Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Lin Yuan and Version 3 by Sirius Huang.
Cell membrane (CM) is a phospholipid bilayer that maintains integrity of a whole cell and relates to many physiological and pathological processes. Developing CM imaging tools is a feasible method for visualizing membrane-related events. Small-molecular fluorescent probes in the near-infrared (NIR) region have been pursued extensively for CM staining to investigate its functions and related events.
- cell membrane imaging
- small-molecular fluorescent probe
- near-infrared imaging
1. Introduction
Cell membrane (CM) is the first barrier that separates interior of cells from the extracellular environment and plays a crucial role in physiological processes, such as signal transduction and biomolecular transport [1][2][3][4][1,2,3,4]. A CM is an amphipathic bilayer membrane consisting of a mix of lipids and proteins [5][6][7][5,6,7]. It is closely related to signal transduction from the extracellular environment, causing further responses through changes in shape and morphology [8][9][10][11][8,9,10,11]. CM damage can cause cell swelling and apoptosis, eventually causing various diseases, such as cirrhosis, diabetes and even cancers [12][13][14][15][12,13,14,15]. Therefore, visualization of a CM enables study of related events and evaluation of cell life status and facilitates diagnosis and treatment of CM-related diseases [16][17][16,17].
At present, a certain number of methods have been developed for CM analysis [18][19][20][21][18,19,20,21]. Among them, fluorescence imaging has become a powerful tool benefiting from its advantages of high sensitivity, simplicity and noninvasiveness [22][23][24][25][26][22,23,24,25,26]. Wheat germ agglutinin (WGA) and concanavalin A have served as popular fluorescent CM probes due to their efficiency and ease of use [27][28][29][27,28,29], but these probes are expensive and have large size. Small-molecular fluorescent probes have been widely used for bioimaging due to their easy preparation, modifiable molecular structures and tunable fluorescence signals [30][31][32][33][34][30,31,32,33,34]. The small size of this probe enables its precise location in the lipid bilayer, which can serve as a flexible tool for study of CM-related events. Small-molecular fluorescent probes with excitation/emission in the near-infrared (NIR) region (650−900 nm) are much more desirable for bioimaging due to attenuated biological autofluorescence, reduced light damage and increased imaging penetration depth [35][36][37][38][35,36,37,38]. In recent decades, various NIR CM-targeting molecular probes have been developed for visualization of cell activities, metabolism and cell-to-cell communication on CMs. These probes are commonly designed by incorporating CM-targeting units to NIR fluorophore. In this process, screening of CM-targeting units is very important. Considering the amphipathic characteristics of CMs, designing amphipathic probes that have similarity and intermiscibility to a CM is an effective way to realize CM imaging. NIR fluorophore with large π-conjugation can serve as a lipophilic moiety. In this case, amphipathic CM probes can be obtained by linking hydrophilic moiety, especially positively or negatively charged groups, to NIR fluorophore. Sometimes, long alkyl chains are needed to assist the probe to insert into a CM by hydrophobic interaction with the alkyl chain of a phospholipid [39][40][39,40]. In addition to amphipathic characteristics of CMs, many proteins with important biological functions are embedded in CMs. Another approach to realize CM imaging is to design probes that can specifically target these proteins or be self-assembled on the surface of a CM after being activated by these proteins. The recent progress of NIR CM molecular probes for visualization of CM-related events are summarized (Scheme 1). The unique molecular structure and photophysicality of NIR dyes are fully considered in design and application of CM probes. These CM probes are divided into two classes based on different targeting mechanisms.
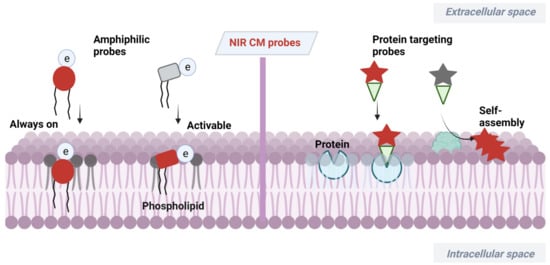
Scheme 1.
Design strategies of NIR CM-targeting molecular probes.
2. NIR Molecular Probes for CM Imaging
NIR dyes with large π-conjugate systems usually have strong lipophilicity [41][42][41,42]. Owing to the glycerophospholipid bilayer structure of CMs, lipophilic NIR dyes are easily transferred into a lipid bilayer through lipophilic–lipophilic interaction. However, in most cases, lipophilic dye molecules are readily internalized into cells, losing their membrane staining ability. Designing fluorescent probes that mimic the amphiphilic structure of CMs [43] is a feasible way to image bilayer membranes. Many proteins with important biological functions are embedded in CMs of live organisms. Another CM staining approach is to incorporate a membrane proteins targeting group to probes or relying on self-assembly of the probes on a CM after being activated by proteins. Herein, advances in development of NIR fluorescent probes for CM staining have been overviewed, including their design strategies, targeting mechanisms and biological applications.
2.1. Amphiphilic NIR Probes for CM Imaging
The amphiphilic NIR CM probe commonly contains hydrophobic moieties to insert into CM through lipophilic–lipophilic interaction and polar headgroups to prevent probe penetrating into cells. The long alkyl chains are sometimes selected to help a probe stay on a CM. Many NIR CM probes retain emission before and after anchoring on the CM, named as “always-on” NIR CM probes. Using these probes, a CM can be stained and the boundary of cells will be observed. However, in this process, repeated washing procedure is required, hindering the real-time and dynamic imaging of CM. To this end, significant effort has been directed at building activatable CM staining probes. One approach to build such probes is to screen for solvatochromic or fluorogenic NIR dyes that are further covalently linked with polar headgroups. These probes have variable emissions (intensity and wavelength), with the environment changed from polar aqueous condition to low polar membrane lipid, thereby achieving CM imaging in an activatable manner. Another approach to construct activatable CM staining probes is relying on disassembly light-up fluorescence strategy. As mentioned above, NIR dyes with large π-conjunction are prone to be aggregated in aqueous solutions, resulting in quenching of their fluorescence [44]. When being inserted into a CM, however, the aggregates are dissolved and dispersed on the CM, leading to significant fluorescence enhancement. In this case, the washing procedure is no longer required because free probes in culture medium are nonfluorescent. Aggregation-induced emission (AIE) dyes are also selected to construct activatable NIR CM probes. These probes are freely rotating in solutions [45], but their intramolecular rotation can be restricted when inserted into a CM, leading to dramatic fluorescence enhancement.
