
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rama Bansil | -- | 2170 | 2023-03-18 20:37:29 | | | |
| 2 | Catherine Yang | + 1 word(s) | 2171 | 2023-03-20 02:16:28 | | | | |
| 3 | Catherine Yang | -3 word(s) | 2168 | 2023-03-20 09:18:44 | | | | |
| 4 | Catherine Yang | Meta information modification | 2168 | 2023-03-20 09:19:25 | | | | |
| 5 | Rama Bansil | -31 word(s) | 2137 | 2023-03-20 16:45:40 | | |
Video Upload Options
Helicobacter spp., including the well-known human gastric pathogen H. pylori, can cause gastric diseases in humans and other mammals. They are Gram-negative bacteria that colonize the gastric epithelium and use their multiple flagella to move across the protective gastric mucus layer. Different Helicobacter spp. differ in the number and arrangement of flagella, as well as in the size and shape of their cell body. The most well-studied of these is H. pylori, which is unipolar and lophotrichous (multiple flagella at one pole). Some of these bacteria are bipolar, e.g., the lophotrichous H. suis, and the monotrichous H. cetorum. The motility of H. pylori, H. suis, and H. cetorum was described in the review in Microorganisms 2023 linked above.
1. Dependence of H. pylori Swimming on Cell Shape and Number of Flagella
The motility and chemotaxis of H. pylori have been discussed in earlier reviews focused on explaining the flagella and chemotaxis molecular machinery [1]. By tracking bacteria microscopically several researchers examined the motility of H. pylori. These studies show that the swimming characteristics of H. pylori depend on the shape of the cell, the size of the bacterium, and the number of flagella. Early studies examined the swimming of H. pylori in viscous polymer solutions to mimic the viscous mucus environment [2] and compared the helical H. pylori with straight–rod E. coli to address the effect of the helical shape [3]. Karim et al. [3] showed that both H. pylori and C. jejuni swam faster than E. coli in aqueous cultures, presumably due to their helical body shape [3]. Martinez et al. [4] examined the effect of the helical shape of H. pylori in detail, using time-resolved phase contrast microscopy to compare the swimming of wild-type, helical H. pylori strains with isogenic, straight-rod mutants (Δcsd4 and Δcsd6) which lacked the helical-shape-determining peptidoglycan peptidases, Csd4 or Csd6 [5]. They noted that the helical shape of the cell body confers about a 7–20% advantage in swimming speed, depending on the strain. They also found that the swimming speed of H. pylori varies considerably among different strains due to differences in cell size and the number of flagella. The distribution of speeds in any given sample is very broad due to variations in cell size, shape, and the number of flagella, as well as temporal changes in speed during swimming. To examine the effect of varying the number of flagella, they compared the wild-type B128 strain of H. pylori (median number of flagella = 3) with its isogenic flagellar mutants, fliOΔC (median 1 flagellum) and sRNA_T (median 4 flagella). They observed that the speed was increased in the sRNA_T mutant, which has an extra flagellum compared to the wild-type, and decreased in the case of fliOΔC, which has on average only one flagellum [4].
Bacteria tracking studies show that H. pylori exhibit a run-reverse-reorient swimming mechanism [6][7][8][9][10] in aqueous broth as well as in viscous solutions. In the case of H. pylori, an unbundled state of tumbling or re-orientation can occur [6][9][10] between the forward and reverse runs. The analysis of trajectories using the methods developed by Theves et al. [11] to obtain the distributions of run speeds, change in orientation angle, and reversal frequency shows that the reversal frequency decreases for H. pylori swimming in viscous methylcellulose and porcine gastric mucin (PGM) solutions compared to Brucella broth (BB10) [6].
2. Motility of the Bipolarly Flagellated H. suis

3. Motility of H. cetorum, a Monotrichous Bipolar Fusiform Bacterium
4. Comparison of the Motility of H. pylori, H. suis, and H. cetorum
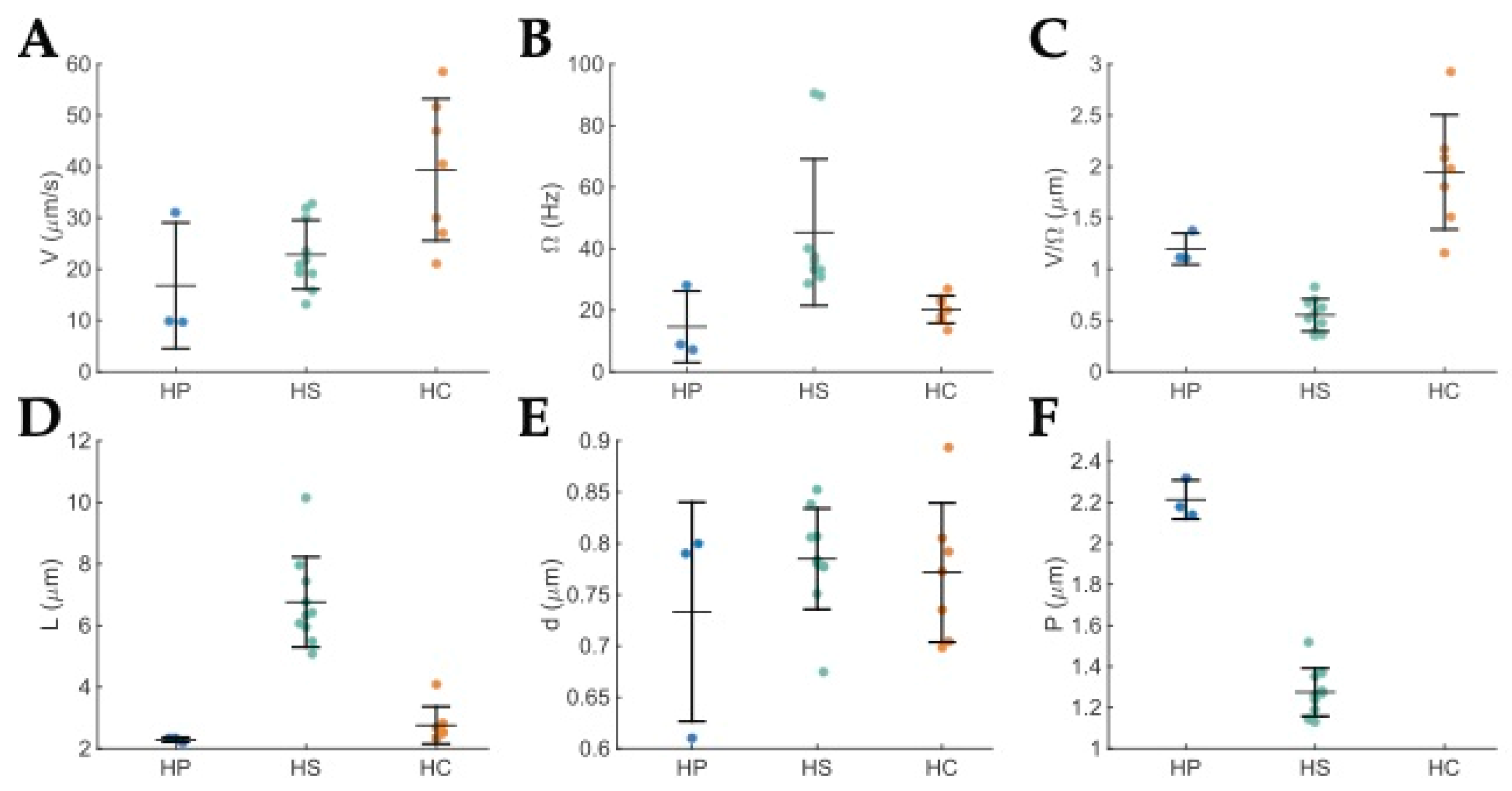
| H. pylori | H. suis | H. cetorum | |
|---|---|---|---|
| V (μm/s) | 17 ± 12 | 23 ± 7 | 39 ± 14 |
| Ω (s−1) | 15 ± 12 | 45± 24 | 20 ± 4 |
| V/Ω (μm) | 1.2 ± 0.2 | 0.6 ±0.2 | 1.9 ± 0.6 |
| L (μm) | 2.29 ± 0.08 | 7 ± 1 | 2.8 ± 0.6 |
| d (μm) | 0.7 ± 0.1 | 0.8 ± 0.05 | 0.77 ± 0.07 |
| P (μm) | 2.21 ± 0.09 | 0.8 ± 0.07 | Not measured |
References
- Lertsethtakarn, P.; Ottemann, K.M.; Hedrixson, D.R. Motility and chemotaxis in Campylobacter and Helicobacter. Annu. Rev. Microbiol. 2011, 65, 389–410.
- Yoshiyama, H.; Nakamura, H.; Kimoto, M.; Okita, K.; Nakazawa, T. Chemotaxis and motility of Helicobacter pylori in a viscous environment. J. Gastroenterol. 1999, 34, 18–23.
- Karim, Q.N.; Logan, R.P.; Puels, J.; Karnholz, A.; Worku, M.L. Measurement of motility of Helicobacter pylori, Campylobacter jejuni, and Escherichia coli by real time computer tracking using the Hobson BacTracker. J. Clin. Pathol. 1998, 51, 623–628.
- Martínez, L.E.; Hardcastle, J.M.; Wang, J.; Pincus, Z.; Tsang, J.; Hoover, T.R.; Bansil, R.; Salama, N.R. Helicobacter pylori strains vary cell shape and flagellum number to maintain robust motility in viscous environments. Mol. Microbiol. 2016, 99, 88–110.
- Sycuro, L.K.; Wyckoff, T.J.; Biboy, J.; Born, P.; Pincus, Z.; Vollmer, W.; Salama, N.R. Multiple Peptidoglycan Modification Networks Modulate Helicobacter pylori’s Cell Shape, Motility, and Colonization Potential. PLoS Pathog. 2012, 8, e1002603.
- Martínez, L.E.; Hardcastle, J.M.; Wang, J.; Pincus, Z.; Tsang, J.; Hoover, T.R.; Bansil, R.; Salama, N.R. Helicobacter pylori strains vary cell shape and flagellum number to maintain robust motility in viscous environments. Mol. Microbiol. 2016, 99, 88–110.
- Antani, J.D.; Sumali, A.X.; Lele, T.P.; Lele, P.P. Asymmetric random walks reveal that the chemotaxis network modulates flagellar rotational bias in Helicobacter pylori. eLife 2021, 10, e63936.
- Howitt, M.R.; Lee, J.Y.; Lertsethtakarn, P.; Vogelmann, R.; Joubert, L.M.; Ottemann, K.M.; Amieva, M.R. Chepep controls helicobacter pylori infection of the gastric glands and chemotaxis in the epsilonproteobacteria. MBio 2011, 2, e00098-11.
- Constantino, M.A.; Jabbarzadeh, M.; Fu, H.C.; Bansil, R. Helical and rod-shaped bacteria swim in helical trajectories with little additional propulsion from helical shape. Sci. Adv. 2016, 2, e1601661.
- Su, C.; Bieniek, K.; Liao, W.; Constantino, M.A.; Decker, S.M.; Turner, B.S.; Bansil, R. Comparison of motility of H. pylori in broth and mucin reveals the interplay of effect of acid on the bacterium and the rheology of the medium it swims in. bioRxiv 2020.
- Theves, M.; Taktikos, J.; Zaburdaev, V.; Stark, H.; Beta, C. A bacterial swimmer with two alternating speeds of propagation. Biophys. J. 2013, 105, 1915–1924.
- Worku, M.L.; Sidebotham, R.L.; Baron, H.J.; Misiewicz, J.J.; Logan, R.P.; Keshavarz, T.; Karim, Q.N. Motility of Helicobacter pylori in a viscous environment. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1143–1150.
- Celli, J.P.; Turner, B.S.; Afdhal, N.H.; Keates, S.; Ghiran, I.; Kelly, C.P.; Ewoldt, R.H.; McKinley, G.H.; So, P.T.C.; Erramilli, S.; et al. Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. Proc. Natl. Acad. Sci. USA 2009, 106, 14321–14326.
- Bansil, R.; Celli, J.P.; Hardcastle, J.M.; Turner, B.S. The influence of mucus microstructure and rheology in Helicobacter pylori infection. Front. Immunol. 2013, 4, 310.
- Celli, J.P.; Turner, B.S.; Afdhal, N.H.; Ewoldt, R.H.; Mckinley, G.H.; Bansil, R.; Erramilli, S. Rheology of gastric mucin exhibits a pH-dependent sol-gel transition. Biomacromolecules 2007, 8, 1580–1586.
- Clyne, M.; Labigne, A.; Drumm, B. Helicobacter pylori requires an acidic environment to survive in the presence of urea. Infect. Immun. 1995, 63, 1669–1673.
- Grognot, M.; Taute, K. More than propellers: How flagella shape bacterial motility behaviors. Curr. Opin. Microbiol. 2021, 61, 73–81.
- Thormann, K.M.; Beta, C.; Kühn, J.M. Wrapped up: The Motility of Polarly Flagellated Bacteria. Annu. Rev. Microbiol. 2022, 76, 349–367.
- Murat, D.; Hérisse, M.; Espinosa, L.; Bossa, A.; Alberto, F.; Wu, L.F. Opposite coordinated rotation of amphitrichous flagella governs oriented swimming and reversals in a magnetotactic spirillum. J. Bacteriol. 2015, 197, 3275–3282.
- Hintsche, M.; Waljor, V.; Großmann, R.; Kühn, J.M.; Thormann, K.M.; Peruani, F.; Beta, C. A polar bundle of flagella can drive bacterial swimming by pushing, pulling, or coiling around the cell body. Sci. Rep. 2017, 7, 16771.
- Constantino, M.A.; Jabbarzadeh, M.; Fu, H.C.; Shen, Z.; Fox, J.G.; Haesebrouck, F.; Lindén, S.; Bansil, R. Bipolar lophotrichous Helicobacter suis combine extended and wrapped flagella bundles to exhibit multiple modes of motility. Sci. Rep. 2018, 8, 14415.
- Harper, C.G.; Feng, Y.; Xu, S.; Taylor, N.S.; Kinsel, M.; Dewhirst, F.E.; Paster, B.J.; Greenwell, M.; Levine, G.; Rogers, A.; et al. Helicobacter cetorum sp. nov., a Urease Positive Helicobacter Species Isolated from Dolphins and Whales. J. Clin. Microbiol. 2002, 40, 4536–4543.
- Constantino, M.A. Investigating Effects of Morphology and Flagella Dynamics on Swimming Kinematics of Different Helicobacter Species Using Single-Cell Imaging. Ph.D. Thesis, Boston University, Boston, MA, USA, 2017.




