Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lin Yuan | -- | 1778 | 2023-03-15 10:23:05 | | | |
| 2 | Sirius Huang | Meta information modification | 1778 | 2023-03-16 02:48:31 | | | | |
| 3 | Sirius Huang | Meta information modification | 1778 | 2023-03-17 07:32:09 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Xu, S.; Pan, W.; Song, Z.; Yuan, L. Near-Infrared Fluorescent Probes for Cell Membrane Imaging. Encyclopedia. Available online: https://encyclopedia.pub/entry/42224 (accessed on 03 March 2026).
Xu S, Pan W, Song Z, Yuan L. Near-Infrared Fluorescent Probes for Cell Membrane Imaging. Encyclopedia. Available at: https://encyclopedia.pub/entry/42224. Accessed March 03, 2026.
Xu, Shuai, Wenjing Pan, Zhi-Ling Song, Lin Yuan. "Near-Infrared Fluorescent Probes for Cell Membrane Imaging" Encyclopedia, https://encyclopedia.pub/entry/42224 (accessed March 03, 2026).
Xu, S., Pan, W., Song, Z., & Yuan, L. (2023, March 15). Near-Infrared Fluorescent Probes for Cell Membrane Imaging. In Encyclopedia. https://encyclopedia.pub/entry/42224
Xu, Shuai, et al. "Near-Infrared Fluorescent Probes for Cell Membrane Imaging." Encyclopedia. Web. 15 March, 2023.
Copy Citation
Cell membrane (CM) is a phospholipid bilayer that maintains integrity of a whole cell and relates to many physiological and pathological processes. Developing CM imaging tools is a feasible method for visualizing membrane-related events. Small-molecular fluorescent probes in the near-infrared (NIR) region have been pursued extensively for CM staining to investigate its functions and related events.
cell membrane imaging
small-molecular fluorescent probe
near-infrared imaging
1. Introduction
Cell membrane (CM) is the first barrier that separates interior of cells from the extracellular environment and plays a crucial role in physiological processes, such as signal transduction and biomolecular transport [1][2][3][4]. A CM is an amphipathic bilayer membrane consisting of a mix of lipids and proteins [5][6][7]. It is closely related to signal transduction from the extracellular environment, causing further responses through changes in shape and morphology [8][9][10][11]. CM damage can cause cell swelling and apoptosis, eventually causing various diseases, such as cirrhosis, diabetes and even cancers [12][13][14][15]. Therefore, visualization of a CM enables study of related events and evaluation of cell life status and facilitates diagnosis and treatment of CM-related diseases [16][17].
At present, a certain number of methods have been developed for CM analysis [18][19][20][21]. Among them, fluorescence imaging has become a powerful tool benefiting from its advantages of high sensitivity, simplicity and noninvasiveness [22][23][24][25][26]. Wheat germ agglutinin (WGA) and concanavalin A have served as popular fluorescent CM probes due to their efficiency and ease of use [27][28][29], but these probes are expensive and have large size. Small-molecular fluorescent probes have been widely used for bioimaging due to their easy preparation, modifiable molecular structures and tunable fluorescence signals [30][31][32][33][34]. The small size of this probe enables its precise location in the lipid bilayer, which can serve as a flexible tool for study of CM-related events. Small-molecular fluorescent probes with excitation/emission in the near-infrared (NIR) region (650−900 nm) are much more desirable for bioimaging due to attenuated biological autofluorescence, reduced light damage and increased imaging penetration depth [35][36][37][38]. In recent decades, various NIR CM-targeting molecular probes have been developed for visualization of cell activities, metabolism and cell-to-cell communication on CMs. These probes are commonly designed by incorporating CM-targeting units to NIR fluorophore. In this process, screening of CM-targeting units is very important. Considering the amphipathic characteristics of CMs, designing amphipathic probes that have similarity and intermiscibility to a CM is an effective way to realize CM imaging. NIR fluorophore with large π-conjugation can serve as a lipophilic moiety. In this case, amphipathic CM probes can be obtained by linking hydrophilic moiety, especially positively or negatively charged groups, to NIR fluorophore. Sometimes, long alkyl chains are needed to assist the probe to insert into a CM by hydrophobic interaction with the alkyl chain of a phospholipid [39][40]. In addition to amphipathic characteristics of CMs, many proteins with important biological functions are embedded in CMs. Another approach to realize CM imaging is to design probes that can specifically target these proteins or be self-assembled on the surface of a CM after being activated by these proteins. The recent progress of NIR CM molecular probes for visualization of CM-related events are summarized (Scheme 1). The unique molecular structure and photophysicality of NIR dyes are fully considered in design and application of CM probes. These CM probes are divided into two classes based on different targeting mechanisms.
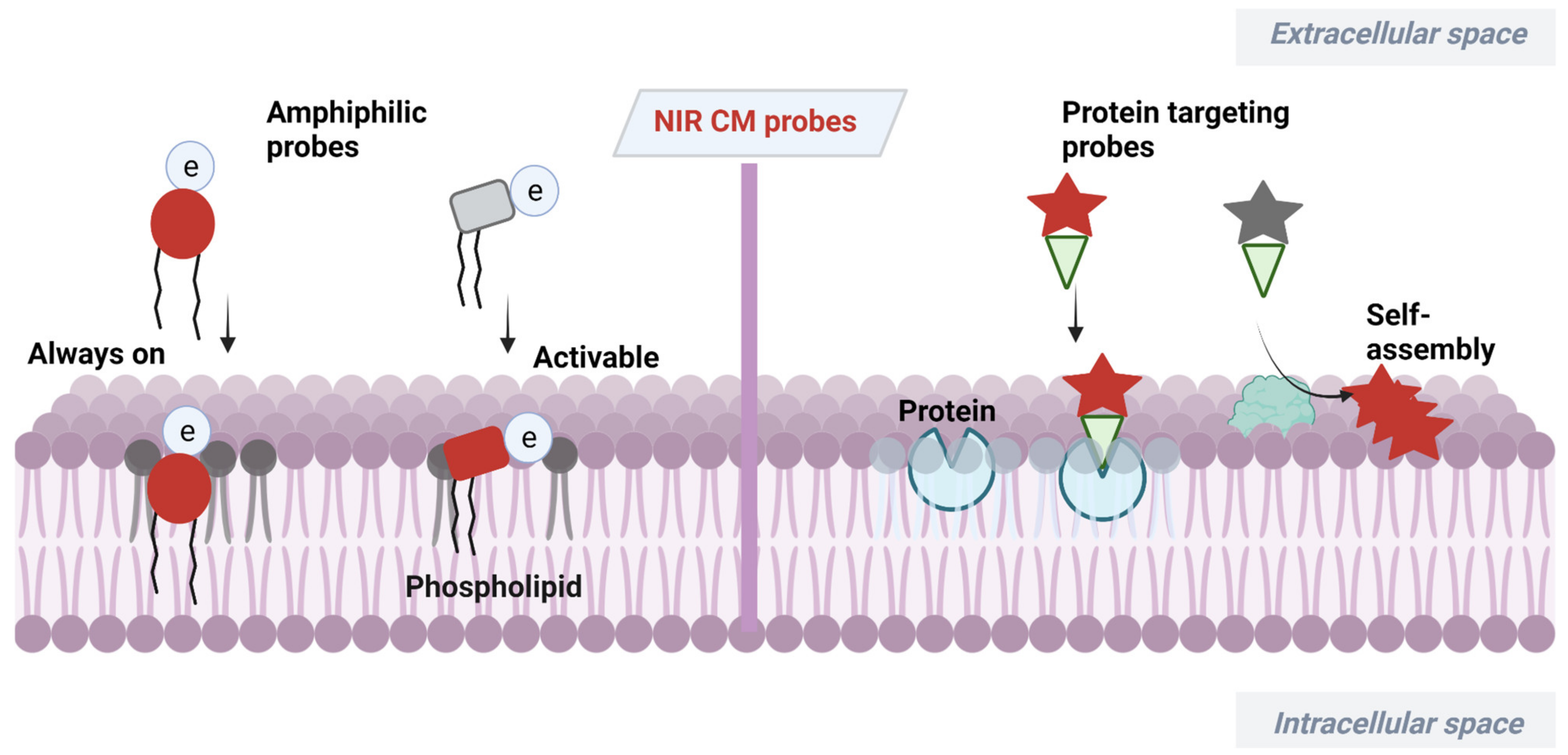
Scheme 1. Design strategies of NIR CM-targeting molecular probes.
2. NIR Molecular Probes for CM Imaging
NIR dyes with large π-conjugate systems usually have strong lipophilicity [41][42]. Owing to the glycerophospholipid bilayer structure of CMs, lipophilic NIR dyes are easily transferred into a lipid bilayer through lipophilic–lipophilic interaction. However, in most cases, lipophilic dye molecules are readily internalized into cells, losing their membrane staining ability. Designing fluorescent probes that mimic the amphiphilic structure of CMs [43] is a feasible way to image bilayer membranes. Many proteins with important biological functions are embedded in CMs of live organisms. Another CM staining approach is to incorporate a membrane proteins targeting group to probes or relying on self-assembly of the probes on a CM after being activated by proteins. Herein, advances in development of NIR fluorescent probes for CM staining have been overviewed, including their design strategies, targeting mechanisms and biological applications.
2.1. Amphiphilic NIR Probes for CM Imaging
The amphiphilic NIR CM probe commonly contains hydrophobic moieties to insert into CM through lipophilic–lipophilic interaction and polar headgroups to prevent probe penetrating into cells. The long alkyl chains are sometimes selected to help a probe stay on a CM. Many NIR CM probes retain emission before and after anchoring on the CM, named as “always-on” NIR CM probes. Using these probes, a CM can be stained and the boundary of cells will be observed. However, in this process, repeated washing procedure is required, hindering the real-time and dynamic imaging of CM. To this end, significant effort has been directed at building activatable CM staining probes. One approach to build such probes is to screen for solvatochromic or fluorogenic NIR dyes that are further covalently linked with polar headgroups. These probes have variable emissions (intensity and wavelength), with the environment changed from polar aqueous condition to low polar membrane lipid, thereby achieving CM imaging in an activatable manner. Another approach to construct activatable CM staining probes is relying on disassembly light-up fluorescence strategy. As mentioned above, NIR dyes with large π-conjunction are prone to be aggregated in aqueous solutions, resulting in quenching of their fluorescence [44]. When being inserted into a CM, however, the aggregates are dissolved and dispersed on the CM, leading to significant fluorescence enhancement. In this case, the washing procedure is no longer required because free probes in culture medium are nonfluorescent. Aggregation-induced emission (AIE) dyes are also selected to construct activatable NIR CM probes. These probes are freely rotating in solutions [45], but their intramolecular rotation can be restricted when inserted into a CM, leading to dramatic fluorescence enhancement.
2.2. Proteins Targeting Probes
Many proteins with important biological functions are embedded in CMs of live organisms. Therefore, design of CM protein targeting probes or probes that can self-assemble on a CM after being activated by proteins is another efficient way for understanding the physiological processes of CMs.
Carbonic anhydrase IX (CA IX), a transmembrane protein induced under hypoxic conditions, is a promising tumor marker. In 2022, Chen et al. developed pH-sensitive CA IX-targeted NIR probe 23 for fluorescence imaging of hypoxic osteosarcoma [46]. This probe was built based on an NIR heptamethine cyanine dye IR783 by connecting with a CA IX targeting group benzenesulfonamide moiety. In buffer solutions, the emission intensity of this probe at 760 nm increased with pH, which was attributed to protonation of amine group under acidic conditions. After incubation of 143B cells with probe 23 for 1 h or even as long as 24 h, this probe was consistently dispersed on CMs, which showed its excellent long-term CM imaging performance. CM imaging of 143B cells under simulated normoxia, hypoxia and acetazolamide conditions showed that probe 23 was able to visualize level changes of biomarker CA IX. Meanwhile, intracellular pH change was also detected by monitoring the fluorescence intensity on CMs. Moreover, in vivo imaging showed that probe 23 had good selectivity for tumors relative to normal tissues, indicating its great potential application in biological and medical fields.
For accurate visualization of prostate cancer boundary and lymphatic metastasis, in 2022, Hu et al. developed self-quenched NIR fluorescence probe 24 with dual prostate cancer membrane affinity [47]. Glutamate–urea–lysine (KUE)-based moieties were introduced into this probe as a ligand for prostate-specific membrane antigen (PSMA), which was overexpressed in >90% of prostate cancer cells. Oleic acid (OA) motif was further introduced as an additional CM-targeting scaffold to maximize the targeting efficiency of probe 24 for prostate cancer. The dual targeted probe was weakly fluorescent in Tris buffer saline due to a strong self-aggregation effect. Upon adding purified PSMA protein into probe solution, an obvious increase in absorbance and fluorescence was observed, indicating that probe 24 could be activated by PSMA. Activation of probe 24 by PSMA was also studied in C4-2PSMA-high cells highly expressing PSMA, and the results showed that the CM was specifically lit up, but its signals were erased regarding cells pre-treated with PSMA inhibitor. In vivo studies revealed that probe 24 could specifically activate in PSMA-positive tumors rather than PSMA-negative tumors, showing its potential for fluorescence-guided accurate and complete resection of prostate tumors.
CyA-B2 molecule (probe 25) was previously selected from library screening for endothelial cells binding. However, the mechanisms of this probe binding on endothelial cells were unclear. In 2022, by studying the competition assays of CyA-B2 using several potential surface markers of endothelial cells, Matsusaki et al. observed that CD133 provided the lowest IC50 value [48]. Therefore, the CD133 protein expressed on endothelial CM was considered as the binding site of probe 25 due to its suppression on blood capillaries by competition assays . Since CD133 is expressed on many types of cancer cells, there would be great potential to use this probe as a bioprobe to monitor or diagnose tumor growth.
Conventional lipid-conjugated amphiphilicity CM probes usually face the problem of gradually diffusing into the cell from the CM surface after a certain period of time, thereby losing in situ information in long-term bioimaging. In 2021, Zhang et al. developed antidiffusion NIR probe 26 based on a fluorochrome HYPQ characterized by strong hydrophobicity and low lipophilicity to address this challenge using γ-glutamyltranspeptidase (GGT) as an example [49]. HYPQ was designed by conjugating strong hydrophobic solid-state fluorochrome 6-chloro–2-(2-hydroxyphenyl) quinazolin-4(3H)-one (HPQ) with a 2-(2-methyl–4H-chromen–4-ylidene) malononitrile group. After probe 26 was activated by GGT, the fluorescence signal on the CM remained unchanged, even with incubation time extending to 6 h, which was attributed to the precipitating and stable signal properties of HYPQ and was significant for in situ monitoring of GGT activity. In vivo imaging revealed that probe 26 could accurately define tumor regions after long-term in situ imaging of tumor-bearing mice . The excellent performance of HYPQ makes it an ideal alternative to construction of universal antidiffusion fluorescent probes and provides an efficient method for accurate imaging-guided surgery in the future.
Another stimuli-responsive in situ self-assembly of NIR small molecules on the surface of CM was reported by Ye et al. in 2019 [50]. Probe 27 consisted of a pre-quenched NIR fluorophore caged by an ALP recognition phosphate group, a paramagnetic DOTA-Gd chelate for MRI and a hydrophobic dipeptide Phe-Phe (FF) linker to promote self-assembly. This probe had high water solubility, weak NIR fluorescence and low r1 relaxivity. After HeLa cells were incubated with probe 27, bright NIR fluorescence was observed on the CM where ALP tended to locate. In situ self-assembled NPs anchored on the HeLa CM were detected with cryo-SEM and TEM. This probe with activatable NIR fluorescence and MRI via in situ self-assembly was suitable for noninvasively measuring and localizing ALP activity in live tumor cells and living mice.
References
- Kreder, R.; Oncul, S.; Kucherak, O.A.; Pyrshev, K.A.; Real, E.; Mély, Y.; Klymchenko, A.S. Blue fluorogenic probes for cell plasma membranes fill the gap in multicolour imaging. RSC Adv. 2015, 5, 22899–22905.
- Sakai, N.; Matile, S. Conjugated polyimine dynamers as phase-sensitive membrane probes. J. Am. Chem. Soc. 2018, 140, 11438–11443.
- Suzuki, K.G. Lipid rafts generate digital-like signal transduction in cell plasma membranes. Biotechnol. J. 2012, 7, 753–761.
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572.
- Kozlov, M.M.; Campelo, F.; Liska, N.; Chernomordik, L.V.; Marrink, S.J.; McMahon, H.T. Mechanisms shaping cell membranes. Curr. Opin. Cell Biol. 2014, 29, 53–60.
- Masters, T.A.; Pontes, B.; Viasnoff, V.; Li, Y.; Gauthier, N.C. Plasma membrane tension orchestrates membrane trafficking, cytoskeletal remodeling, and biochemical signaling during phagocytosis. Proc. Natl. Acad. Sci. USA 2013, 110, 11875–11880.
- Surrey, T.; Jähnig, F. Refolding and oriented insertion of a membrane protein into a lipid bilayer. Proc. Natl. Acad. Sci. USA 1992, 89, 7457–7461.
- Cardone, A.; Lopez, F.; Affortunato, F.; Busco, G.; Hofer, A.M.; Mallamaci, R.; Martinelli, C.; Colella, M.; Farinola, G.M. An aryleneethynylene fluorophore for cell membrane staining. Biochim. Biophys. Acta 2012, 1818, 2808–2817.
- Kahya, N.; Scherfeld, D.; Bacia, K.; Poolman, B.; Schwille, P. Probing lipid mobility of raft-exhibiting model membranes by fluorescence correlation spectroscopy. J. Biol. Chem. 2003, 278, 28109–28115.
- Koivusalo, M.; Welch, C.; Hayashi, H.; Scott, C.C.; Kim, M.; Alexander, T.; Touret, N.; Hahn, K.M.; Grinstein, S. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 2010, 188, 547–563.
- Zhang, C.; Jin, S.; Yang, K.; Xue, X.; Li, Z.; Jiang, Y.; Chen, W.Q.; Dai, L.; Zou, G.; Liang, X.J. Cell membrane tracker based on restriction of intramolecular rotation. ACS Appl. Mater. Interfaces 2014, 6, 8971–8975.
- Dal Molin, M.; Verolet, Q.; Colom, A.; Letrun, R.; Derivery, E.; Gonzalez-Gaitan, M.; Vauthey, E.; Roux, A.; Sakai, N.; Matile, S. Fluorescent flippers for mechanosensitive membrane probes. J. Am. Chem. Soc. 2015, 137, 568–571.
- Wang, K.-N.; Qi, G.; Chu, H.; Chao, X.-J.; Liu, L.-Y.; Li, G.; Cao, Q.; Mao, Z.-W.; Liu, B. Probing cell membrane damage using a molecular rotor probe with membrane-to-nucleus translocation. Mater. Horiz. 2020, 7, 3226–3233.
- Weissleder, R.; Pittet, M.J. Imaging in the era of molecular oncology. Nature 2008, 452, 580–589.
- Jain, S.K.; Shohet, S.B. A novel phospholipid in irreversibly sickled cells: Evidence for in vivo peroxidative membrane damage in sickle cell disease. Blood 1984, 63, 362–367.
- Chen, Y.; Pan, R.; Wang, Y.; Guo, P.; Liu, X.; Ji, F.; Hu, J.; Yan, X.; Wang, G.P.; Zhang, L.; et al. Carbon helical nanorobots capable of cell membrane penetration for single cell targeted SERS bio-sensing and photothermal cancer therapy. Adv. Funct. Mater. 2022, 32, 2200600.
- Bu, Y.; Xu, T.; Zhu, X.; Zhang, J.; Wang, L.; Yu, Z.; Yu, J.; Wang, A.; Tian, Y.; Zhou, H.; et al. A NIR-I light-responsive superoxide radical generator with cancer cell membrane targeting ability for enhanced imaging-guided photodynamic therapy. Chem. Sci. 2020, 11, 10279–10286.
- Chen, H.; Zheng, Y.; Jiang, J.H.; Wu, H.L.; Shen, G.L.; Yu, R.Q. An ultrasensitive chemiluminescence biosensor for cholera toxin based on ganglioside-functionalized supported lipid membrane and liposome. Biosens. Bioelectron. 2008, 24, 684–689.
- Garcia-Saez, A.J.; Schwille, P. Surface analysis of membrane dynamics. Biochim. Biophys. Acta 2010, 1798, 766–776.
- Ogiso, H.; Taniguchi, M.; Okazaki, T. Analysis of lipid-composition changes in plasma membrane microdomains. J. Lipid Res. 2015, 56, 1594–1605.
- Zhao, W.; Tian, Y.; Cai, M.; Wang, F.; Wu, J.; Gao, J.; Liu, S.; Jiang, J.; Jiang, S.; Wang, H. Studying the nucleated mammalian cell membrane by single molecule approaches. PLoS ONE 2014, 9, e91595.
- Li, K.; Ren, T.B.; Huan, S.; Yuan, L.; Zhang, X.B. Progress and perspective of solid-state organic fluorophores for biomedical applications. J. Am. Chem. Soc. 2021, 143, 21143–21160.
- Ning, J.; Liu, T.; Dong, P.; Wang, W.; Ge, G.; Wang, B.; Yu, Z.; Shi, L.; Tian, X.; Huo, X.; et al. Molecular design strategy to construct the near-infrared fluorescent probe for selectively sensing human cytochrome P450 2J2. J. Am. Chem. Soc. 2019, 141, 1126–1134.
- Ren, T.B.; Xu, W.; Zhang, W.; Zhang, X.X.; Wang, Z.Y.; Xiang, Z.; Yuan, L.; Zhang, X.B. A general method to increase stokes shift by introducing alternating vibronic structures. J. Am. Chem. Soc. 2018, 140, 7716–7722.
- Schaferling, M. The art of fluorescence imaging with chemical sensors. Angew. Chem. Int. Ed. 2012, 51, 3532–3554.
- Paige, J.; Nguyen-Duc, T.; Song, W.; Jaffrey, S. Fluorescence imaging of cellular metabolites with RNA. Science 2012, 335, 1194.
- Chazotte, B. Labeling membrane glycoproteins or glycolipids with fluorescent wheat germ agglutinin. Cold Spring Harb. Protoc. 2011, 2011, 570–572.
- Klymchenko, A.S.; Kreder, R. Fluorescent probes for lipid rafts: From model membranes to living cells. Chem. Biol. 2014, 21, 97–113.
- Ben-Bassat, H.; Goldblum, N. Concanavalin a receptors on the surface membrane of lymphocytes from patient’s with Hodgkin’s disease and other malignant lymphomas. Proc. Natl. Acad. Sci. USA 1975, 72, 1046–1049.
- Cheng, D.; Peng, J.; Lv, Y.; Su, D.; Liu, D.; Chen, M.; Yuan, L.; Zhang, X. De Novo Design of Chemical Stability Near-Infrared Molecular Probes for High-Fidelity Hepatotoxicity Evaluation In Vivo. J. Am. Chem. Soc. 2019, 141, 6352–6361.
- Li, K.; Xu, S.; Xiong, M.; Huan, S.Y.; Yuan, L.; Zhang, X.B. Molecular engineering of organic-based agents for in situ bioimaging and phototherapeutics. Chem. Soc. Rev. 2021, 50, 11766–11784.
- Li, X.; Gao, X.; Shi, W.; Ma, H. Design strategies for water-soluble small molecular chromogenic and fluorogenic probes. Chem. Rev. 2014, 114, 590–659.
- Liu, H.W.; Chen, L.; Xu, C.; Li, Z.; Zhang, H.; Zhang, X.B.; Tan, W. Recent progresses in small-molecule enzymatic fluorescent probes for cancer imaging. Chem. Soc. Rev. 2018, 47, 7140–7180.
- Liu, Y.; Teng, L.; Xu, C.; Ren, T.-B.; Xu, S.; Lou, X.; Yuan, L.; Zhang, X.-B. An integration strategy to develop dual-state luminophores with tunable spectra, large stokes shift, and activatable fluorescence for high-contrast imaging. CCS Chem. 2022, 4, 2153–2164.
- Cheng, D.; Pan, Y.; Wang, L.; Zeng, Z.; Yuan, L.; Zhang, X.; Chang, Y.-T. Selective visualization of the endogenous peroxynitrite in an inflamed mouse model by a mitochondria-targetable two-photon ratiometric fluorescent probe. J. Am. Chem. Soc. 2016, 139, 285–292.
- Karton-Lifshin, N.; Albertazzi, L.; Bendikov, M.; Baran, P.S.; Shabat, D. “Donor-two-acceptor” dye design: A distinct gateway to NIR fluorescence. J. Am. Chem. Soc. 2012, 134, 20412–20420.
- Liu, Y.; Teng, L.; Xu, C.; Liu, H.W.; Xu, S.; Guo, H.; Yuan, L.; Zhang, X.B. A “Double-Locked” and enzyme-activated molecular probe for accurate bioimaging and hepatopathy differentiation. Chem. Sci. 2019, 10, 10931–10936.
- Lv, Y.; Dan, C.; Dongdong, S.; Chen, M.; Yin, B.C.; Yuan, L.; Zhang, X.B. Visualization of oxidative injury in the mouse kidney using selective superoxide anion fluorescent probes. Chem. Sci. 2018, 9, 7606–7613.
- Koo, C.K.; Wong, K.L.; Man, C.W.; Tam, H.L.; Tsao, S.W.; Cheah, K.W.; Lam, M.H. Two-photon plasma membrane imaging in live cells by an amphiphilic, water-soluble cyctometalated platinum(II) complex. Inorg. Chem. 2009, 48, 7501–7503.
- Liu, H.; Kwong, B.; Irvine, D.J. Membrane anchored immunostimulatory oligonucleotides for in vivo cell modification and localized immunotherapy. Angew. Chem. Int. Ed. 2011, 50, 7052–7055.
- Kalchenko, V.; Shivtiel, S.; Malina, V.; Lapid, K.; Haramati, S.; Lapidot, T.; Brill, A.; Harmelin, A. Use of lipophilic near-infrared dye in whole-body optical imaging of hematopoietic cell homing. J. Biomed. Opt. 2006, 11, 050507.
- Zhang, C.; Liu, T.; Su, Y.; Luo, S.; Zhu, Y.; Tan, X.; Fan, S.; Zhang, L.; Zhou, Y.; Cheng, T.; et al. A near-infrared fluorescent heptamethine indocyanine dye with preferential tumor accumulation for in vivo imaging. Biomaterials 2010, 31, 6612–6617.
- Schmidt, T.; Schütz, G.; Baumgartner, W.; Gruber, H.; Schindler, H. Characterization of photophysics and mobility of single molecules in a fluid lipid membrane. J. Phys. Chem. 1995, 99, 17662–17668.
- Huang, Y.; Xing, J.; Gong, Q.; Chen, L.C.; Liu, G.; Yao, C.; Wang, Z.; Zhang, H.L.; Chen, Z.; Zhang, Q. Reducing aggregation caused quenching effect through co-assembly of PAH chromophores and molecular barriers. Nat. Commun. 2019, 10, 169.
- Zhang, W.; Huang, Y.; Chen, Y.; Zhao, E.; Hong, Y.; Chen, S.; Lam, J.W.Y.; Chen, Y.; Hou, J.; Tang, B.Z. Amphiphilic tetraphenylethene-based pyridinium salt for selective cell-membrane imaging and room-light-induced special reactive oxygen species generation. ACS Appl. Mater. Interfaces 2019, 11, 10567–10577.
- Hu, Z.; Li, R.; Cui, X.; Hu, C.; Chen, Z. A pH-sensitive carbonic anhydrase IX-targeted near-infrared probe for fluorescent sensing and imaging of hypoxic osteosarcoma. Sens. Actuators B Chem. 2023, 379, 133171.
- Wu, L.-L.; Zhao, Q.; Wang, Q.; Zhang, Q.; Yang, F.; Zheng, B.; Hu, H.-Y.; Xing, N. Membrane dual-targeting probes: A promising strategy for fluorescence-guided prostate cancer surgery and lymph node metastases detection. Acta Pharm. Sin. B, 2022; in press.
- Abdul Sisak, M.A.; Louis, F.; Miyao, T.; Lee, S.H.; Chang, Y.T.; Matsusaki, M. Mechanism assay of interaction between blood vessels-near infrared probe and cell surface marker proteins of endothelial cells. Mater. Today Bio 2022, 15, 100332.
- Li, K.; Lyu, Y.; Huang, Y.; Xu, S.; Liu, H.W.; Chen, L.; Ren, T.B.; Xiong, M.; Huan, S.; Yuan, L.; et al. A de novo strategy to develop NIR precipitating fluorochrome for long-term in situ cell membrane bioimaging. Proc. Natl. Acad. Sci. USA 2021, 118, e2018033118.
- Yan, R.; Hu, Y.; Liu, F.; Wei, S.; Fang, D.; Shuhendler, A.J.; Liu, H.; Chen, H.Y.; Ye, D. Activatable NIR fluorescence/MRI bimodal probes for in vivo imaging by enzyme-mediated fluorogenic reaction and self-assembly. J. Am. Chem. Soc. 2019, 141, 10331–10341.
More
Information
Subjects:
Chemistry, Analytical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
865
Revisions:
3 times
(View History)
Update Date:
17 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





