; Perucatti, A.; Incarnato, D. Comparison of the human with the sheep genomes by use of human chromosome-specific painting probes. Mammal. Genome 1999, 10, 719–723.
- Iannuzzi, L.; Di Meo, G.P.; Perucatti, A.; Incarnato, D.; Lopez-Corrales, N.; Smith, J. Chromosomal localization of four HSA2 type I loci in river buffalo (Bubalus bubalis, 2n=50) chromosomes 2q and 12. Mammal. Genome 2000, 11, 241–242.
- Hayes, H.; Bonfils, J.; Faure, M.; Papet, I. Assignment of BCAT1, the gene encoding cytosolic branched chain aminotransferase, to sheep chromosome band 3q33 and to cattle and goat chromosome bands 5q33 by in situ hybridization. Cytogenet. Cell Genet. 2000, 90, 84–85.
- Iannuzzi, L.; Di Meo, G.P.; Perucatti, A.; Incarnato, D.; Schibler, L.; Cribiu, E.P. Comparative FISH-mapping of bovid X chromosomes reveals homologies and divergences between the subfamilies Bovinae and Caprinae. Cytogenet. Cell Genet. 2000, 89, 171–176.
- Iannuzzi, L.; Di Meo, G.P.; Perucatti, A.; Schibler, L.; Incarnato, D.; Ferrara, L.; Bardaro, T.; Cribiu, E.P. Sixteen type I loci from six chromosomes were comparatively fluorescent in-situ mapped to river buffalo (Bubalus bubalis) and sheep (Ovis aries) chromosomes. Chromosome Res. 2000, 8, 447–450.
- Di Meo, G.P.; Perucatti, A.; Schibler, L.; Incarnato, D.; Ferrara, L.; Cribiu, E.P.; Iannuzzi, L. Thirteen type I loci from HSA4q, HSA6p, HSA7q and HSA12q were comparatively FISH-mapped in four river buffalo and sheep chromosomes. Cytogenet. Cell Genet. 2000, 90, 102–105.
- Iannuzzi, L.; Di Meo, G.P.; Perucatti, A.; Schibler, L.; Incarnato, D.; Cribiu, E.P. Comparative FISH-mapping in river buffalo and sheep chromosomes: Assignment of forty autosomal type I loci from sixteen human chromosomes. Cytogenet. Cell Genet. 2001, 94, 43–48.
- Gautier, M.; Hayes, H.; Taourit, S.; Laurent, P.; Eggen, A. Assignment of eight additional genes from human chromosome 11 to bovine chromosomes 15 and 29: Refinement of the comparative map. Cytogenet. Cell Genet. 2001, 93, 60–64.
- Di Meo, G.P.; Perucatti, A.; Incarnato, D.; Ferretti, L.; Di Berardino, D.; Caputi Jambrenghi, A.; Vonghia, G.; Iannuzzi, L. Comparative mapping of twenty-eight bovine loci in sheep (Ovis aries, 2n = 54) and river buffalo (Bubalus bubalis, 2n = 50) by FISH. Cytogenet. Genome Res. 2002, 98, 262–264.
- Cosseddu, G.M.; Oustry-Vaiman, A.; Jego, B.; Moreno, C.; Taourit, S.; Cribiu, E.P.; Elsen, J.M.; Vaiman, D. Sheep/human comparative map in a chromosome region involved in scrapie incubation time shows multiple breakpoints between human chromosomes 14 and 15 and sheep chromosomes 7 and 18. Chromosome Res. 2002, 10, 369–378.
- Hayes, H.; Elduque, C.; Gautier, M.; Schibler, L.; Cribiu, E.; Eggen, A. Mapping of 195 genes in cattle and updated comparative map with man, mouse, rat and pig. Cytogenet. Genome Res. 2003, 102, 16–24.
- Goldammer, T.; Amaral, M.E.; Brunner, R.M.; Owens, E.; Kata, S.R.; Schwerin, M.; Womack, J.E. Clarifications on breakpoints in HSAX and BTAX by comparative mapping of F9, HPRT, and XIST in cattle. Cytogenet. Genome Res. 2003, 101, 39–42.
- Brunner, R.M.; Sanftleben, H.; Goldammer, T.; Kühn, C.; Weikard, R.; Kata, S.R.; Womack, J.E.; Schwerin, M. The telomeric region of BTA18 containing a potential QTL region for health in cattle exhibits high similarity to the HSA19q region in humans. Genomics 2003, 81, 270–278.
- Ahrens, E.; Graphodatskaya, D.; Nguyen, B.X.; Stranzinger, G. Cytogenetic comparison of saola (Pseudoryx nghetinhensis) and cattle (Bos taurus) using G- and Q-banding and FISH. Cytogenet. Genome Res. 2005, 111, 147–151.
- Di Meo, G.P.; Perucatti, A.; Uboldi, C.; Roperto, S.; Incarnato, D.; Roperto, F.; Williams, J.; Eggen, A.; Ferretti, L.; Iannuzzi, L. Comparative mapping of the fragile histidine triad (FHIT) gene in cattle, river buffalo, sheep and goat by FISH and assignment to BTA22 by RH-mapping: A comparison with HSA3. Anim. Genet 2005, 36, 363–364.
- Di Meo, G.P.; Perucatti, A.; Floriot, S.; Incarnato, D.; Rullo, R.; Caputi Jambrenghi, A.; Ferretti, L.; Vonghia, G.; Cribiu, E.; Eggen, A.; et al. Chromosome evolution and improved cytogenetic maps of the Y chromosome in cattle, zebu, river buffalo, sheep and goat. Chromosome Res. 2005, 13, 349–355.
- Perucatti, A.; Floriot, S.; Di Meo, G.P.; Soglia, D.; Rullo, R.; Maione, S.; Incarnato, D.; Eggen, A.; Sacchi, P.; Rasero, R.; et al. Comparative FISH mapping of mucin 1, transmembrane (MUC1) among cattle, river buffalo, sheep and goat chromosomes: Comparison between bovine chromosome 3 and human chromosome 1. Cytogenet. Genome Res. 2006, 112, 103–105.
- Perucatti, A.; Di Meo, G.P.; Vallinoto, M.; Kierstein, G.; Schneider, M.P.; Incarnato, D.; Caputi Jambrenghi, A.; Mohammadi, G.; Vonghia, G.; Silva, A.; et al. FISH-mapping of LEP and SLC26A2 genes in sheep, goat and cattle R-banded chromosomes: Comparison between bovine, ovine and caprine chromosome 4 (BTA4/OAR4/CHI4) and human chromosome 7 (HSA7). Cytogenet. Genome Res. 2006, 115, 7–9.
- Schibler, L.; Roig, A.; Mahe, M.-F.; Laurent, P.; Hayes, H.; Rodolphe, F.; Cribiu, E.P. High-resolution comparative mapping among man, cattle and mouse suggests a role for repeat sequences in mammalian genome evolution. BMC Genom. 2006, 7, 194.
- Antonacci, R.; Vaccarelli, G.; Di Meo, G.P.; Piccinni, B.; Miccoli, M.C.; Cribiu, E.P.; Perucatti, A.; Iannuzzi, L.; Ciccarese, S. Molecular in situ hybridization analysis of sheep and goat BAC clones identifies the transcriptional orientation of T cell receptor gamma genes on chromosome 4 in bovids. Vet. Res. Comm. 2007, 31, 977–983.
- Perucatti, A.; Di Meo, G.P.; Goldammer, T.; Incarnato, D.; Brunner, R.; Iannuzzi, L. Comparative FISH-mapping of twelve loci in river buffalo and sheep chromosomes: Comparison with HSA8p and HSA4q. Cytogenet. Genome. Res. 2007, 119, 242–244.
- Goldammer, T.; Brunner, R.M.; Weikard, R.; Kuehn, C.; Wimmers, K. Generation of an improved cytogenetic and comparative map of Bos taurus chromosome BTA27. Chromosome Res. 2007, 15, 203–213.
- Ropiquet, A.; Gerbault-Seureau, M.; Deuve, J.L.; Gilbert, C.; Pagacova, E.; Chai, N.; Rubes, J.; Hassanin, A. Chromosome evolution in the subtribe Bovina (Mammalia, Bovidae): The karyotype of the Cambodian banteng (Bos javanicus birmanicus) suggests that Robertsonian translocations are related to interspecific hybridization. Chromosome Res. 2008, 16, 1107–1118.
- Nguyen, T.T.; Aniskin, V.M.; Gerbault-Seureau, M.; Planton, H.; Renard, J.P.; Nguyen, B.X.; Hassanin, A.; Volobouev, V.T. Phylogenetic position of the saola (Pseudoryx nghetinhensis) inferred from cytogenetic analysis of eleven species of Bovidae. Cytogenet. Genome Res. 2008, 122, 41–54.
- Bratuś, A.; Bugno, M.; Klukowska-Rötzler, J.; Sawińska, M.; Eggen, A.; Słota, E. Chromosomal homology between the human and the bovine DMRT1 genes. Folia Biol. 2009, 57, 29–32.
- Bratuś, A.; Słota, E. Comparative cytogenetic and molecular studies of DM domain genes in pig and cattle. Cytogenet. Genome Res. 2009, 126, 180–185.
- Perucatti, A.; Di Meo, G.P.; Goldammer, T.; Incarnato, D.; Nicolae, I.; Brunner, R.; Iannuzzi, L. FISH-mapping comparison between river buffalo chromosome 7 and sheep chromosome 6: Assignment of new loci and comparison with HSA4. Cytogenet. Genome Res. 2009, 124, 106–111.
- Goldammer, T.; Brunner, R.M.; Rebl, A.; Wu, C.H.; Nomura, K.; Hadfield, T.; Maddox, J.F.; Cockett, N.E. Cytogenetic anchoring of radiation hybrid and virtual maps of sheep chromosome X and comparison of X chromosomes in sheep, cattle, and human. Chromosome Res. 2009, 17, 497–506.
- Manera, S.; Bonfiglio, S.; Malusà, A.; Denis, C.; Boussaha, M.; Russo, V.; Roperto, F.; Perucatti, A.; Di Meo, G.P.; Eggen, A.; et al. Comparative mapping and genomic annotation of the bovine oncosuppressor gene WWOX. Cytogenet. Genome Res. 2009, 126, 186–193.
- Goldammer, T.; Di Meo, G.P.; Lühken, G.; Drögemüller, C.; Wu, C.H.; Kijas, J.; Dalrymple, B.P.; Nicholas, F.W.; Maddox, J.F.; Iannuzzi, L.; et al. Molecular cytogenetics and gene mapping in sheep (Ovis aries, 2n = 54). Cytogenet. Genome Res. 2009, 126, 63–76.
- Schibler, L.; Di Meo, G.P.; Cribiu, E.P.; Iannuzzi, L. Molecular cytogenetics and comparative mapping in goats (Capra hircus, 2n = 60). Cytogenet. Genome Res. 2009, 126, 77–85.
- Genualdo, V.; Spalenza, V.; Perucatti, A.; Iannuzzi, A.; Di Meo, G.P.; Caputi-Jambrenghi, A.; Vonghia, G.; Rasero, R.; Nebbia, C.; Sacchi, P.; et al. Fluorescence in situ hybridization mapping of six loci containing genes involved in the dioxin metabolism of domestic bovids. J. Appl. Genet. 2011, 52, 229–232.
- Di Meo, G.P.; Goldammer, T.; Perucatti, A.; Genualdo, V.; Iannuzzi, A.; Incarnato, D.; Rebl, A.; Di Berardino, D.; Iannuzzi, L. Extended cytogenetic maps of sheep chromosome 1 and their cattle and river buffalo homoeologues: Comparison with the OAR1 RH map and human chromosomes 2, 3, 21 and 1q. Cytogenet. Genome Res. 2011, 133, 16–24.
- Cernohorska, H.; Kubickova, S.; Vahala, J.; Rubes, J. Molecular insights into X;BTA5 chromosome rearrangements in the tribe Antilopini (Bovidae). Cytogenet. Genome Res. 2012, 136, 188–198.
- Kopecna, O.; Kubickova, S.; Cernohorska, H.; Cabelova, K.; Vahala, J.; Rubes, J. Isolation and comparison of tribe-specific centromeric repeats within Bovidae. J. Appl. Genet. 2012, 53, 193–202.
- Perucatti, A.; Genualdo, V.; Iannuzzi, A.; Rebl, A.; Di Berardino, D.; Goldammer, T.; Iannuzzi, L. Advanced comparative cytogenetic analysis of X chromosomes in river buffalo, cattle, sheep, and human. Chromosome Res. 2012, 20, 413–425.
- Kolesárová, V.; Šiviková, K.; Holečková, B.; Dianovský, J. A comparative FISH mapping of LCA5L gene in cattle, sheep, and goats. Anim. Biotechnol. 2015, 26, 37–39.
- De Lorenzi, L.; Planas, J.; Rossi, E.; Malagutti, L.; Parma, P. New cryptic karyotypic differences between cattle (Bos taurus) and goat (Capra hircus). Chromosome Res. 2015, 23, 225–235.
- De Lorenzi, L.; Pauciullo, A.; Iannuzzi, A.; Parma, P. Cytogenetic Characterization of a Small Evolutionary Rearrangement Involving Chromosomes BTA21 and OAR18. Cytogenet. Genome Res. 2020, 160, 193–198.
- Perucatti, A.; Iannuzzi, A.; Armezzani, A.; Palmarini, M.; Iannuzzi, L. Comparative fluorescence in Situ hybridization (FISH) mapping of twenty-three endogenous Jaagsiekte sheep retrovirus (enJSRVs) in sheep (Ovis aries) and river buffalo (Bubalus bubalis) chromosomes. Animals 2022, 12, 2834.
- Hayes, H. Chromosome painting with human chromosome-specific DNA libraries reveals the extent and distribution of conserved segments in bovine chromosomes. Cytogenet. Cell Genet. 1995, 71, 168–174.
- Chowdhary, B.P.; Frönicke, L.; Gustavsson, I.; Scherthan, H. Comparative analysis of the cattle and human genomes: Detection of ZOO-FISH and gene mapping-based chromosomal homologies. Mamm. Genome 1996, 7, 297–302.
- Solinas-Toldo, S.; Lengauer, C.; Fries, R. Comparative genome map of human and cattle. Genomics 1995, 27, 489–496.
- Pauciullo, A.; Cosenza, G.; Peretti, V.; Iannuzzi, A.; Di Meo, G.P.; Ramunno, L.; Iannuzzi, L.; Rubes, J.; Di Berardino, D. Incidence of X-Y aneuploidy in sperm of two indigenous cattle breeds by using dual color fluorescent in situ hybridization (FISH). Theriogenology 2011, 76, 328–333.
- Pauciullo, A.; Nicodemo, D.; Peretti, V.; Marino, G.; Iannuzzi, A.; Cosenza, G.; Di Meo, G.P.; Ramunno, L.; Iannuzzi, L.; Rubes, J.; et al. X-Y aneuploidy rate in sperm of two “minor” breeds of cattle (Bos taurus) by using dual color fluorescent in situ hybridization (FISH). Theriogenology 2012, 78, 688–695.
- Pauciullo, A.; Knorr, C.; Perucatti, A.; Iannuzzi, A.; Iannuzzi, L.; Erhardt, G. Characterization of a very rare case of living ewe-buck hybrid using classical and molecular cytogenetics. Sci. Rep. 2016, 6, 34781.
- Iannuzzi, A.; Pereira, J.; Iannuzzi, C.; Fu, B.; Ferguson-Smith, M. Pooling strategy and chromosome painting characterize a living zebroid for the first time. PLoS ONE 2017, 12, e0180158.
- Iannuzzi, L.; King, W.A.; Di Berardino, D. Chromosome evolution in domestic bovids as revealed by chromosome banding and FISH-mapping techniques. Cytogenet. Genome Res. 2009, 126, 49–62.
- Amaral, M.E.; Grant, J.R.; Riggs, P.K.; Stafuzza, N.B.; Filho, E.A.; Goldammer, T.; Weikard, R.; Brunner, R.M.; Kochan, K.J.; Greco, A.J.; et al. A first generation whole genome RH map of the river buffalo with comparison to domestic cattle. BMC Genom. 2008, 9, 631.
- Faraut, T.; de Givry, S.; Hitte, C.; Lahbib-Mansais, Y.; Morisson, M.; Milan, D.; Schiex, T.; Servin, B.; Vignal, A.; Galibert, F.; et al. Contribution of radiation hybrids to genome mapping in domestic animals. Cytogenet. Genome Res. 2009, 126, 21–33.
- Stafuzza, N.B.; Abbassi, H.; Grant, J.R.; Rodrigues-Filho, E.A.; Ianella, P.; Kadri, S.M.; Amarante, M.V.; Stohard, P.; Womack, J.E.; de León, F.A.; et al. Comparative RH maps of the river buffalo and bovine Y chromosomes. Cytogenet. Genome Res. 2009, 126, 132–138.
- Durmaz, A.A.; Karaca, E.; Demkow, U.; Toruner, G.; Schoumans, J.; Cogulu, O. Evolution of Genetic Techniques: Past, Present, and Beyond. BioMed. Res. Intern. 2015, 2015, 461524.
- Fidlerova, H.; Senger, G.; Kost, M.; Sanseau, P.; Sheer, D. Two simple procedures for releasing chromatin from routinely fixed cells for fluorescence in situ hybridization. Cytogenet. Cell Genet. 1994, 65, 203–205.
- Brunner, R.M.; Goldammer, T.; Fürbass, R.; Vanselow, J.; Schwerin, M. Genomic organization of the bovine aromatase encoding gene and a homologous pseudogene as revealed by DNA fiber FISH. Cytogenet. Cell Genet. 1998, 82, 37–40.
- Seyfert, H.M.; Pitra, C.; Meyer, L.; Brunner, R.M.; Wheeler, T.T.; Molenaar, A.; McCracken, J.Y.; Herrmann, J.; Thiesen, H.J.; Schwerin, M. Molecular characterization of STAT5A- and STAT5B-encoding genes reveals extended intragenic sequence homogeneity in cattle and mouse and different degrees of divergent evolution of various domains. J Mol. Evol. 2000, 50, 550–561.
- Hamilton, C.K.; Favetta, L.A.; Di Meo, G.P.; Floriot, S.; Perucatti, A.; Peippo, J.; Kantanen, J.; Eggen, A.; Iannuzzi, L.; King, W.A. Copy number variation of testis-specific protein, Y-encoded (TSPY) in 14 different breeds of cattle (Bos taurus). Sex Dev. 2009, 3, 205–213.
- Pauciullo, A.; Fleck, K.; Lühken, G.; Di Berardino, D.; Erhardt, G. Dual-color high-resolution fiber-FISH analysis on lethal white syndrome carriers in sheep. Cytogenet. Genome Res. 2013, 140, 46–54.
- du Manoir, S.; Speicher, M.R.; Joos, S.; Schröck, E.; Popp, S.; Döhner, H.; Kovacs, G.; Robert-Nicoud, M.; Lichter, P.; Cremer, T. Detection of complete and partial chromosome gains and losses by comparative genomic in situ hybridization. Hum. Gene.t 1993, 90, 590–610.
- Kallioniemi, A.; Kallioniemi, O.P.; Sudar, D.; Rutovitz, D.; Gray, J.W.; Waldman, F.; Pinkel, D. Comparative genomic hybridization for molecular cytogenetic analysis of solid tumors. N. Y. Sci. J. 1992, 258, 818–821.
- Solinas-Toldo, S.; Lampel, S.; Stilgenbauer, S.; Nickolenko, J.; Benner, A.; Döhner, H.; Cremer, T.; Lichter, P. Matrix-based comparative genomic hybridization: Biochips to screen for genomic imbalances. Genes Chromosom. Cancer. 1997, 20, 399–407.
- Brennan, C.; Zhang, Y.; Leo, C.; Feng, B.; Cauwels, C.; Aguirre, A.J.; Kim, M.; Protopopov, A.; Chin, L. High-resolution global profiling of genomic alterations with long oligonucleotide microarray. Cancer Res. 2004, 64, 4744–4748.
- Carvalho, B.; Ouwerkerk, E.; Meijer, G.A.; Ylstra, B. High resolution microarray comparative genomic hybridisation analysis using spotted oligonucleotides. J. Clin. Pathol. 2004, 57, 644–646.
- Pinkel, D.; Albertson, D.G. Array comparative genomic hybridization and its applications in cancer. Nat. Genet. 2005, 37, S11–S17.
- Iafrate, A.J.; Feuk, L.; Rivera, M.N.; Listewnik, M.L.; Donahoe, P.K.; Qi, Y.; Scherer, S.W.; Lee, C. Detection of large-scale variation in the human genome. Nat. Genet. 2004, 36, 949–951.
- Redon, R.; Ishikawa, S.; Fitch, K.R.; Feuk, L.; Perry, G.H.; Andrews, T.D.; Fiegler, H.; Shapero, M.H.; Carson, A.R.; Hurles, M.E.; et al. Global variation in copy number in the human genome. Nature 2006, 444, 444–454.
- Sebat, J.; Lakshmi, B.; Troge, J.; Alexander, J.; Young, J.; Lundin, P.; Månér, S.; Massa, H.; Walker, M.; Chi, M.; et al. Large-scale copy number polymorphism in the human genome. N. Y. Sci. J. 2004, 305, 525–528.
- Zarrei, M.; MacDonald, J.R.; Merico, D.; Scherer, S.W. A copy number variation map of the human genome. Nat. Rev. Genet. 2015, 16, 172–183.
- Liu, G.E.; Van Tassell, C.P.; Sonstegard, T.S.; Li, R.W.; Alexander, L.J.; Keele, J.W.; Matukumalli, L.K.; Smith, T.P.; Gasbarre, L.C. Detection of germline and somatic copy number variations in cattle. Dev. Biol. 2008, 132, 231–237.
- Liu, G.E.; Hou, Y.L.; Zhu, B.; Cardone, M.F.; Jiang, L.; Cellamare, A.; Mitra, A.; Alexander, L.J.; Coutinho, L.L.; Dell’Aquila, M.E.; et al. Analysis of copy number variations among diverse cattle breeds. Genome Res. 2010, 20, 693–703.
- Fadista, J.; Thomsen, B.; Holm, L.E.; Bendixen, C. Copy number variation in the bovine genome. BMC Genom. 2010, 11, 284.
- Liu, M.; Fang, L.; Liu, S.; Pan, M.G.; Seroussi, E.; Cole, J.B.; Ma, L.; Chen, H.; Liu, G.E. Array CGH-based detection of CNV regions and their potential association with reproduction and other economic traits in Holsteins. BMC Genom. 2019, 20, 181.
- Zhang, L.; Jia, S.; Yang, M.; Xu, Y.; Li, C.; Sun, J.; Huang, Y.; Lan, X.; Lei, C.; Zhou, Y.; et al. Detection of copy number variations and their effects in Chinese bulls. BMC Genom. 2014, 15, 480.
- Kijas, J.W.; Barendse, W.; Barris, W.; Harrison, B.; McCulloch, R.; McWilliam, S.; Whan, V. Analysis of copy number variants in the cattle genome. Gene 2011, 482, 73–77.
- Jenkins, G.M.; Goddard, M.E.; Black, M.A.; Brauning, R.; Auvray, B.; Dodds, K.G.; Kijas, J.W.; Cockett, N.; McEwan, J.C. Copy number variants in the sheep genome detected using multiple approaches. BMC Genom. 2016, 17, 441.
- Fontanesi, L.; Beretti, F.; Martelli, P.L.; Colombo, M.; Dall’olio, S.; Occidente, M.; Portolano, B.; Casadio, R.; Matassino, D.; Russo, V. A first comparative map of copy number variations in the sheep genome. Genomics 2011, 97, 158–165.
- Fontanesi, L.; Martelli, P.L.; Beretti, F.; Riggio, V.; Dall’Olio, S.; Colombo, M.; Casadio, R.; Russo, V.; Portolano, B. An initial comparative map of copy number variations in the goat (Capra hircus) genome. BMC Genom. 2010, 11, 639.




