Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Stephen H. Safe | -- | 2525 | 2023-03-22 15:09:33 | | | |
| 2 | Rita Xu | Meta information modification | 2525 | 2023-03-23 02:50:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Safe, S. Specificity Proteins and Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/42437 (accessed on 07 February 2026).
Safe S. Specificity Proteins and Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/42437. Accessed February 07, 2026.
Safe, Stephen. "Specificity Proteins and Cancer" Encyclopedia, https://encyclopedia.pub/entry/42437 (accessed February 07, 2026).
Safe, S. (2023, March 22). Specificity Proteins and Cancer. In Encyclopedia. https://encyclopedia.pub/entry/42437
Safe, Stephen. "Specificity Proteins and Cancer." Encyclopedia. Web. 22 March, 2023.
Copy Citation
The specificity protein (Sp) transcription factors (TFs) Sp1, Sp2, Sp3 and Sp4 exhibit structural and functional similarities in cancer cells and extensive studies of Sp1 show that it is a negative prognostic factor for patients with multiple tumor types.
Sp1
Sp3
Sp4
non-oncogene addiction
1. Background
Specificity protein 1 (Sp1) was among the first transcription factors (TFs) identified and is a member of the Sp/Kruppel-like factor (Sp/KLF) family. Members of this family exhibit variable structural domains and functions but all contain conserved zinc fingers in their DNA binding domains that bind GC-rich (Sps) and CACC (KLFs) boxes [1][2][3][4][5][6][7]. Not surprisingly, within the Sp and KLF sub-families there can be some overlap and competition for the same cis-elements, although for many Sp-regulated genes, differences in cell context and levels of expression dictate which Sp transcription factor is active. Among the 9 Sp genes, Sp1-Sp4 are most similar in terms of both structure and function (Figure 1). It should also be pointed out that among Sp1-Sp4, most research has focused on Sp1 and to a lesser extent Sp3 and it is possible that for some genes and pathways, the potential contributions of Sp2 and Sp4 have been understudied. There has been extensive research on the mechanisms of Sp-regulated gene expression, which frequently is observed in genes that lack a TATA box. Many Sp-regulated genes bind and activate gene expression through one or more GC-rich sequences proximal to the start sites where there are ordered assemblies of nuclear cofactors to form a transcriptionally active complex that includes DNA-bound Sp1, Sp3 or Sp4. The composition of transcription complexes includes polymerase II, transcription factor IID (TFIID), TATA box binding protein (TBP) and associated factors (TAFs) and members of the cofactor required for Sp1 activation/mediator (CRISP/MED) complexes [8]. The overall complex is highly variable and both gene- and cell-context-dependent. Moreover, there is also evidence that Sp TFs bind imperfect/variable GC-rich sequences and also interact with distal enhancer sequences, as described for the Topoisomerase IIa promoter [9]. There is a focus on the interactions of Sp TFs with non-coding RNAs and their functions; however, it should also be noted that Sp1 physically interacts with over 55 other proteins [2]. Sp1 function is also influenced by post-transcriptional modifications that include phosphorylation, acetylation, glycosylation and cleavage, and these changes can enhance or inhibit protein stability. Unfortunately, data for Sp3-Sp4 in terms of transcriptional function, post-transcriptional modifications and interactions with other factors have not been extensively investigated.
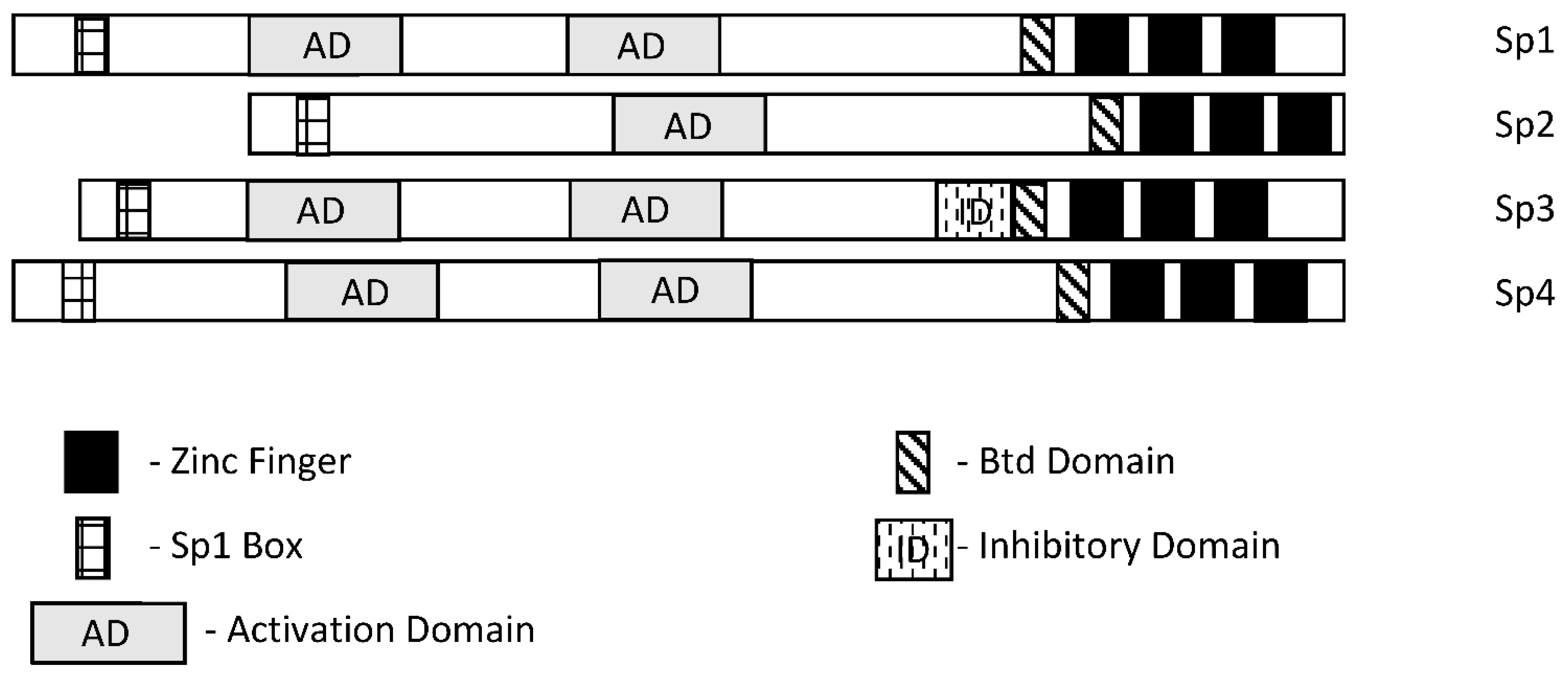
Figure 1. Schematic structures of Sp1, Sp2, Sp3 and Sp4. These transcription factors exhibit several common structural features; however, Sp3 expresses an inhibitory domain that results in gene-specific decreased expression in some cell lines.
2. Sp TFs as Cancer Prognostic Factors
Extensive analysis of tumor and non-tumor tissues has identified many prognostic factors that can be used to predict patient outcomes. Moreover, in some cases, the results dictate the application of specific treatment regimens, and this is particularly true of early-stage breast cancer where expression of estrogen receptor α (ERα, ESR1) in mammary tumors usually results in treatment with endocrine therapies [10]. Table 1 illustrates the important role of Sp1 as a negative prognostic factor for multiple cancers where Sp1 is generally more highly expressed in tumors compared to normal tissue and overexpression is correlated with decreased disease-free patient survival or another negative outcome. With the exception of highly variable results for lung cancer, most tumors overexpress Sp1 (or Sp3) compared to non-tumor tissue and poorer outcomes are observed in patients with tumors overexpressing this TF. In liver cancer, both Sp1 and Sp2 are negative prognostic factors for survival [11][12][13]. In many cases, manuscripts reporting the role of Sp1 as a diagnostic factor are accompanied by laboratory studies showing the pro-oncogenic functional activities of Sp1.
Table 1. Clinical/prognostic Significance of Sp transcription factors.
| Tumor | Sp TF | Prognosis | Refs. |
|---|---|---|---|
| Prostate | Sp1/Sp3/FLIP | Overexpression correlated with a high Gleason score and predicted recurrence | [14] |
| Esophageal squamous cell carcinoma | Sp1 | High Sp1 predicts poor patient survival | [15] |
| Astrocytoma | Sp1 | Poor patient prognosis | [16] |
| Bladder urothelial carcinoma | Sp | Poor clinical outcomes | [17] |
| Glioma | Sp1 | Poor outcomes, higher expression in higher grades, immune invasion | [18][19][20] |
| Head and Neck | Sp3 | Predicted poor survival | [21] |
| Pancreatic | Sp1 (Sp1/LOXL2) | Decreased survival, higher grade, dual prognostic factor (with LOXL2) | [22][23][24] |
| Oral squamous cell carcinoma | Sp1 | Overexpressed and prometastatic | [25] |
| Gastric cancer | Sp1 | Overexpressed, poor prognosis, increased in higher stages | [26][27][28][29][30] |
| Liver cancer | Sp1 | Overexpressed, poor prognosis | [11][12] |
| Colin cancer | Sp1/Sp3 | Overexpressed, decreased survival | [31][32] |
| Breast cancer | Sp1/Par3 | Lower levels/advanced stage, poor prognosis | [33][34][35][36] |
| Lung cancer | Sp1 | Variable prognosis, decreased Sp1 with increasing stage | [37][38][39] |
| Ovarian cancer | Sp1/DANCR | Sp1 overexpression in tumor, correlates with DANCR | [40] |
| Liver cancer | Sp2 | Decreased survival | [13] |
Meta-analysis of multiple studies has also been used to probe the role of Sp1 in gastric cancer, and higher Sp1 expression is correlated with increased depth of invasion and lymph node metastasis, increased TNM staging and Lauren’s classification [41]. A similar meta-analysis approach was used to examine multiple tumor types [42] and similar associations were observed as reported for gastric cancer.
3. Role of Sp in Cell Transformation
Sp1 is clearly a negative prognostic factor for multiple cancers, and this is accompanied by increased expression of Sp transcription factors in tumors compared to non-tumor tissues. These observations suggest that the process that drives the transformation of a normal cell to a tumor cell may also involve Sp transcription factors. This was investigated in a classical study that examined the effects of carcinogen or oncogene-induced transformation of human fibroblasts into fibrosarcoma cells in which the fibrosarcoma, but not the fibroblasts, had the ability to form tumors in athymic nude mice [43][44]. This dramatic change in the phenotype of fibrosarcoma cells compared to the fibroblasts was accompanied by an 8- to 18-fold increased expression of Sp1 protein, which is enhanced during fibroblast cell transformation. Moreover, it was also demonstrated that knockdown of Sp1 in the fibrosarcomas resulted in cells that did not form tumors in athymic nude mice. Other studies show that EGF-induced transformation of bladder epithelial cells and Kras induced transformation of MCFI0A cells also involved Sp1 or an Sp1-regulated gene [45][46]. CYP1B1 also enhanced the proliferation, migration and invasion of MCFI0A and MCF7 cells and this was also accompanied by increased expression of Sp1 and Sp1 regulated genes and silencing or inhibition of Sp1 inhibited CYP1B1-mediated transformation [47].
Arsenic is a carcinogen and considered to be a public health hazard. Exposures of human bronchial epithelial Beas-2B cells to arsenic over a period of several months lead to cell transformation and this was due, in part, to induction DNA methyltransferase 1 (DNMT1) [48]. However, further examination found that arsenic induced Sp1, which in part enhanced DNMT1 expression and loss of miR-199a-5p, which was critical for arsenic-induced transformation. The proposed mechanism involves arsenic-induced Sp1, which in turn activates DNMT1 and suppresses miR-199a-5p. These results demonstrate a role for Sp1 in arsenic-induced transformation of Beas-2B cells; however, the direct effect of Sp1-mediated suppression of miR-199a-5p is unexpected and needs further investigation. Rhabdomyosarcomas (RMS) express high levels of Sp1 compared to non-transformed muscle tissue and RMS cell lines express high levels of Sp1, Sp3 and Sp4. Transformation of human smooth muscles with telomerase, the PAX3-FOXO1 oncogene and NMyc transforms these muscle cell lines; however, expression of only one or two of these factors is not sufficient for transformation [49]. Interestingly, transfection of one or two of these genes dramatically induces expression of Sp1 and Sp3 but not Sp4. This suggests that the process of cell transformation is accompanied by early induction of Sp1 and Sp3 prior to conversion of the muscle cell into a cancer cell [50].
The role of Sp TFs in the process of transformation has also been investigated in cancer stem cells, where they directly regulate genes associated with “stemness” or cooperate with other genes and non-coding RNAs to enhance stemness. At present, there is strong evidence for the role of Sp1 in inducing stemness, and the cooperating factors vary with tumor type. Stemness in breast cancer is maintained by the long non-coding RNA408 (Lnc408)—dependent recruitment of Sp3 to CBY1 gene promoters to inhibit expression of CBY1, which indirectly enhances levels of nuclear β-catenin and β-catenin regulated cancer stem cell-related genes [51]. In gastric cancer, Sp1 regulates expression of leucine-rich repeat-containing receptor 5 (LGR5), a key stem cell factor [52], and in hepatocellular carcinoma, Sp1 induced LncRNA DPPA2 upstream binding RNA (DUBR) [53]. DUBR not only promotes stemness, but also oxaliplatin resistance through an Sp1/DUBR/E2F1-CIP2A axis. The cancer stem-cell-related protein BMI1 is overexpressed in lung cancer and is important for maintaining this phenotype and resistance to pemetrexed [54]. BMI1 also regulates Sp1 expression and knockdown of Sp1 or treatment mithramycin reverses many of the effects of BMI1, including chug resistance. The pro-oncogenic LncRNA HOTAIR interacts with and upregulates Sp1, which induces DNMI1, and transcriptional repression of miR-199a-5p and targeting downregulation of Sp1 or DNMI1 was found to decrease stemness and progression of cutaneous squamous cell carcinoma [55]. In papillary thyroid carcinoma, the LncRNA DOCK9-AS2 interacts with and induces Sp1, which in turn induces β-catenin, which is further induced by DOCK9-AS2 interacting with miR-1972, resulting in increased β-catenin and Wnt signaling [56]. Sp1 is overexpressed in glioblastoma cells [18][19][20] and plays a role in maintaining stemness and drug resistance in this tumor type. It was also reported that ANGPTL4 and Sp4 were overexpressed in GBM and predicted poor patient prognosis [57]. Sp4 also regulates ANGPTL4 and downstream EGFR/AKT/4E-BP1, which is associated with temozolomide resistance and expression of cancer stem cell markers. Drug resistance and stemness in GBM were also associated with Sp1 in another study [58] and in glioma, HDAC/Sp1 regulation of BMI1 enhanced stemness [59]; this exhibited some overlap with lung cancer cells and BMI1 [54].
4. Sp TFs and Regulation of Protein-Encoding Genes in Cancer Cells
In 1983–1984, Tjian and coworkers initially identified Sp1 as a factor that stimulated SV40 early promoter transcription by 40-fold and bound to GC-rich elements in target gene promoters [60][61]. This same group also identified Sp2 as another TF that bound SV40 [60], and approximately a decade later, Sp3 and Sp4 were also characterized [62][63][64][65][66] as a structurally related sub-class of the Sp/KLF family. Subsequent research has demonstrated that Sp1-Sp4 TFs directly regulate or co-regulate thousands of protein-encoding genes associated with cell proliferation, survival, migration and invasion [7]. A detailed study of the role of Sp1, Sp3 and Sp4 in cancer was investigated in multiple cancer cell lines by individual knockdown of the three genes and their combination coupled with analysis of the resulting functional and genomic effects and their overlap [66]. Knockdown of Sp1 (siSp1), Sp3 (siSp3) and Sp4 (siSp4) and their combination (siSp1, 3, 4) decreased growth, increased Annexin V staining (apoptosis) and decreased invasion in A549 lung, MiaPaca2 (pancreatic), SW480 (colon), 786-0 (kidney), SKBR3 (breast), MDA-MB231 (breast), Panc1 (pancreatic) and L3.6 pL (pancreatic) cancer cells. Knockdown efficiencies were high and cell context-dependent differences in functional response potencies were < three-fold for most responses. For most responses, cells deficient in Sp1, Sp3 and Sp4 (triple knockdown) exhibited the highest effect on growth inhibition, induction of Annexin V staining and inhibition of invasion; however, the magnitude of the differences between single and triple knockdown was relatively modest. These results indicate that Sp1, Sp3 and Sp4 individually regulate proliferation, survival and invasion of cancer cells and the loss of one of these TFs is not compensated or rescued by the other two. One possible explanation is that Sp1, Sp3 and Sp4 cooperatively regulate many of the same pro-oncogenic genes and loss of a single TF compromises any possible rescue by the other two.
The highly invasive Panc1 pancreatic cancer cell line was used as a model to investigate the differential expression of genes after knockdown of Sp1, Sp3 and Sp4. Figure 2 illustrates the number of DEGs after knockdown of Sp1, Sp3 and Sp4, including 3532, 4826 and 4232 genes, respectively. Further analysis shows that the common DEGs after knockdown of Sp1/Sp3, Sp1/Sp4 and Sp3/Sp4 were 1113, 1140 and 2753, respectively, indicating that pairs of the three Sp TFs regulated a relatively high percentage of genes in common. This was particularly true for Sp3/Sp4, in which 2753 genes were commonly regulated by both transcription factors, which includes 57 and 64% of all Sp3 and Sp4 regulated genes, respectively. This would suggest that particularly for Sp3 and Sp4 and also the other pairs (Sp1/Sp3, Sp1/Sp4), there may be significant cooperative regulation of genes that requires more than one Sp TF. As demonstrated in Figure 2 and Figure 3, Sp1, Sp3 and Sp4 regulate expression of several thousand genes, with many of them associated with cancer proliferation, survival, and migration/invasion. Moreover, the three transcription factors also regulate genes in common and also genes that are Sp- specific and vary with cell context. Sp (Sp1, Sp3 and Sp4) regulated genes include epidermal growth factor receptor 1 (EGFR), other tyrosine kinases, cMyc, bcl2, survivin, vascular endothelial growth factor receptors (VEGFR1 and VEGFR2), matrix metalloproteinases and many other genes.
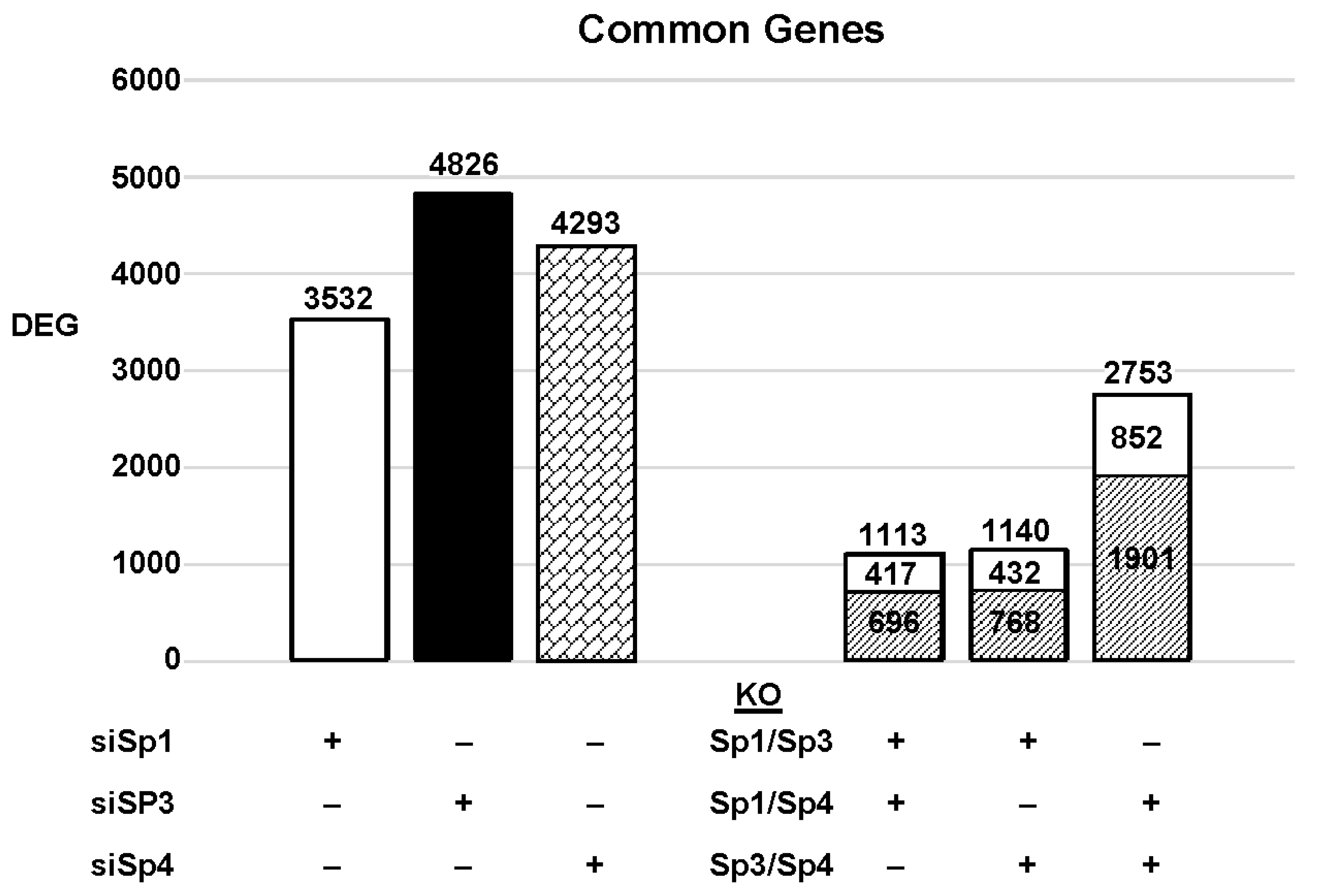
Figure 2. Sp knockdown and changes in gene expression. Panc1 cells were transfected with siRNAs, and after Sp knockdown, the changes in gene expression and the genes commonly induced/repressed by siSp1/siSp3, siSp1/siSp4, and siSp4/siSp3 were determined ( : decreased and
: decreased and  : increased expression in the double knockout groups).
: increased expression in the double knockout groups).
 : decreased and
: decreased and  : increased expression in the double knockout groups).
: increased expression in the double knockout groups).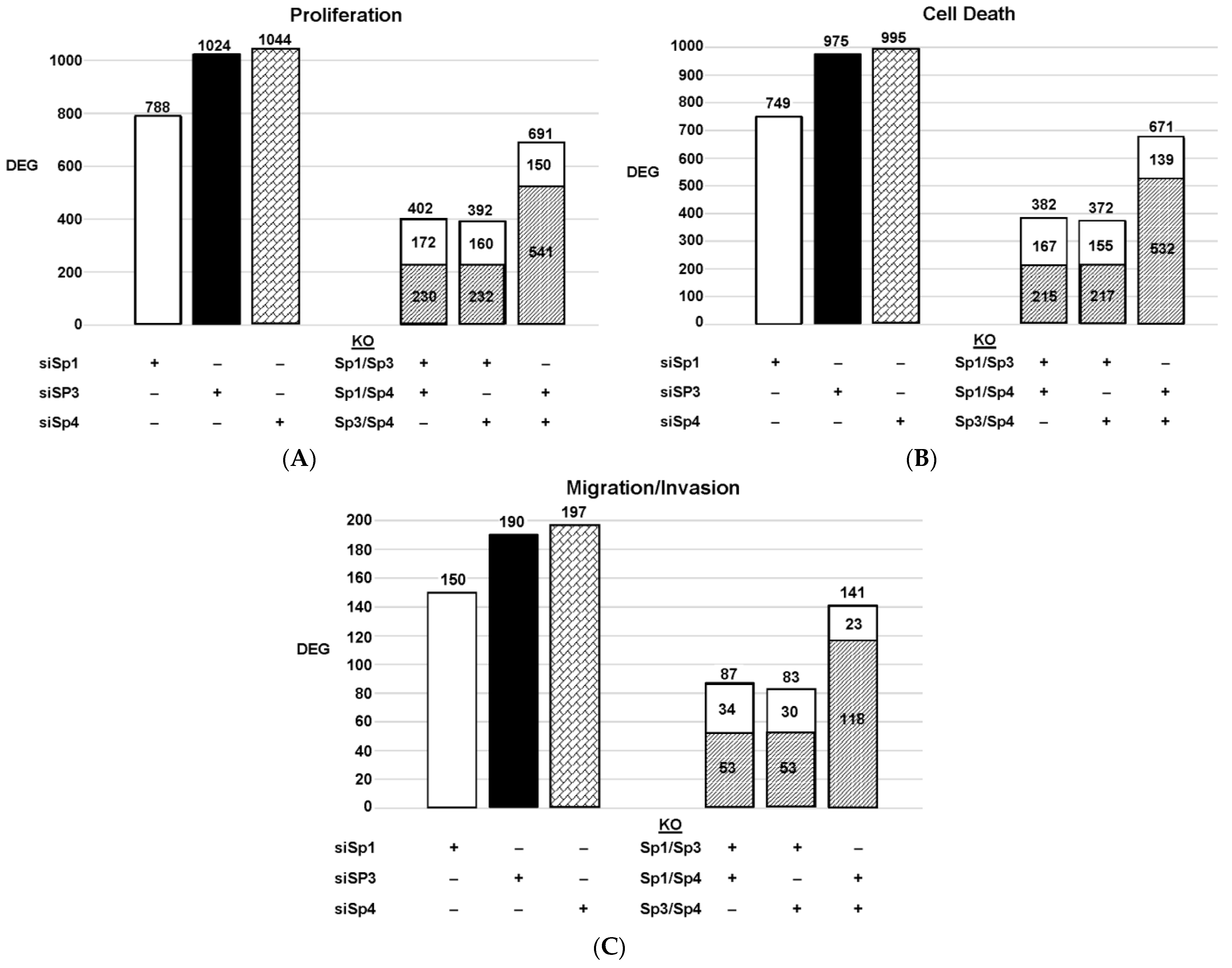
Figure 3. Effects of Sp knockdown by RNAi: IPA analysis of the differentially expressed genes in Panc1 cells associated with cell proliferation (A), Annexin V staining (B), and invasion (C). In these same samples, the common genes observed after knockdown of Sp1/Sp3, Sp1/Sp4 and Sp3/Sp4 are given. ( : decreased and
: decreased and  : increased expression in the double knockout group).
: increased expression in the double knockout group).
 : decreased and
: decreased and  : increased expression in the double knockout group).
: increased expression in the double knockout group).Since Sp TF regulate genes associated with cell proliferation, survival, and invasion, we used ingenuity pathway analysis (IPA) to analyze DEGs for each pathway after knockdown of individual Sps and their combination. The relative expressions of DEGs were determined and the results are illustrated in Figure 3. The patterns of DEGs associated with Panc1 cell proliferation, survival, and invasion after knockdown of Sp1, Sp3 and Sp4 were similar; however, the number of genes involved followed the order of proliferation ≥cell death > invasion. In addition, the pattern of the number of DEGs commonly expressed by Sp1/Sp3, Sp1/Sp4 and Sp3/Sp4 associated with cell proliferation, survival and invasion was higher than that observed for the total genes. The percentage of common genes/total genes was the highest for Sp3/Sp4, where the percentages were 67%, 68% and 74% (Sp3), and 66%, 67% and 72% (Sp4) for cell proliferation, survival, and invasion respectively. Casual IPA analysis also confirmed by their z scores (>2.0 or <−2.0) that the DEGs in each group were strongly associated with the functional responses.
There is evidence from the large number of publications that not only do Sp1, Sp3 and Sp4 regulate pro-oncogenic pathways and genes, but there are also reports that Sp2 performs similar functions [13][67][68]. For example, Sp2 knockdown in hepatocellular carcinoma cells decreases cell migration, proliferation and survival of hepatocellular carcinoma cells and this is due, in part, to decreasing the expression of the TRIB3 gene [13]. Additionally, Sp2-dependent suppression carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) [67] and overexpression of Sp2 increase susceptibility to wound- and carcinogen-induced tumorigenesis [68]. Thus, Sp1-Sp4 regulation of protein-encoding genes plays an important role in cell transformation and tumorigenesis.
References
- Kim, C.-K.; He, P.; Bialkowska, A.B.; Yang, V.W. SP and KLF Transcription Factors in Digestive Physiology and Diseases. Gastroenterology 2017, 152, 1845–1875.
- Beishline, K.; Azizkhan-Clifford, J. Sp1 and the ‘hallmarks of cancer’. FEBS J. 2015, 282, 224–258.
- Li, L.; Davie, J.R. The role of Sp1 and Sp3 in normal and cancer cell biology. Ann. Anat. Anat. Anzeiger. 2010, 192, 275–283.
- Safe, S.; Abbruzzese, J.; Abdelrahim, M.; Hedrick, E. Specificity Protein Transcription Factors and Cancer: Opportunities for Drug Development. Cancer Prev. Res. 2018, 11, 371–382.
- Orzechowska-Licari, E.J.; LaComb, J.F.; Mojumdar, A.; Bialkowska, A.B. SP and KLF Transcription Factors in Cancer Metabolism. Int. J. Mol. Sci. 2022, 23, 9956.
- Vizcaíno, C.; Mansilla, S.; Portugal, J. Sp1 transcription factor: A long-standing target in cancer chemotherapy. Pharmacol. Ther. 2015, 152, 111–124.
- Hedrick, E.; Cheng, Y.; Jin, U.-H.; Kim, K.; Safe, S. Specificity protein (Sp) transcription factors Sp1, Sp3 and Sp4 are non-oncogene addiction genes in cancer cells. Oncotarget 2016, 7, 22245–22256.
- D’Alessio, J.A.; Ng, R.; Willenbring, H.; Tjian, R. Core promoter recognition complex changes accompany liver development. Proc. Natl. Acad. Sci. USA 2011, 108, 3906–3911.
- Williams, A.O.; Isaacs, R.J.; Stowell, K.M. Down-regulation of human topoisomerase IIalpha expression correlates with relative amounts of specificity factors Sp1 and Sp3 bound at proximal and distal promoter regions. BMC Mol. Biol. 2007, 8, 36.
- Francis, P.A.; Pagani, O.; Fleming, G.F.; Walley, B.A.; Colleoni, M.; Láng, I.; Gómez, H.L.; Tondini, C.; Ciruelos, E.; Burstein, H.J.; et al. Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer. N. Engl. J. Med. 2018, 379, 122–137.
- Liu, L.; Ji, P.; Qu, N.; Pu, W.L.; Jiang, D.W.; Liu, W.Y.; Li, Y.-Q.; Shi, R.-L. The impact of high co-expression of Sp1 and HIF1α on prognosis of patients with hepatocellular cancer. Oncol. Lett. 2016, 12, 504–512.
- Kong, L.-M.; Yao, L.; Lu, N.; Dong, Y.-L.; Zhang, J.; Wang, Y.-Q.; Liu, L.; Zhang, H.-L.; Huang, J.-G.; Liao, C.-G. Interaction of KLF6 and Sp1 regulates basigin-2 expression mediated proliferation, invasion and metastasis in hepatocellular carcinoma. Oncotarget 2016, 7, 27975.
- Yue, Z.; Jie, C.; Jiatao, L.; Wei, H.; Wei, W.; Guoping, S. Sp2 promotes invasion and metastasis of hepatocellular carcinoma by targeting TRIB3 protein. Cancer Med. 2020, 9, 3592–3603.
- Bedolla, R.G.; Gong, J.; Prihoda, T.J.; Yeh, I.T.; Thompson, I.M.; Ghosh, R.; Kumar, A.P. Predictive Value of Sp1/Sp3/FLIP Signature for Prostate Cancer Recurrence. PLoS ONE 2012, 7, e44917.
- Gu, L.; Sang, M.; Li, J.; Liu, F.; Wu, Y.; Liu, S.; Wang, P.; Shan, B. Expression and prognostic significance of MAGE-A11 and transcription factors (SP1,TFCP2 and ZEB1) in ESCC tissues. Pathol. Res. Pract. 2019, 215, 152446.
- Chen, Y.-T.; Tsai, H.-P.; Wu, C.-C.; Chen, C.-Y.; Chai, C.-Y.; Kwan, A.-L. High-level Sp1 is Associated with Proliferation, Invasion, and Poor Prognosis in Astrocytoma. Pathol. Oncol. Res. 2019, 25, 1003–1013.
- Zhu, J.; Lu, Z.; Ke, M.; Cai, X. Sp1 is overexpressed and associated with progression and poor prognosis in bladder urothelial carcinoma patients. Int. Urol. Nephrol. 2022, 54, 1505–1512.
- Guan, H.; Cai, J.; Zhang, N.; Wu, J.; Yuan, J.; Li, J.; Li, M. Sp1 is upregulated in human glioma, promotes MMP-2-mediated cell invasion and predicts poor clinical outcome. Int. J. Cancer 2012, 130, 593–601.
- Dong, Q.; Cai, N.; Tao, T.; Zhang, R.; Yan, W.; Li, R.; Zhang, J.; Luo, H.; Shi, Y.; Luan, W.; et al. An Axis Involving SNAI1, microRNA-128 and SP1 Modulates Glioma Progression. PLoS ONE 2014, 9, e98651.
- Yu, Y.; Cao, F.; Xiong, Y.; Zhou, H. SP1 transcriptionally activates NLRP6 inflammasome and induces immune evasion and radioresistance in glioma cells. Int. Immunopharmacol. 2021, 98, 107858.
- Essafi-Benkhadir, K.; Grosso, S.; Puissant, A.; Robert, G.; Essafi, M.; Deckert, M.; Chamorey, E.; Dassonville, O.; Milano, G.; Auberger, P.; et al. Dual Role of Sp3 Transcription Factor as an Inducer of Apoptosis and a Marker of Tumour Aggressiveness. PLoS ONE 2009, 4, e4478.
- Jiang, N.Y.; Woda, B.A.; Banner, B.F.; Whalen, G.F.; Dresser, K.A.; Lu, D. Sp1, a New Biomarker That Identifies a Subset of Aggressive Pancreatic Ductal Adenocarcinoma. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1648–1652.
- Hu, J.; Hu, H.; Hang J-j Yang H-y Wang Z-y Wang, L.; Chen, D.-H.; Wang, L.-W. Simultaneous high expression of PLD1 and Sp1 predicts a poor prognosis for pancreatic ductal adenocarcinoma patients. Oncotarget 2016, 7, 78557–78565.
- Kim, I.-k.; Lee, Y.S.; Kim, H.S.; Dong, S.M.; Park, J.S.; Yoon, D.S. Specific protein 1(SP1) regulates the epithelial-mesenchymal transition via lysyl oxidase-like 2(LOXL2) in pancreatic ductal adenocarcinoma. Sci. Rep. 2019, 9, 5933.
- Liu, X.-b.; Wang, J.; Li, K.; Fan, X.-N. Sp1 promotes cell migration and invasion in oral squamous cell carcinoma by upregulating Annexin A2 transcription. Mol. Cell. Probes 2019, 46, 101417.
- Wang, L.; Wei, D.; Huang, S.; Peng, Z.; Le, X.; Wu, T.T.; Yao, J.; Ajani, J.; Xie, K. Transcription factor Sp1 expression is a significant predictor of survival in human gastric cancer. Clin. Cancer Res. 2003, 9, 6371–6380.
- Lee, H.S.; Park, C.-K.; Oh, E.; Erkin, Ö.C.; Jung, H.S.; Cho, M.-H.; Kwon, M.J.; Chae, S.W.; Kim, S.-H.; Wang, L.-H.; et al. Low SP1 Expression Differentially Affects Intestinal-Type Compared with Diffuse-Type Gastric Adenocarcinoma. PLoS ONE 2013, 8, e55522.
- Yao, J.C.; Wang, L.; Wei, D.; Gong, W.; Hassan, M.; Wu, T.-T.; Mansfield, P.; Ajani, J.; Xie, K. Association between Expression of Transcription Factor Sp1 and Increased Vascular Endothelial Growth Factor Expression, Advanced Stage, and Poor Survival in Patients with Resected Gastric Cancer. Clin. Cancer Res. 2004, 10, 4109–4117.
- Zhang, J.; Zhu, Z.-G.; Ji, J.; Yuan, F.; Yu, Y.-Y.; Liu, B.-Y.; Lin, Y.-Z. Transcription factor Sp1 expression in gastric cancer and its relationship to long-term prognosis. World J. Gastroenterol. 2005, 11, 2213–2217.
- Chen, J.-J.; Ren, Y.-L.; Shu, C.-J.; Zhang, Y.; Chen, M.-J.; Xu, J.; Li, J.; Li, A.-P.; Chen, D.-Y.; He, J.-D.; et al. JP3, an antiangiogenic peptide, inhibits growth and metastasis of gastric cancer through TRIM25/SP1/MMP2 axis. J. Exp. Clin. Cancer Res. 2020, 39, 118.
- Maurer, G.D.; Leupold, J.H.; Schewe, D.M.; Biller, T.; Kates, R.E.; Hornung, H.-M.; Lau-Werner, U.; Post, S.; Allgayer, H. Analysis of Specific Transcriptional Regulators as Early Predictors of Independent Prognostic Relevance in Resected Colorectal Cancer. Clin. Cancer Res. 2007, 13, 1123–1132.
- Wang, F.; Ma, Y.-L.; Zhang, P.; Shen, T.-Y.; Shi, C.-Z.; Yang, Y.-Z.; Moyer, M.-P.; Zhang, H.-Z.; Chen, H.-Q.; Liang, Y.; et al. SP1 mediates the link between methylation of the tumour suppressor miR-149 and outcome in colorectal cancer. J. Pathol. 2013, 229, 12–24.
- Li, L.; Gao, P.; Li, Y.; Shen, Y.; Xie, J.; Sun, D.; Xue, A.; Zhao, Z.; Xu, Z.; Zhang, M.; et al. JMJD2A-dependent silencing of Sp1 in advanced breast cancer promotes metastasis by downregulation of DIRAS3. Breast Cancer Res. Treat. 2014, 147, 487–500.
- Wang, X.B.; Peng, W.Q.; Yi, Z.J.; Zhu, S.L.; Gan, Q.H. Expression and prognostic value of transcriptional factor sp1 in breast cancer. Ai Zheng 2007, 26, 996–1000.
- Kim, J.-Y.; Jung, H.H.; Ahn, S.; Bae, S.; Lee, S.K.; Kim, S.W.; Lee, J.E.; Nam, S.J.; Ahn, J.S.; Im, Y.-H.; et al. The relationship between nuclear factor (NF)-κB family gene expression and prognosis in triple-negative breast cancer (TNBC) patients receiving adjuvant doxorubicin treatment. Sci. Rep. 2016, 6, 31804.
- Zhao, Y.; Yao, D.; Li, Y.; Zhang, S.; Tao, Z.; Zhang, L.; Hu, X.; Wang, B.; Chen, S. Loss of polarity protein Par3 is mediated by transcription factor Sp1 in breast cancer. Biochem. Biophys. Res. Commun. 2021, 561, 172–179.
- Hsu, T.I.; Wang, M.C.; Chen, S.Y.; Yeh, Y.M.; Su, W.C.; Chang, W.C.; Hung, J.-J. Sp1 expression regulates lung tumor progression. Oncogene 2012, 31, 3973–3988.
- Kong, L.-M.; Liao, C.-G.; Fei, F.; Guo, X.; Xing, J.-L.; Chen, Z.-N. Transcription factor Sp1 regulates expression of cancer-associated molecule CD147 in human lung cancer. Cancer Sci. 2010, 101, 1463–1470.
- Zhang, H.-W.; Wang, E.-W.; Li, L.-X.; Yi, S.-H.; Li, L.-C.; Xu, F.-L.; Wang, D.-L.; Wu, Y.-Z.; Nian, W.-Q. A regulatory loop involving miR-29c and Sp1 elevates the TGF-β1 mediated epithelial-to-mesenchymal transition in lung cancer. Oncotarget 2016, 7, 85905–85916.
- Cui, P.-H.; Li, Z.-Y.; Li, D.-H.; Han, S.-Y.; Zhang, Y.-J. SP1-induced lncRNA DANCR contributes to proliferation and invasion of ovarian cancer. Kaohsiung J. Med. Sci. 2021, 37, 371–378.
- Shi, S.; Zhang, Z.G. Role of Sp1 expression in gastric cancer: A meta-analysis and bioinformatics analysis. Oncol. Lett. 2019, 18, 4126–4135.
- Chen, M.; Gao, Y.; Gan, K.; Liu, K.; Xu, B. SP1 Expression and the Clinicopathological Features of Tumors: A Meta-Analysis and Bioinformatics Analysis. Pathol. Oncol. Res. 2021, 27, 581998.
- Lou, Z.; O’Reilly, S.; Liang, H.; Maher, V.M.; Sleight, S.D.; McCormick, J.J. Down-regulation of overexpressed sp1 protein in human fibrosarcoma cell lines inhibits tumor formation. Cancer Res. 2005, 65, 1007–1017.
- McCormick, J.J.; Maher, V.M. Malignant Transformation of Human Skin Fibroblasts by Two Alternative Pathways. In Human Cell Transformation: Role of Stem Cells and the Microenvironment; Rhim, J.S., Kremer, R., Eds.; Springer: New York, NY, USA, 2012; pp. 191–207.
- Jin, H.; Xu, J.; Guo, X.; Huang, H.; Li, J.; Peng, M.; Zhu, J.; Tian, Z.; Wu, X.-R.; Tang, M.-S.; et al. XIAP RING domain mediates miR-4295 expression and subsequently inhibiting p63α protein translation and promoting transformation of bladder epithelial cells. Oncotarget 2016, 7, 56540–56557.
- Zhong, X.; Zheng, L.; Shen, J.; Zhang, D.; Xiong, M.; Zhang, Y.; He, X.; Tanyi, J.L.; Yang, F.; Montone, K.T.; et al. Suppression of MicroRNA 200 Family Expression by Oncogenic KRAS Activation Promotes Cell Survival and Epithelial-Mesenchymal Transition in KRAS-Driven Cancer. Mol. Cell. Biol. 2016, 36, 2742–2754.
- Kwon, Y.-J.; Baek, H.-S.; Ye, D.-J.; Shin, S.; Kim, D.; Chun, Y.-J. CYP1B1 Enhances Cell Proliferation and Metastasis through Induction of EMT and Activation of Wnt/β-Catenin Signaling via Sp1 Upregulation. PLoS ONE 2016, 11, e0151598.
- He, J.; Liu, W.; Ge, X.; Wang, G.-C.; Desai, V.; Wang, S.; Mu, W.; Bhardwaj, V.; Seifert, E.; Liu, L.-Z.; et al. Arsenic-induced metabolic shift triggered by the loss of miR-199a-5p through Sp1-dependent DNA methylation. Toxicol. Appl. Pharmacol. 2019, 378, 114606.
- Naini, S.; Etheridge, K.T.; Adam, S.J.; Qualman, S.J.; Bentley, R.C.; Counter, C.M.; Linardic, C.M. Defining the Cooperative Genetic Changes That Temporally Drive Alveolar Rhabdomyosarcoma. Cancer Res. 2008, 68, 9583–9588.
- Chadalapaka, G.; Jutooru, I.; Sreevalsan, S.; Pathi, S.; Kim, K.; Chen, C.; Crose, L.; Linardic, C.; Safe, S. Inhibition of rhabdomyosarcoma cell and tumor growth by targeting specificity protein (Sp) transcription factors. Int. J. Cancer 2013, 132, 795–806.
- Wen, S.; Qin, Y.; Wang, R.; Yang, L.; Zeng, H.; Zhu, P.; Li, Q.; Qiu, Y.; Chen, S.; Liu, Y.; et al. A novel Lnc408 maintains breast cancer stem cell stemness by recruiting SP3 to suppress CBY1 transcription and increasing nuclear β-catenin levels. Cell Death Dis. 2021, 12, 437.
- Wilhelm, F.; Simon, E.; Böger, C.; Behrens, H.-M.; Krüger, S.; Röcken, C. Novel Insights into Gastric Cancer: Methylation of R-spondins and Regulation of LGR5 by SP1. Mol. Cancer Res. 2017, 15, 776–785.
- Liu, S.; Bu, X.; Kan, A.; Luo, L.; Xu, Y.; Chen, H.; Lin, X.; Lai, Z.; Wen, D.; Huang, L.; et al. SP1-induced lncRNA DUBR promotes stemness and oxaliplatin resistance of hepatocellular carcinoma via E2F1-CIP2A feedback. Cancer Lett. 2022, 528, 16–30.
- Shen, H.-T.; Chien, P.-J.; Chen, S.-H.; Sheu, G.-T.; Jan, M.-S.; Wang, B.-Y.; Chang, W.-W. BMI1-Mediated Pemetrexed Resistance in Non-Small Cell Lung Cancer Cells Is Associated with Increased SP1 Activation and Cancer Stemness. Cancers 2020, 12, 2069.
- Chen, J.; Hou, S.-F.; Tang, F.-J.; Liu, D.-S.; Chen, Z.-Z.; Zhang, H.-L.; Wang, S.-H. HOTAIR/Sp1/miR-199a critically regulates cancer stemness and malignant progression of cutaneous squamous cell carcinoma. Oncogene 2022, 41, 99–111.
- Dai, W.; Jin, X.; Han, L.; Huang, H.; Ji, Z.; Xu, X.; Tang, M.; Jiang, B.; Chen, W. Exosomal lncRNA DOCK9-AS2 derived from cancer stem cell-like cells activated Wnt/β-catenin pathway to aggravate stemness, proliferation, migration, and invasion in papillary thyroid carcinoma. Cell Death Dis. 2020, 11, 743.
- Tsai, Y.-T.; Wu, A.-C.; Yang, W.-B.; Kao, T.-J.; Chuang, J.-Y.; Chang, W.-C.; Hsu, T.-I. ANGPTL4 Induces TMZ Resistance of Glioblastoma by Promoting Cancer Stemness Enrichment via the EGFR/AKT/4E-BP1 Cascade. Int. J. Mol. Sci. 2019, 20, 5625.
- Chang, K.-Y.; Huang, C.-T.; Hsu, T.-I.; Hsu, C.-C.; Liu, J.-J.; Chuang, C.-K.; Hung, J.-J.; Chang, W.-C.; Tsai, K.K.; Chuang, J.-Y. Stress stimuli induce cancer-stemness gene expression via Sp1 activation leading to therapeutic resistance in glioblastoma. Biochem. Biophys. Res. Commun. 2017, 493, 14–19.
- Tsai, Y.-T.; Wu, C.-C.; Ko, C.-Y.; Hsu, T.-I.; Chang, W.-C.; Lo, W.-L.; Chuang, J.-Y. Correlation between the expression of cancer stem cell marker BMI1 and glioma prognosis. Biochem. Biophys. Res. Commun. 2021, 550, 113–119.
- Dynan, W.S.; Tjian, R. Isolation of transcription factors that discriminate between different promoters recognized by RNA polymerase II. Cell 1983, 32, 669–680.
- Gidoni, D.; Dynan, W.S.; Tjian, R. Multiple specific contacts between a mammalian transcription factor and its cognate promoters. Nature 1984, 312, 409–413.
- Kingsley, C.; Winoto, A. Cloning of GT box-binding proteins: A novel Sp1 multigene family regulating T-cell receptor gene expression. Mol. Cell. Biol. 1992, 12, 4251–4261.
- Hagen, G.; Müller, S.; Beato, M.; Suske, G. Sp1-mediated transcriptional activation is repressed by Sp3. EMBO J. 1994, 13, 3843–3851.
- Hagen, G.; Dennig, J.; Preiß, A.; Beato, M.; Suske, G. Functional Analyses of the Transcription Factor Sp4 Reveal Properties Distinct from Sp1 and Sp3. J. Biol. Chem. 1995, 270, 24989–24994.
- Kalff-Suske, M.; Kunz, J.; Grzeschik, K.H.; Suske, G. Human Sp4 transcription factor gene (SP4) maps to chromosome 7p15. Genomics 1995, 26, 631–633.
- Kalff-Suske, M.; Kunz, J.; Grzeschik, K.H.; Suske, G. Human Sp3 transcriptional regulator gene (SP3) maps to chromosome 2q31. Genomics 1996, 37, 410–412.
- Phan, D.; Cheng, C.-J.; Galfione, M.; Vakar-Lopez, F.; Tunstead, J.; Thompson, N.E.; Burgess, R.R.; Najjar, S.M.; Yu-Lee, L.-Y.; Lin, S.-H. Identification of Sp2 as a Transcriptional Repressor of Carcinoembryonic Antigen-Related Cell Adhesion Molecule 1 in Tumorigenesis. Cancer Res. 2004, 64, 3072–3078.
- Kim, T.-H.; Chiera, S.L.; Linder, K.E.; Trempus, C.S.; Smart, R.C.; Horowitz, J.M. Overexpression of Transcription Factor Sp2 Inhibits Epidermal Differentiation and Increases Susceptibility to Wound- and Carcinogen-Induced Tumorigenesis. Cancer Res. 2010, 70, 8507–8516.
More
Information
Subjects:
Oncology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
510
Revisions:
2 times
(View History)
Update Date:
23 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




