Polyphenols comprise a number of natural substances, such as flavonoids, that show interesting biological effects. Among these substances is naringin, a naturally occurring flavanone glycoside found in citrus fruits and Chinese medicinal herbs. Several studies have shown that naringin has numerous biological properties, including cardioprotective, cholesterol-lowering, anti-Alzheimer’s, nephroprotective, antiageing, antihyperglycemic, antiosteoporotic and gastroprotective, anti-inflammatory, antioxidant, antiapoptotic, anticancer and antiulcer effects. Despite its multiple benefits, the clinical application of naringin is severely restricted due to its susceptibility to oxidation, poor water solubility, and dissolution rate.
1. Introduction
Polyphenols comprise a number of naturally occurring and biologically-active substances, including flavonoids. Flavonoids are an important group of secondary metabolites and a permanent source of compounds with a wide spectrum of biological actions that may be able to stimulate steps altered in different diseases. Citrus flavonoids establish an important stream of flavonoids, with naringin (NRG) standing out in this classification
[1].
NRG was first discovered in grapefruit flowers by De Vry in 1857, however, the results of his research were not published at that time
[2]. NRG (4′,5,7-trihydroxy flavanone 7-rhamnoglucoside) is a naturally occurring flavanone glycoside present in several plant species, and it is one of the most widely used active compounds in Chinese herbal medicine. Subsequent research reported that NRG accumulates at a high level in various citrus fruits, such as grapefruit and sour orange. The characteristic bitter taste of citrus juices is produced due to the accumulation of NRG.
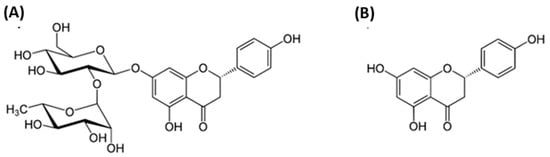
Figure 1A shows the structure of NRG, which consists of flavanone naringenin (NRGN), Figure 1B) and disaccharide neohesperidose linked through a glycosidic bond. The molecular formula of NRG is C27H32O14 and its molecular weight is 580.53.
Figure 1. Chemical structures of naringin (NRG) (A) and naringenin (NRGN) (B).
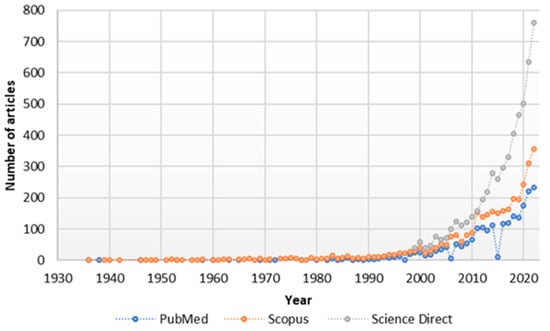
Since 2010, there has been a marked scientific interest in NRG, as can be clearly seen in Figure 2, which shows an increasing number of articles published with the keyword naringin in different databases (Science Direct, Pubmed, and Scopus).
Figure 2. Number of Publications by Year/Database.
ABased on this research, a large number of scientific articles have been conducted on NRG describing its structure, physicochemical properties, and its therapeutic uses in different diseases.
The concentration of NRG in fruit is dependent on numerous factors, such as the time of fruit collection, the part of the fruit analyzed, whether the peel is the reservoir of NRG, and the fruit’s drying time
[3].
The process of obtaining NRG from fruits is similar to that of other flavonoids such as hesperidin, and it basically consists of three steps: extraction, separation, and purification.
Maceration, percolation, thermal reflux, Soxhlet, supercritical, microwave assisted, and ultrasound assisted are some of the often-employed methods of NRG extraction
[4].
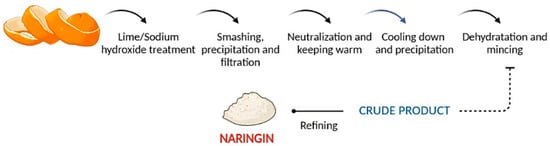
One of the most conventional methods applied, used for its simplicity, is the extraction of NRG with an alkaline solution of pH 9–10, such as lime or sodium hydroxide, and its posterior precipitation in cold acidic water. Figure 3 shows, representatively, the steps necessary to isolate NRG.
Figure 3. Steps in the naringin alkaline extraction process.
Kanokorn et al. have developed an efficient and simple method to isolate high-purity NRG from grapefruit of Citrus grandis Osbeck peel in high yield. The described process, consisting of a simple extraction with methanol followed by crystallization in water with the addition of 14–15% (
v/
v) dichloromethane, gave a yield four times higher compared to the same method without the addition of dichloromethane and five times higher than compared to conventional direct extraction with hot water. The yield obtained from different cultivars was 16–24 mg per gram of dry weight of peel
[5].
2. Bioavailability and Pharmacokinetic Properties of Naringin
NRG is slightly soluble in water (1 mg/mL at 40 °C)
[6][7]. In organic solvents, NRG shows a higher solubility in polar solvents (methanol > ethanol > ethyl acetate) compared to nonpolar solvents (petroleum ether > hexane), probably due to the hydrophilic sugar residues in its structure.
The solubility of NRG in different solvents also becomes more plausible with increasing temperatures in certain ranges
[7][8].
However, according to the Biopharmaceutical Classification of Drugs (BCS), NRG is a class IV drug, which indicates that it is a compound with significant limitations for oral administration. Lack of solubility and low permeability result in a limited bioavailability of NRG due to its large hydrophobic ring structure
[8][9].
The degree of conjugation with sugar moieties
[9][10][11][10,11,12] and its corresponding elimination by intestinal bacterial species are directly related to NRG metabolism. In addition, NRG is unstable at acidic pH and enzymatically cleaved by β-glycosidase in the stomach
[12][13][13,14].
NRG is also resistant to enzymatic attacks in the stomach and small intestine, so it will eventually reach the colon.
Oral absorption of NRG from citrus products suggested that NRG is poorly absorbed from the gastrointestinal tract in its original form
[14][15] and it is often exposed to enzymes secreted by the microbiome of the gut region. The process begins with the elimination, first of rhamnose, and then of glucose via enzymes β-glucosidases.
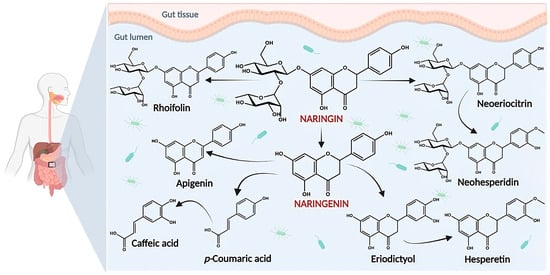
As shown in
Figure 4, NRG is broken down into its aglycone NRGN in the gut by intestinal microflora, a process which further affects its bioavailability
[15][16], metabolizes it into other flavonoid metabolites, and degrades it into a variety of ring fission products
[15][16][17][16,17,18].
Figure 4. Some potential pathways for the metabolism of NRG by the gut microbiota.
Recent studies have demonstrated the important role of these microbial metabolites in the overall bioactivity of NRG
[18][19][19,20]. In fact, the physiological effects of NRG cannot be fully achieved in the absence of microbial metabolites due to the extensive biotransformation of the parent compound
[20][21]. Although significant conversion takes place in the colon, some breakdown also occurs in the distal portion of the small intestine
[21][22].
During its release, NRG is absorbed through the intestinal epithelium by passive diffusion and proton-coupled active transport. It is further metabolized into phenolic acids by the cleavage of the pyran ring, demethylation, and dehydroxylation with the help of bacterial enzymes.
It is therefore important to outline the intestinal microbial metabolism of NRG.
The free form of NRG can be found transiently in the plasma of rats and humans, and NRGN glucuronide appears to be the predominant metabolite
[22][23][23,24]. It has been described that NRG is rapidly absorbed in a first concentration peak at 15 min and in a consequent one at 3 h after oral administration of monomeric NRG. Following 8 h of dosing, the authors found no evidence of rapid metabolism (5.075 mg/kg). The area under the curve (AUC) and the mean residence time (MRT) resulted in 274.8 mg/L and 114.0 min, respectively
[24][25].
3. Biological Activities of Naringin
NRG has been explored broadly for its biological and pharmacological effects. Most of the health benefits of NRG are related to the common chemical structure of natural flavonoids and to the presence of the sugar moieties and hydroxyl groups attached to both aromatic rings, structures that provide particular physicochemical and physiological characteristics able to perform numerous functions and distinguish themselves from others. It has been recently demonstrated that NRG exerts potential therapeutic actions by modulating various protein and enzyme expressions
[25][26].
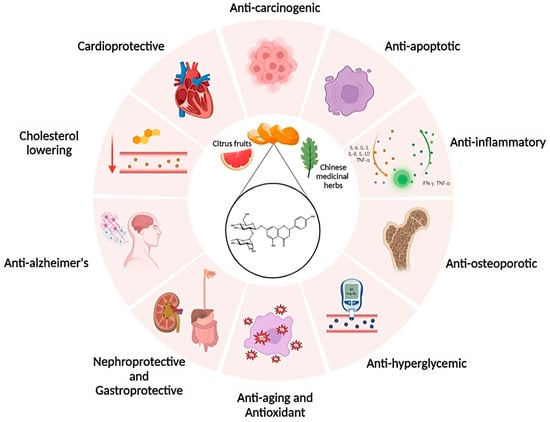
As shown in
Figure 5, NRG has been documented to possess antioxidant, neuroprotective, anti-inflammatory, antiapoptotic, antiulcer, antiosteoporotic, and anticancer properties
[26][27][28][29][30][31][27,28,29,30,31,32].
Figure 5. Health effects of naringin on different biological systems.
In recent years, NRG has also been increasingly used as a phytopharmaceutical in dietary supplement formulations
[32][33].
4. Clinical Translation and Challenges for Its Therapeutic Application
Despite its benefits in the prevention and treatment of various diseases
[33][34][34,35], NRG has not yet been approved for clinical administration either as a single therapy or in combination with other bioactive compounds, mainly due to the intense in vivo metabolism of flavonoids that restricts its therapeutic efficacy
[33][34][34,35].
In addition, the low solubility and dissolution rate of NRG has resulted in low bioavailability (approximately 8.8%) when administered orally. The limiting step in its absorption in the organism is caused due to the poor water solubility of NRG, which results in lower therapeutic efficacy
[14][35][15,36].
Furthermore, this specific drug degrades in an acidic pH environment and is easily metabolized in the intestinal region by the enzyme β-glucosidase, an inherent property of the intestinal microflora
[36][37].
In most cases, significant exploration of NRG occurs mainly through the oral route, however, its absorption in the intestinal tract does not take place in the same manner. In comparison, NRG’s absorption in the GIT tends to be rather slow and erratic
[12][13].
In addition, the microbes present in the gut play an important role in the bioavailability and clinical efficacy of flavonoids such as NRG
[37][38].
Currently, several efforts have been concentrated on overcoming certain disadvantages of NRGs for use in clinical applications. In vitro attempts have been made to improve the bioavailability and absorption of flavonoids by modifying their solubility and dissolution rates along with the prevention of their degradation due to gut microbes, remarkably through the encapsulation of either nanoparticles or microparticles
[12][37][38][13,38,39].
When administered intravenously (IV), NRG can also degrade during blood circulation. This structure is generally not stable when present in the bloodstream and readily undergoes oxidation in the serum and liver, where it is degraded by β-glucosidases
[36][37].
According to the above report, NRG interacts with bovine serum albumin immediately under physiological conditions defining its pharmacokinetic profile, which promotes excretion and thus influences the bioavailability of NRG
[39][40].
Various strategies are often proposed to improve the bioavailability and bioactivity of biologically active compounds for medicinal purposes. These strategies include advances in drug delivery systems from nanotechnology, pharmaceutical technology, and colloidal systems, as well as chemical structural modifications of a drug candidate, use of appropriate bioenhancers, and inhibition of intestinal cell transporters, among others.
The development of micro- and nanoformulations is recognized as one of the most potent lines of research to overcome the physicochemical and biopharmaceutical limitations of drugs in the treatment of pathologies and diseases. Numerous micro- and nanotechnology-based drug delivery systems have improved the bioavailability and pharmacokinetics of NRG, which ultimately protects it from degradation and random interactions when it has prolonged circulation times
[40][41][41,42].
These systems have the potential to exhibit a sustained release profile after appropriate modification with precise targeting of elements to enhance accumulation at specific sites
[42][43]. Thus, it is possible to improve the bioavailability of NRG by conjugating it to a suitable micro- or nanotransporter
[43][44].