
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | SANTIAGO DANIEL PALMA | -- | 1676 | 2023-03-23 13:07:52 | | | |
| 2 | Jason Zhu | -4 word(s) | 1672 | 2023-03-24 02:36:32 | | |
Video Upload Options
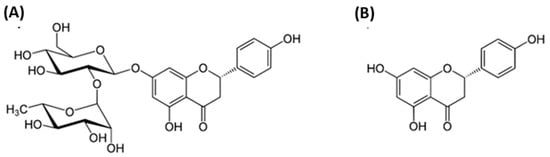
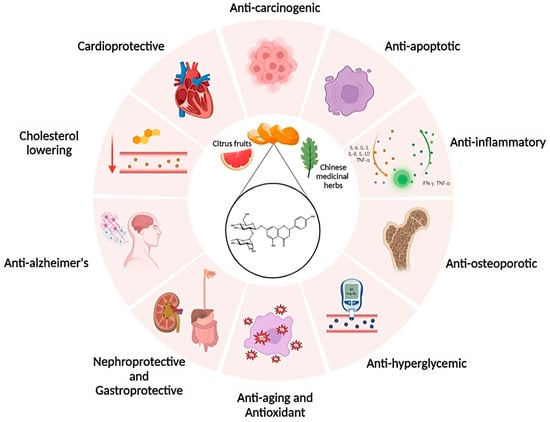
Polyphenols comprise a number of natural substances, such as flavonoids, that show interesting biological effects. Among these substances is naringin, a naturally occurring flavanone glycoside found in citrus fruits and Chinese medicinal herbs. Several studies have shown that naringin has numerous biological properties, including cardioprotective, cholesterol-lowering, anti-Alzheimer’s, nephroprotective, antiageing, antihyperglycemic, antiosteoporotic and gastroprotective, anti-inflammatory, antioxidant, antiapoptotic, anticancer and antiulcer effects. Despite its multiple benefits, the clinical application of naringin is severely restricted due to its susceptibility to oxidation, poor water solubility, and dissolution rate.
1. Introduction
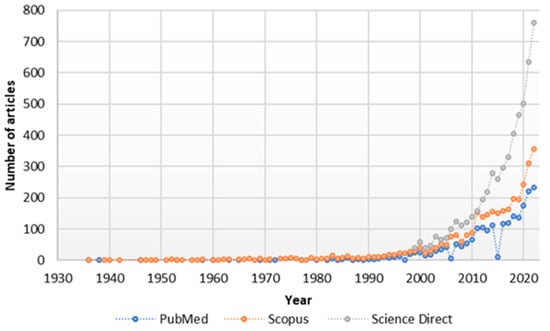
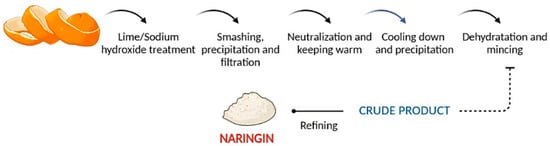
2. Bioavailability and Pharmacokinetic Properties of Naringin
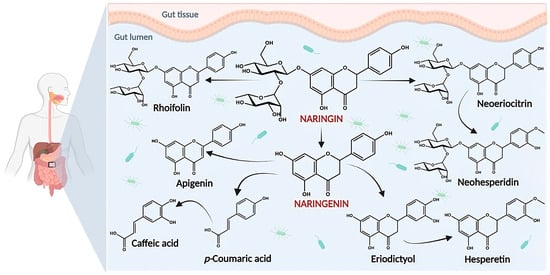
3. Biological Activities of Naringin
4. Clinical Translation and Challenges for Its Therapeutic Application
References
- Chen, R.; Qi, Q.L.; Wang, M.T.; Li, Q.Y. Therapeutic potential of naringin: An overview. Pharm. Biol. 2016, 54, 3203–3210.
- Rangaswami, S.; Seshadri, T.R.; Veeraraghaviah, J. Constitution of naringin. The position of the sugar group. J. Proc. Ind. Acad. Sci. 1939, 9, 328–332.
- Zhao, B.T.; Kim, E.J.; Son, K.H.; Son, J.K.; Min, B.S.; Woo, M.H. Quality evaluation and pattern recognition analyses of marker compounds from five medicinal drugs of Rutaceae family by HPLC/PDA. Arch. Pharm. Res. 2015, 38, 1512–1520.
- Stabrauskiene, J.; Marksa, M.; Ivanauskas, L.; Bernatoniene, J. Optimization of naringin and naringenin extraction from Citrus × paradisi L. using hydrolysis and excipients as adsorbent. Pharmaceutics 2022, 14, 890.
- Kanokorn, S.; Surachai, P.; Supason, W. An efficient method for the large scale isolation of naringin from pomelo (Citrus grandis) peel. Int. J. Food Sci. Technol. 2009, 44, 1737–1742.
- PubChem . Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004. PubChem Compound Summary for CID 442428, Naringin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Naringin (accessed on 11 January 2023).
- Zhang, L.; Song, L.; Zhang, P.; Liu, T.; Zhou, L.; Yang, G.; Lin, R.; Zhang, J. Solubilities of naringin and naringenin in different solvents and dissociation constants of naringenin. J. Chem. Eng. Data 2015, 60, 932–940.
- Budel, R.G.; da Silva, D.A.; Moreira, M.P.; Dalcin, A.J.F.; da Silva, A.F.; Nazario, L.R.; Majolo, J.H.; Lopes, L.Q.S.; Santos, R.C.V.; Antunes Soares, F.A.; et al. Toxicological evaluation of naringin-loaded nanocapsules in vitro and in vivo. Colloids Surf. B Biointerfaces 2020, 188, 110754.
- Carrier, R.L.; Miller, L.A.; Ahmed, I. The utility of cyclodextrins for enhancing oral bioavailability. J. Control. Release 2007, 123, 78–99.
- Ilangumaran, S.; Hoessli, D.C. Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem. J. 1998, 335 Pt 2, 433–440.
- Liu, L.; Shan, S.; Zhang, K.; Ning, Z.Q.; Lu, X.P.; Cheng, Y.Y. Naringenin and hesperetin, two flavonoids derived from Citrus aurantium up-regulate transcription of adiponectin. Phytother. Res. 2008, 22, 1400–1403.
- Lauro, M.R.; De Simone, F.; Sansone, F.; Iannelli, P.; Aquino, R.P. Preparations and release characteristics of naringin and naringenin gastro-resistant microparticles by spray-drying. J. Drug Deliv. Sci. Technol. 2007, 17, 119–124.
- Sharma, A.; Bhardwaj, P.; Arya, S.K. Naringin: A potential natural product in the field of biomedical applications. Carbohydrate Polymer Technol. Appl. 2021, 2, 100068.
- Kanaze, F.I.; Bounartzi, M.I.; Georgarakis, M.; Niopas, I. Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur. J. Clin. Nutr. 2007, 61, 472–477.
- Choudhury, R.; Chowrimootoo, G.; Srai, K.; Debnam, E.; Rice-Evans, C.A. Interactions of the flavonoid naringenin in the gastrointestinal tract and the influence of glycosylation. Biochem. Biophys. Res. Commun. 1999, 265, 410–415.
- Khan, A.W.; Kotta, S.; Ansari, S.H.; Sharma, R.K.; Ali, J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid Naringenin: Design, characterization, in vitro and in vivo evaluation. Drug Deliv. 2015, 22, 552–561.
- Jeon, S.M.; Kim, H.K.; Kim, H.J.; Do, G.M.; Jeong, T.S.; Park, Y.B.; Choi, M.S. Hypocholesterolemic and antioxidative effects of naringenin and its two metabolites in high-cholesterol fed rats. Transl. Res. 2007, 149, 15–21.
- Steed, A.L.; Christophi, G.P.; Kaiko, G.E.; Sun, L.; Goodwin, V.M.; Jain, U.; Esaulova, E.; Artyomov, M.N.; Morales, D.J.; Holtzman, M.J.; et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science 2017, 357, 498–502.
- Wei, Y.; Gao, J.; Kou, Y.; Liu, M.; Meng, L.; Zheng, X.; Xu, S.; Liang, M.; Sun, H.; Liu, Z.; et al. The intestinal microbial metabolite desaminotyrosine is an anti-inflammatory molecule that modulates local and systemic immune homeostasis. FASEB J. 2020, 34, 16117–16128.
- Zeng, X.; Zheng, Y.; He, Y.; Zhang, J.; Peng, W.; Su, W. Microbial Metabolism of Naringin and the Impact on Antioxidant Capacity. Nutrients 2022, 14, 3765.
- Hixson, A.W.; Crowell, J.H. Dependence of reaction velocity upon surface and agitation. J. Ind. Eng. Chem. 1931, 23, 923–931.
- Felgines, C.; Texier, O.; Morand, C.; Manach, C.; Scalbert, A.; Régerat, F.; Rémésy, C. Bioavailability of the flavanone naringenin and its glycosides in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G1148–G1154.
- Fang, T.Z.; Wang, Y.G.; Ma, Y.; Su, W.W.; Bai, Y.; Zhao, P.Y. A rapid LC/MS/MS quantitation assay for naringin and its two metabolites in rats plasma. J. Pharmaceut. Biomed. 2006, 40, 454–459.
- Li, S.Q.; Dong, S.; Su, Z.H.; Zhang, H.W.; Peng, J.B.; Yu, C.Y.; Zou, Z.M. Comparative pharmacokinetics of naringin in rat after oral administration of chaihu-shu-gan-san aqueous extract and naringin alone. Metabolites 2013, 3, 867–880.
- Ghanbari-Movahed, M.; Jackson, G.; Farzaei, M.H.; Bishayee, A. A systematic review of the preventive and therapeutic effects of naringin against human malignancies. Front. Pharmacol. 2021, 12, 639840.
- Heidary Moghaddam, R.; Samimi, Z.; Moradi, S.Z.; Little, P.J.; Xu, S.; Farzaei, M.H. Naringenin and naringin in cardiovascular disease prevention: A preclinical review. Eur. J. Pharmacol. 2020, 887, 173535.
- Raja Kumar, S.; Mohd Ramli, E.S.; Abdul Nasir, N.A.; Ismail, N.H.M.; Mohd Fahami, N.A. Preventive effect of naringin on metabolic syndrome and its mechanism of action: A systematic review. Evid. Based Complement Alternat. Med. 2019, 2019, 9752826.
- Zeng, X.; Su, W.; Liu, B.; Chai, L.; Shi, R.; Yao, H. A Review on the pharmacokinetic properties of naringin and its therapeutic efficacies in respiratory diseases. Mini Rev. Med. Chem. 2020, 20, 286–293.
- Ahmed, S.; Khan, H.; Aschner, M.; Hasan, M.M.; Hassan, S.T.S. Therapeutic potential of naringin in neurological disorders. Food Chem. Toxicol. 2019, 132, 110646.
- Yang, Y.; Trevethan, M.; Wang, S.; Zhao, L. Beneficial effects of citrus flavanones naringin and naringenin and their food sources on lipid metabolism: An update on bioavailability, pharmacokinetics, and mechanisms. J. Nutr. Biochem. 2022, 104, 108967.
- Miles, E.A.; Calder, P.C. Effects of citrus fruit juices and their bioactive components on inflammation and immunity: A narrative review. Front. Immunol. 2021, 12, 712608.
- Rivoira, M.A.; Rodriguez, V.; Talamoni, G.; Tolosa de Talamoni, N. New Perspectives in the pharmacological potential of naringin in medicine. Curr. Med. Chem. 2021, 28, 1987–2007.
- Shulman, M.; Cohen, M.; Soto-Gutierrez, A.; Yagi, H.; Wang, H.; Goldwasser, J.; Lee-Parsons, C.W.; Benny-Ratsaby, O.; Yarmush, M.L.; Nahmias, Y. Enhancement of naringenin bioavailability by complexation with hydroxypropyl-β-cyclodextrin. PLoS ONE 2011, 6, e18033, Erratum in PLoS ONE 2012, 7.
- Wang, M.J.; Chao, P.D.L.; Hou, Y.C.; Hsiu, S.L.; Wen, K.C.; and Tsai, S.Y. Pharmacokinetics and conjugation metabolism of naringin and naringenin in rats after single dose and multiple dose administrations. J. Food Drug Anal. 2006, 14, 247–253.
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. 1), 230S–242S.
- Walle, T. Absorption and metabolism of flavonoids. Free Radic. Biol. Med. 2004, 36, 829–837.
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22.
- Rao, K.; Imran, M.; Jabri, T.; Ali, I.; Perveen, S.; Shafiullah, A.S.; Shah, M.R. Gum tragacanth stabilized green gold nanoparticles as cargos for Naringin loading: A morphological investigation through AFM. Carbohydr. Polym. 2017, 174, 243–252.
- Roy, A.; Tripathy, D.; Chatterjee, A.; Dasgupta, S. A spectroscopic study of the interaction of the antioxidant naringin with bovine serum albumin. J. Biophys. Chem. 2010, 1, 141–152.
- Santo, V.E.; Gomes, M.E.; Mano, J.F.; Reis, R.L. From nano- to macro-scale: Nanotechnology approaches for spatially controlled delivery of bioactive factors for bone and cartilage engineering. Nanomedicine 2012, 7, 1045–1066.
- Singh, R.; Lillard, J.W. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223.
- Low, S.A.; Kopeček, J. Targeting polymer therapeutics to bone. Adv. Drug Deliv. Rev. 2012, 64, 1189–1204.
- Lavrador, P.; Gaspar, V.M.; Mano, J.F. Bioinstructive Naringin-Loaded Micelles for Guiding Stem Cell Osteodifferentiation. Adv. Healthc. Mater. 2018, 7, 1800890.




