Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xianjun Yu | -- | 2519 | 2023-03-14 14:12:44 | | | |
| 2 | Lindsay Dong | + 5 word(s) | 2524 | 2023-03-15 08:49:42 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ma, Z.; Hua, J.; Liu, J.; Zhang, B.; Wang, W.; Yu, X.; Xu, J. Mesenchymal Stromal Cell-Based Targeted Therapy Pancreatic Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/42185 (accessed on 07 February 2026).
Ma Z, Hua J, Liu J, Zhang B, Wang W, Yu X, et al. Mesenchymal Stromal Cell-Based Targeted Therapy Pancreatic Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/42185. Accessed February 07, 2026.
Ma, Zhilong, Jie Hua, Jiang Liu, Bo Zhang, Wei Wang, Xianjun Yu, Jin Xu. "Mesenchymal Stromal Cell-Based Targeted Therapy Pancreatic Cancer" Encyclopedia, https://encyclopedia.pub/entry/42185 (accessed February 07, 2026).
Ma, Z., Hua, J., Liu, J., Zhang, B., Wang, W., Yu, X., & Xu, J. (2023, March 14). Mesenchymal Stromal Cell-Based Targeted Therapy Pancreatic Cancer. In Encyclopedia. https://encyclopedia.pub/entry/42185
Ma, Zhilong, et al. "Mesenchymal Stromal Cell-Based Targeted Therapy Pancreatic Cancer." Encyclopedia. Web. 14 March, 2023.
Copy Citation
Pancreatic cancer is an aggressive malignancy with high mortality rates and poor prognoses. Despite rapid progress in the diagnosis and treatment of pancreatic cancer, the efficacy of current therapeutic strategies remains limited. Hence, better alternative therapeutic options for treating pancreatic cancer need to be urgently explored. Mesenchymal stromal cells (MSCs) have recently received much attention as a potential therapy for pancreatic cancer owing to their tumor-homing properties.
pancreatic cancer
mesenchymal stromal cells
exosomes
tumor-targeted therapy
1. Introduction
Pancreatic cancer, an aggressive human malignant tumor, is often termed a silent killer owing to its poor prognosis, and its incidence has been increasing over the years [1][2][3]. The mortality rate within one year after diagnosis is approximately 75%, and the 5-year survival rate is no more than 8% [4][5][6]. Pancreatic ductal adenocarcinoma (PDAC) accounts for 90% of all pancreatic tumors, and other subtypes include acinar carcinoma, pancreatoblastoma, and neuroendocrine neoplasms [3]. Approximately 50% of patients with PDAC display no symptoms during the early stage, and by the time a diagnosis is confirmed, they are in the late stage of PDAC [3][7][8]. Thus, most patients miss opportunities for radical surgical resection in the early stage and can only undergo radiotherapy and chemotherapy later. However, owing to the special extracellular matrix barrier of pancreatic cancer and resistance to chemotherapeutic drugs, some cancer cells cannot be killed [9][10]. Furthermore, approximately 40% of patients with PDAC experience tumor recurrence even after surgical resection and die within one year [11][12][13]. Despite the rapid progress in the diagnosis and therapy of pancreatic cancer, the efficacy of present therapeutic measures remains poor [7]. Therefore, identifying alternative treatment strategies for the better management of pancreatic cancer is an urgent requirement.
As a carrier of anti-tumor drugs, mesenchymal stromal cells (MSCs) can be genetically engineered to release various agents such as treatment proteins, suicide genes, and oncolytic viruses to decrease cancer growth and progression [14][15]. The application of MSCs as therapeutic biological carriers in cytotherapy has some distinct advantages, including low immunogenicity, tumor tropism, a massive expansion in vitro, and the ability to transfer various therapeutic agents [16][17][18][19]. Reportedly, MSCs can home to tumor locations and survive in the tumor microenvironment (TME) [16][20][21].
2. Tumor-Homing Properties of MSCs
MSCs are adult stem cells capable of multilineage differentiation and self-renewal [22][23]. MSCs exist in most tissues and are usually extracted from various sources, including bone marrow, umbilical cord, menstrual blood, placenta, adipose tissues, and muscles [19][24][25][26]. To date, MSCs have been shown to treat multiple diseases owing to their immunomodulatory and anti-inflammatory effects and tissue repair ability [18][27][28]. They thus have excellent application prospects in regenerative medicine.
MSCs can accurately migrate to injured tissues and organs and play a key role in inhibiting inflammation, decreasing tissue fibrosis formation, and promoting regeneration, thereby indicating that MSCs can selectively migrate to certain sites in the body [29][30]. Moreover, MSCs have been found to selectively migrate to primary and metastatic tumor locations, thus revealing the tumor-homing capacity of MSCs [16][20][21][31][32][33][34][35]. However, despite reports that MSCs could migrate to tumor locations in various types of tumors, the potential mechanisms by which MSCs home to tumors are still unclear.
MSCs express various chemokines and cell adhesion molecules that coordinate the mobilization of MSCs to the damage locations [15][36][37][38][39][40]. Recent research has found that the tumor-homing capacity of MSCs could be regulated by the cooperation of cytokines, chemokines, and adhesion molecules [15][41][42][43][44][45]. Hence, this observation indicates that the homing capabilities of MSCs could depend on the inflammatory microenvironment of the tumor.
MSCs are involved in the initiation, development, progression, and metastasis of tumors [46]. They can directly affect tumor development through crosstalk with tumors or the release of soluble molecules [14]. Although MSCs are indicated to exhibit pro-tumor effects [47], they can also inhibit the growth of tumors by various mechanisms, such as inhibiting tumor cell proliferation and promoting tumor cell death [48]. Furthermore, owing to their tumor-homing properties, MSCs and their exosomes have been considered promising tools for the accurate and selective release of antitumor molecules, RNA, or anticancer drugs that aid in reducing tumor cell viability and invasive characteristics [16][17][49][50][51]. Therefore, MSCs may serve as a potential tumor-targeted therapeutic strategy.
On infusing MSCs into rats, MSCs could home to pancreatic cancer sites to exert their anticancer effects [33][34]. However, the mechanism by which MSCs crosstalk with tumor cells has not yet been elucidated, and consequently, translational medicine progress has been limited. Thus, the underlying molecular mechanism by which MSCs crosstalk with tumors needs further exploration; this will aid in improving the effectiveness of MSC therapeutic potential.
3. MSC Therapy for Pancreatic Cancer
3.1. Native MSCs
Naïve MSCs have some potential advantages for treatment, such as abundance, low immunogenicity, and ease of isolation and in vitro expansion. Hence, it is widely applied to various diseases including cancer. Cousin et al. found that native human adipose tissue-derived mesenchymal stromal cells (AD-MSCs) inhibit pancreatic cancer cell proliferation and promote tumor cell death by inhibiting the cell cycle at the G1 phase [52]. Doi et al. observed that native rat umbilical cord matrix-derived stem cells (UCMSCs) decrease the growth of pancreatic tumors in mouse peritoneal models and increase the overall survival time of mice [53].
3.2. Genetically Engineered MSCs
Genetically modified MSCs are promising potential cancer therapies to further enhance the efficacy of MSCs to target tumor cells. These MSCs deliver anti-proliferative, pro-apoptotic, and anti-angiogenic molecules to target tumor cells [47][54]. These effects might depend on several mechanisms: MSCs preferentially migrate to locations of inflammation, ischemia, and malignancy; genetically modified MSCs only release therapeutic gene products in the special TME; transgenes encoding biologic agents might themselves exhibit targeted and differential effects in tumor cells [55]. Previous studies have shown that selective targeting of therapeutic gene expression by MSCs is feasible and effective in the treatment of various cancers [56][57][58].
TRAIL is a therapeutic protein that induces tumor cell death; however, pancreatic cancer cells present intrinsic resistance toward TRAIL by the expression of anti-apoptotic proteins like the X-linked inhibitor of apoptosis protein (XIAP) [59][60]. Inhibiting XIAP could promote TRAIL-induced apoptosis of pancreatic cancer cells [61][62]. Mohr et al. found that the TRAIL-modified mouse bone marrow MSCs (BM-MSCs) deliver soluble TRAIL that suppresses the metastatic growth of pancreatic cancers [63]. Moreover, TRAIL-transfected pancreas-derived MSCs can promote pancreatic cancer cell death [64].
In summary, combining MSCs with selective gene treatment results in enhanced therapeutic effects on inhibiting tumor growth; this might aid in developing new tools for pancreatic cancer treatment (Figure 1).
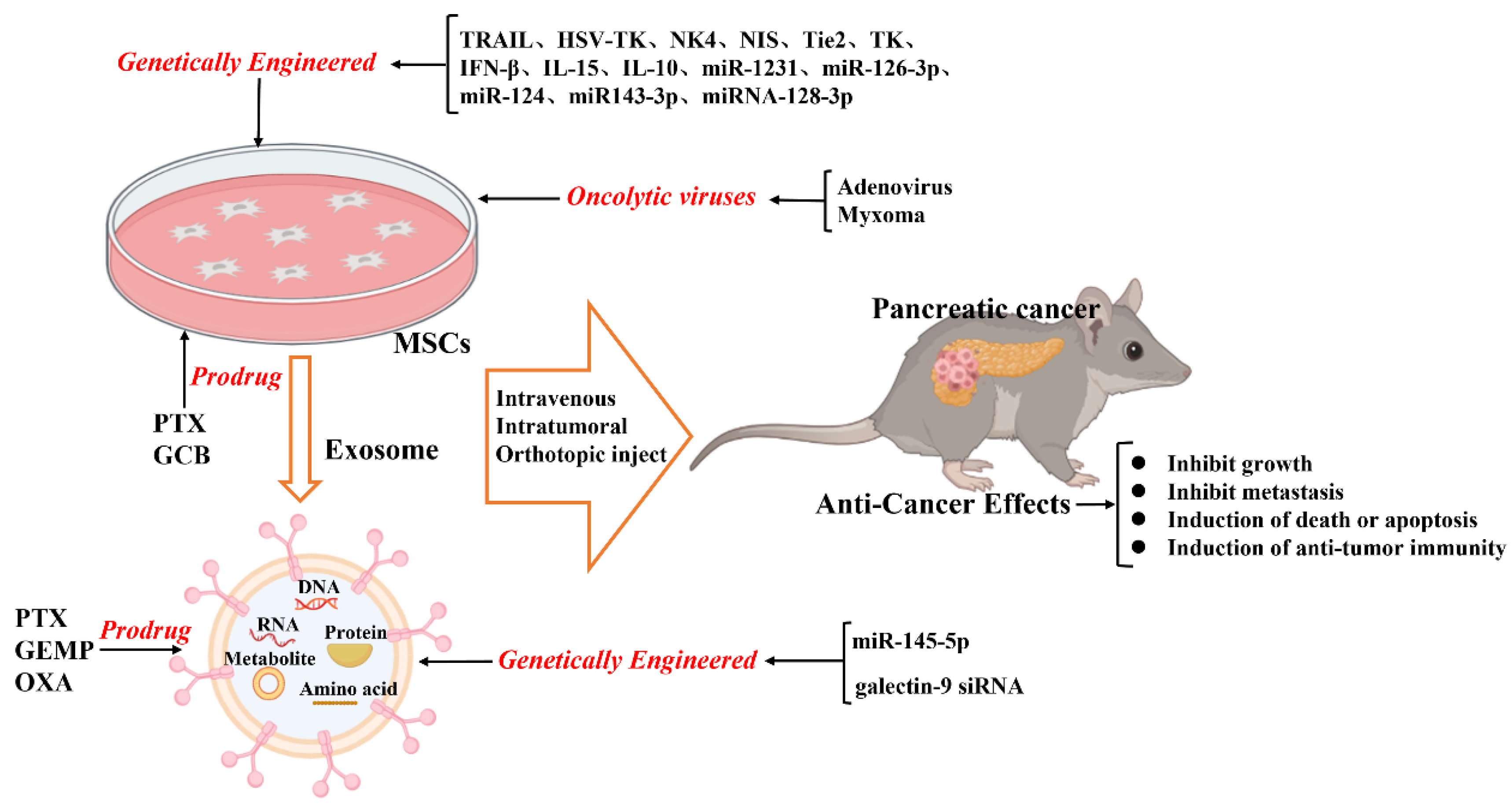
Figure 1. Schematic illustration of the current anticancer treatment based on mesenchymal stromal cells (MSCs) and MSC-derived exosomes.
3.3. Exosomes as a Vehicle for Therapy Delivery
Thus far, numerous studies have found that MSCs can secrete extracellular vesicles), including microvesicles (100–2000 nm in diameter) and exosomes (30–150 nm in diameter), which act as paracrine mediators between MSCs and target cells [65][66][67]. Exosomes can deliver cargo (nucleic acids, proteins, lipids, amino acids, and metabolites) from the originating cells to the target cells [67][68][69]. Compared with those of artificial nanocarriers, exosomes, as natural vesicles secreted by cells, have double lipid membranes, better biocompatibility, lower immunogenicity, stronger targeting specificity, deeper tissue permeability, and longer circulating half-life [67][70][71][72][73]. Based on these advantages, exosomes have been applied for engineering functional cargo loads, such as packaged nucleic acid, functional proteins, and other therapeutic molecules into exosomes [66][67][74][75][76].
Notably, exosomes have been shown to transfer microRNAs (miRNAs) to target cancer cell proliferation, differentiation, and metastasis [75][77][78][79][80]. For example, miRNA-100 carried by MSC-derived exosomes suppress tumor angiogenesis and breast cancer progression via the mTOR/HIF1A/VEGF pathway [81]. Additionally, Li et al. showed that engineered exosomes from UCMSCs enriched with miR-302a significantly inhibit endometrial cancer cell proliferation and migration by decreasing cyclin D1 expression and inhibiting the AKT pathway [82].
On modifying the normal fibroblast-like mesenchymal cell-derived exosomes to deliver short interfering RNA or short hairpin RNA to target oncogenic KrasG12D, tumor growth was found to be decreased in multiple mouse models of pancreatic cancer, thereby increasing the overall survival [83]. The study offers insight into the target therapeutic potential of exosomes in pancreatic cancer. Furthermore, infusing the exosomes derived from miRNA-engineered MSCs, which contain abundant MSC-sourced anti-tumorigenic miRNAs, can represent a potentially new therapeutic measure for pancreatic cancer. For example, the exosomes extracted from miR-1231-modified BM-MSCs with high levels of miR-1231 reduce the proliferation of pancreatic cancer cells [84]. Overexpressed miR-126-3p derived from BM-MSC exosomes inhibit the proliferation, invasion, and metastasis of pancreatic tumor cells and induce their apoptosis in vitro and in vivo by inhibiting the expression of ADAM9 [85]. Exosomes derived from miRNA-MSCs release miR-124 and miR143-3p in pancreatic tumor cells, inhibiting the proliferation of tumor cells [86][87]. The exosomes derived from miRNA-128-3p-modified UCMSCs can inhibit the proliferation, invasion, and migration of pancreatic cancer cells via the miRNA-128-3p/Galectin-3 axis [88].
3.4. MSC-Mediated Drug Delivery
MSCs can incorporate chemotherapeutic drugs in vitro, subsequently releasing the effective concentration of drugs in their conditioned medium to exert therapeutic effects [89][90][91]. Furthermore, tumor-homing properties of MSCs allow them to precisely deliver the drug to the tumor location; this has been widely studied as a targeted delivery agent of anti-cancer drugs [92][93][94].The proliferation of stromal fibroblasts and the deposition of extracellular matrix, which are the defining characteristics of PDAC, lead to a fibrotic state known as desmoplastic or reactive stroma [95][96]. Therefore, this could make it difficult to acquire an effective drug concentration by the common route of administration. Notably, after the MSCs were preconditioned to high doses of paclitaxel (PTX), they intracellularly accumulate the drug and then release it, thereby inhibiting pancreatic tumor cell proliferation [97]. Brini et al. demonstrated that PTX-loaded gingival interdental papilla MSCs can release a sufficient amount of PTX to inhibit the proliferation of pancreatic tumor cells [98].
Exosomes can be loaded with therapeutic drugs and then used to release them into the target cells [99][100]. The methods applied for directly loading drugs inside exosomes include incubation, electroporation, sonication, extrusion, freeze-thaw cycles, and saponin application. Presently, the most commonly applied methods are incubation and electroporation [80][100]. As drug carriers, exosomes are widely studied as therapeutic agents and can potentially be clinically applied. Recently, GEMP- and PTX-loaded exosomes revealed superiorities in homing and penetrating abilities that aided in inhibiting the growth of pancreatic tumors in vivo [101].
3.5. Delivery of Oncolytic Viruses
The oncolytic virus has revealed promising results in the treatment of several cancers in various clinical trials [102][103][104]. It can directly cause oncolysis and spread to adjacent tumor cells to activate an anti-cancer immune response. Oncolytic viruses can replicate and selectively target tumor cells, but they cannot bind or effectively replicate in most normal cells. MSCs have been shown to protect viruses from immune clearance through a unique cell carrier tool before delivering them to metastatic tumor sites [105][106][107]. Although the tumor-homing ability of MSCs makes them a promising candidate for systemically delivering oncolytic viruses to tumor location, infection and particle production by MSCs remain areas of concern. The viruses genetically modified for improved delivery by MSCs are aimed at enhancing oncolysis and improving virus production in tumor cells [108].
4. Challenges of MSCs in Treating Pancreatic Cancer
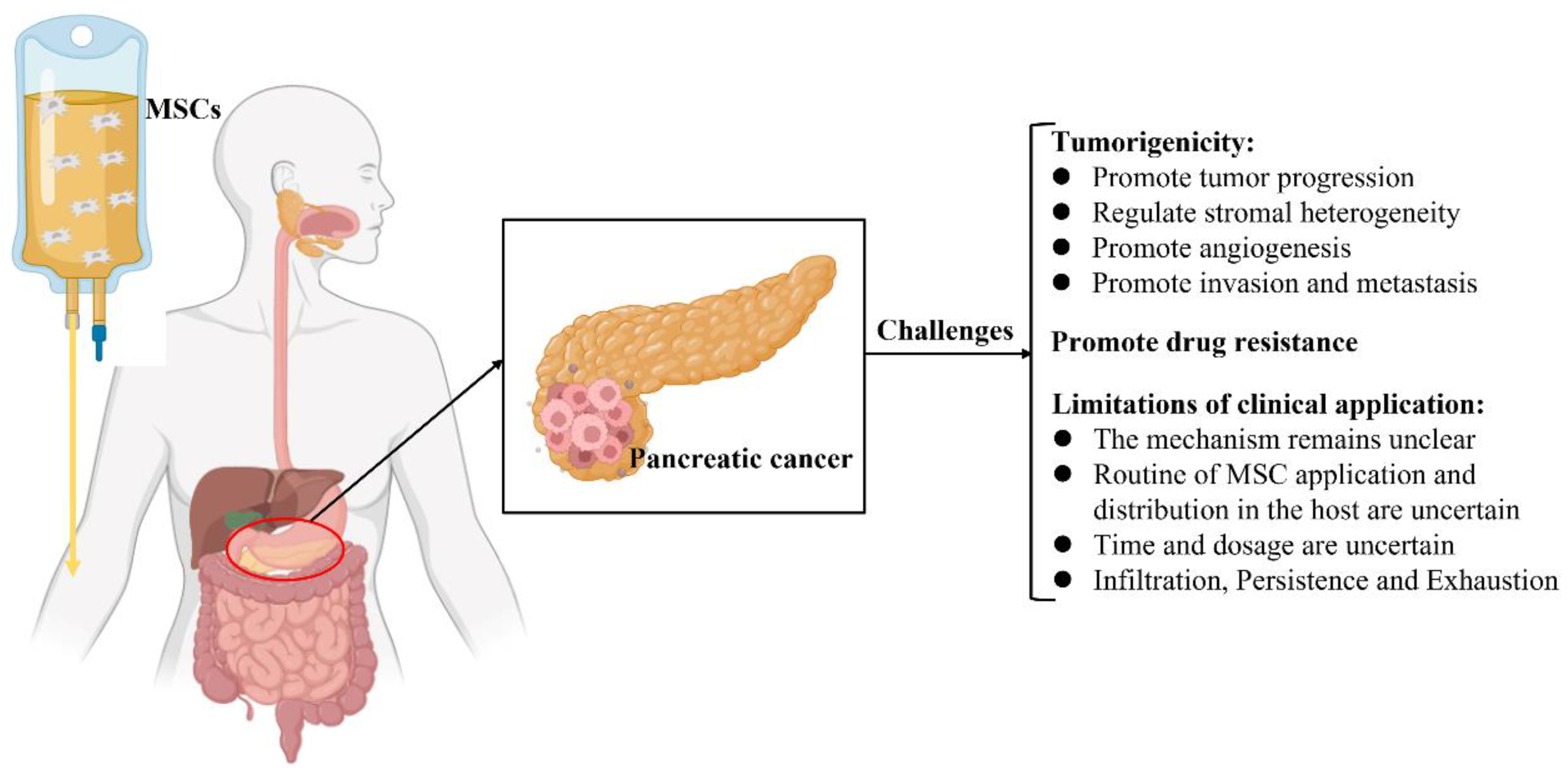
MSCs have been used as a therapeutic intervention for tumors; nevertheless, they are reportedly involved in tumor progression, including tumorigenesis, tumor growth, metastasis, and regulation of the TME [79][109]. Furthermore, the potential mechanisms by which MSCs crosstalk with tumor cells in the TME have not yet been elucidated [41][109][110][111]. Hence, the clinical application of MSCs in the treatment of pancreatic cancer remains controversial and challenging (Figure 2).

Figure 2. Schematic illustration of the current challenges of mesenchymal stromal cell treatment in pancreatic cancer.
4.1. Tumorigenicity
Numerous studies have shown that MSCs have inherent tumorigenicity properties [17][31][112][113][114][115]. MSCs possess the molecular potential to affect and direct several crucial processes, which are important for tumor development, as the cells contain an abundant source of various biochemical mediators [14][110]. MSCs have been successfully isolated from various types of tumor tissues, such as HCC, glioma, gastric cancer, breast cancer, ovarian cancer, prostate cancer, colon cancer, and pancreatic cancer, indicating that MSC is a distinct stromal cell type in the TME that participates in tumor development [17][116]. It consists of stromal cells that include tumor-associated fibroblasts, tumor endothelial cells, immune and inflammatory cells, and bone marrow-derived cells [117][118]. Interactions between tumor cells and the TME tremendously impact tumor development, metastasis, and drug resistance [119][120].
MSCs can reportedly modulate stromal heterogeneity in various solid tumors, including pancreatic cancer [113][121]. Furthermore, MSCs regulate specific secretory molecules in the TME and promote the progression and invasion of pancreatic cancer [114][122]. AD-MSCs migrate to pancreatic cancer locations to serve as a major source of a-SMA+ cells and promote tumor progression [31][32]. Nevertheless, the mechanism underlying the mobilization of these intricate molecules remains unclear. Notably, Ganguly et al. found that MUC5AC acts as a systemic carrier of tumor secretome and can alter stromal maturation in pancreatic cancer by mobilizing AD-MSCs via CD44 and CD29/ITGB1 clustering [123].
4.2. MSCs Promote Drug Resistance
MSCs have been revealed to induce and play an important role in the drug resistance of tumor cells in the TME [99][124]. Several potential mechanisms underlying this phenomenon might include promoting active drug sequestration, decreasing drug concentration, and delivering specific RNA, proteins, and functional small molecules into target cells to induce dysregulation of relevant signaling pathways. For example, Roodhart et al. found that endogenous MSCs are activated on treatment with platinum analogs and release some mediators to protect tumor cells against a range of chemotherapeutics. By a metabolomics method, the results showed that two distinct platinum-induced polyunsaturated fatty acids derived from MSCs, 12-oxo-5,8,10-heptadecatrienoic acid and hexadeca-4,7,10,13-tetraenoic acid [16:4 (n−3)], induce resistance to platinum-based chemotherapy [125]. Furthermore, MSC-derived exosomes induce drug resistance in various tumor cells. Wang et al. showed that BM-MSC-derived exosomes play key roles in drug resistance in multiple myeloma and induce their proliferation, migration, and survival [126].
4.3. Limitations of the Clinical Applications of MSCs
4.3.1. Routines of MSC Application and Distribution in the Host
Numerous studies have reported the tumor-homing properties of MSCs; however, the migration and distribution of MSCs in the body are not yet clearly understood. In animal experiments, the most common method of infusion of MSCs is through the intravenous (IV) or intraperitoneal (i.p.) route [127]. Nevertheless, owing to their size and the small dimensions of the lung vessels, a large number of MSCs are temporarily distributed in the lung after IV administration [128][129][130][131][132]. Notably, three days after IV administration, most of the MSCs are recruited to the tumor locations in an orthotopic pancreatic cancer model of athymic nude mice; only some MSCs are observed in the lung [133].
4.3.2. Timing and Dosage
The anti-tumor effect of exogenous MSCs depends on the time of their inoculation in tumor-bearing animals. MSCs infused during the initial phase of tumor growth exert anti-tumor effects, whereas those infused during the progressive stage of tumor development induce immune escape and promote the growth of the tumor [134]. Thus, the best time for treatment needs to be established.
4.3.3. Infiltration, Persistence, and Exhaustion
Although chimeric antigen receptor (CAR)-modified T cells reveal a new potential option in cancers, current CAR T-cell clinical trials in pancreatic cancer have been unable to increase survival and exhibit any significant response. This might be due to obstacles such as T-cell infiltration, persistence, and exhaustion [95]. The TME in pancreatic cancer comprises tumor cells, endothelial cells, immune cells, stromal cells, extracellular matrix, and a broad spectrum of enzymes, cytokines, and growth factors [135]. As a dense stroma surrounds pancreas cancer, successful immunotherapy and chemotherapy are required to break through the physical and environmental barriers in the TME [136].
References
- Wu, W.; He, X.-K.; Yang, L.; Wang, Q.; Bian, X.; Ye, J.; Li, Y.; Li, L. Rising trends in pancreatic cancer incidence and mortality in 2000–2014. Clin. Epidemiol. 2018, 10, 789–797.
- Saad, A.M.; Turk, T.; Al-Husseini, M.J.; Abdel-Rahman, O. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study. BMC Cancer 2018, 18, 688.
- Park, W.; Chawla, A.; O’Reilly, E. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862.
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.; Biankin, A.; Neale, R.; Tempero, M.; Tuveson, D.; Hruban, R.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022.
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int. J. Mol. Sci. 2017, 18, 1338.
- Siegel, R.; Miller, K.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30.
- Mizrahi, J.; Surana, R.; Valle, J.; Shroff, R. Pancreatic cancer. Lancet 2020, 395, 2008–2020.
- Zhou, B.; Xu, J.-W.; Cheng, Y.-G.; Gao, J.-Y.; Hu, S.-Y.; Wang, L.; Zhan, H.X. Early detection of pancreatic cancer: Where are we now and where are we going? Int. J. Cancer 2017, 141, 231–241.
- Fan, Y.-F.; Shang, W.-T.; Lu, G.-H.; Guo, K.-X.; Deng, H.; Zhu, X.-H.; Wang, C.-C.; Tian, J. Decreasing hyaluronic acid combined with drug-loaded nanoprobes improve the delivery and efficacy of chemotherapeutic drugs for pancreatic cancer. Cancer Lett. 2021, 523, 1–9.
- Cai, H.; Wang, R.; Guo, X.; Song, M.; Yan, F.; Ji, B.; Liu, Y. Combining Gemcitabine-Loaded Macrophage-like Nanoparticles and Erlotinib for Pancreatic Cancer Therapy. Mol. Pharm. 2021, 18, 2495–2506.
- Crippa, S.; Belfiori, G.; Bissolati, M.; Partelli, S.; Pagnanelli, M.; Tamburrino, D.; Gasparini, G.; Rubini, C.; Zamboni, G.; Falconi, M. Recurrence after surgical resection of pancreatic cancer: The importance of postoperative complications beyond tumor biology. HPB 2021, 23, 1666–1673.
- Nienhuijs, S.W.; Akker, S.A.V.D.; de Vries, E.; de Hingh, I.H.; Visser, O.; Lemmens, V.E. Nationwide Improvement of Only Short-Term Survival After Resection for Pancreatic Cancer in The Netherlands. Pancreas 2012, 41, 1063–1066.
- Barugola, G.; Partelli, S.; Marcucci, S.; Sartori, N.; Capelli, P.; Bassi, C.; Pederzoli, P.; Falconi, M. Resectable Pancreatic Cancer: Who Really Benefits From Resection? Ann. Surg. Oncol. 2009, 16, 3316–3322.
- Lan, T.; Luo, M.; Wei, X. Mesenchymal stem/stromal cells in cancer therapy. J. Hematol. Oncol. 2021, 14, 195.
- Hmadcha, A.; Martin-Montalvo, A.; Gauthier, B.; Soria, B.; Capilla-Gonzalez, V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8, 43.
- Timaner, M.; Tsai, K.K.; Shaked, Y. The multifaceted role of mesenchymal stem cells in cancer. Semin. Cancer Biol. 2019, 60, 225–237.
- Yin, Z.; Jiang, K.; Li, R.; Dong, C.; Wang, L. Multipotent mesenchymal stromal cells play critical roles in hepatocellular carcinoma initiation, progression and therapy. Mol. Cancer 2018, 17, 178.
- Galipeau, J.; Sensébé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833.
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and advances in clinical applications of mesenchymal stromal cells. J. Hematol. Oncol. 2021, 14, 24.
- Mirzaei, H.; Sahebkar, A.; Avan, A.; Jaafari, M.R.; Salehi, R.; Salehi, H.; Baharvand, H.; Rezaei, A.; Hadjati, J.; Pawelek, J.M.; et al. Application of Mesenchymal Stem Cells in Melanoma: A Potential Therapeutic Strategy for Delivery of Targeted Agents. Curr. Med. Chem. 2016, 23, 455–463.
- Houghton, J.; Stoicov, C.; Nomura, S.; Rogers, A.B.; Carlson, J.; Li, H.; Cai, X.; Fox, J.G.; Goldenring, J.R.; Wang, T.C. Gastric Cancer Originating from Bone Marrow-Derived Cells. Science 2004, 306, 1568–1571.
- Wei, X.; Yang, X.; Han, Z.-P.; Qu, F.-F.; Shao, L.; Shi, Y.-F. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol. Sin. 2013, 34, 747–754.
- Mishra, P.J.; Banerjee, D. Activation and Differentiation of Mesenchymal Stem Cells. Methods Mol. Biol. 2017, 1554, 201–209.
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313.
- Ma, Z.; Zhou, J.; Yang, T.; Xie, W.; Song, G.; Song, Z.; Chen, J. Mesenchymal stromal cell therapy for pancreatitis: Progress and challenges. Med. Res. Rev. 2021, 41, 2474–2488.
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.; Sensebe, L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT(R)) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy 2019, 21, 1019–1024.
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol. Sci. 2020, 41, 653–664.
- Krampera, M.; Le Blanc, K. Mesenchymal stromal cells: Putative microenvironmental modulators become cell therapy. Cell Stem Cell 2021, 28, 1708–1725.
- Devine, S.M.; Cobbs, C.; Jennings, M.; Bartholomew, A.; Hoffman, R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood 2003, 101, 2999–3001.
- Zhao, G.; Ge, Y.; Zhang, C.; Zhang, L.; Xu, J.; Qi, L.; Li, W. Progress of Mesenchymal Stem Cell-Derived Exosomes in Tissue Repair. Curr. Pharm. Des. 2020, 26, 2022–2037.
- Zhang, Y.; Daquinag, A.; Traktuev, D.O.; Amaya-Manzanares, F.; Simmons, P.J.; March, K.L.; Pasqualini, R.; Arap, W.; Kolonin, M.G. White Adipose Tissue Cells Are Recruited by Experimental Tumors and Promote Cancer Progression in Mouse Models. Cancer Res 2009, 69, 5259–5266.
- Okumura, T.; Ohuchida, K.; Kibe, S.; Iwamoto, C.; Ando, Y.; Takesue, S.; Nakayama, H.; Abe, T.; Endo, S.; Koikawa, K.; et al. Adipose tissue-derived stromal cells are sources of cancer-associated fibroblasts and enhance tumor progression by dense collagen matrix. Int. J. Cancer 2019, 144, 1401–1413.
- Jazowiecka-Rakus, J.; Hadrys, A.; Rahman, M.; McFadden, G.; Fidyk, W.; Chmielik, E.; Pazdzior, M.; Grajek, M.; Kozik, V.; Sochanik, A. Myxoma Virus Expressing LIGHT (TNFSF14) Pre-Loaded into Adipose-Derived Mesenchymal Stem Cells Is Effective Treatment for Murine Pancreatic Adenocarcinoma. Cancers 2021, 13, 1394.
- Han, J.; Hwang, H.S.; Na, K. TRAIL-secreting human mesenchymal stem cells engineered by a non-viral vector and photochemical internalization for pancreatic cancer gene therapy. Biomaterials 2018, 182, 259–268.
- Karp, J.M.; Teo, G.S.L. Mesenchymal Stem Cell Homing: The Devil Is in the Details. Cell Stem Cell 2009, 4, 206–216.
- Lau, T.T.; Wang, D.-A. Stromal cell-derived factor-1 (SDF-1): Homing factor for engineered regenerative medicine. Expert Opin. Biol. Ther. 2011, 11, 189–197.
- Vicinanza, C.; Lombardi, E.; Da Ros, F.; Marangon, M.; Durante, C.; Mazzucato, M.; Agostini, F. Modified mesenchymal stem cells in cancer therapy: A smart weapon requiring upgrades for wider clinical applications. World J. Stem Cells 2022, 14, 54–75.
- Ma, J.; Liu, N.; Yi, B.; Zhang, X.; Gao, B.; Zhang, Y.; Xu, R.; Li, X.; Dai, Y. Transplanted hUCB-MSCs migrated to the damaged area by SDF-1/CXCR4 signaling to promote functional recovery after traumatic brain injury in rats. Neurol. Res. 2015, 37, 50–56.
- Ringe, J.; Strassburg, S.; Neumann, K.; Endres, M.; Notter, M.; Burmester, G.-R.; Kaps, C.; Sittinger, M. Towards in situ tissue repair: Human mesenchymal stem cells express chemokine receptors CXCR1, CXCR2 and CCR2, and migrate upon stimulation with CXCL8 but not CCL2. J. Cell. Biochem. 2007, 101, 135–146.
- De Ugarte, D.A.; Alfonso, Z.; Zuk, P.A.; Elbarbary, A.; Zhu, M.; Ashjian, P.; Benhaim, P.; Hedrick, M.H.; Fraser, J.K. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol. Lett. 2003, 89, 267–270.
- Chopra, N.; Choudhury, S.; Bhargava, S.; Wajid, S.; Ganguly, N. Potentials of “stem cell-therapy” in pancreatic cancer: An update. Pancreatology 2019, 19, 1034–1042.
- Sun, Z.; Wang, S.; Zhao, R.C. The roles of mesenchymal stem cells in tumor inflammatory microenvironment. J. Hematol. Oncol. 2014, 7, 14.
- Volarevic, V.; Ljujic, B.; Stojkovic, P.; Lukic, A.; Arsenijevic, N.; Stojkovic, M. Human stem cell research and regenerative medicine--present and future. Br. Med Bull. 2011, 99, 155–168.
- Momin, E.N.; Vela, G.; Zaidi, H.A.; Quinones-Hinojosa, A. The Oncogenic Potential of Mesenchymal Stem Cells in the Treatment of Cancer: Directions for Future Research. Curr. Immunol. Rev. 2010, 6, 137–148.
- Kucerova, L.; Altanerova, V.; Matuskova, M.; Tyciakova, S.; Altaner, C. Adipose Tissue–Derived Human Mesenchymal Stem Cells Mediated Prodrug Cancer Gene Therapy. Cancer Res 2007, 67, 6304–6313.
- Ridge, S.M.; Sullivan, F.J.; Glynn, S.A. Mesenchymal stem cells: Key players in cancer progression. Mol. Cancer 2017, 16, 31.
- Galland, S.; Stamenkovic, I. Mesenchymal stromal cells in cancer: A review of their immunomodulatory functions and dual effects on tumor progression. J. Pathol. 2019, 250, 555–572.
- Fathi, E.; Sanaat, Z.; Farahzadi, R. Mesenchymal stem cells in acute myeloid leukemia: A focus on mechanisms involved and therapeutic concepts. Blood Res. 2019, 54, 165–174.
- Khakoo, A.Y.; Pati, S.; Anderson, S.A.; Reid, W.; Elshal, M.F.; Rovira, I.I.; Nguyen, A.T.; Malide, D.; Combs, C.A.; Hall, G. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J. Exp. Med. 2006, 203, 1235–1247.
- Dwyer, R.M.; Khan, S.; Barry, F.P.; O’Brien, T.; Kerin, M.J. Advances in mesenchymal stem cell-mediated gene therapy for cancer. Stem Cell Res. Ther. 2010, 1, 25.
- Shah, K. Mesenchymal stem cells engineered for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 739–748.
- Cousin, B.; Ravet, E.; Poglio, S.; De Toni, F.; Bertuzzi, M.; Lulka, H.; Touil, I.; André, M.; Grolleau, J.-L.; Péron, J.-M.; et al. Adult Stromal Cells Derived from Human Adipose Tissue Provoke Pancreatic Cancer Cell Death both In Vitro and In Vivo. PLoS ONE 2009, 4, e6278.
- Doi, C.; Maurya, D.K.; Pyle, M.M.; Troyer, D.; Tamura, M. Cytotherapy with naive rat umbilical cord matrix stem cells significantly attenuates growth of murine pancreatic cancer cells and increases survival in syngeneic mice. Cytotherapy 2010, 12, 408–417.
- Mohammadi, M.; Jaafari, M.R.; Mirzaei, H.R.; Mirzaei, H. Mesenchymal stem cell: A new horizon in cancer gene therapy. Cancer Gene Ther. 2016, 23, 285–286.
- Keung, E.Z.; Nelson, P.J.; Conrad, C. Concise Review: Genetically Engineered Stem Cell Therapy Targeting Angiogenesis and Tumor Stroma in Gastrointestinal Malignancy. Stem Cells 2013, 31, 227–235.
- De Palma, M.; Mazzieri, R.; Politi, L.S.; Pucci, F.; Zonari, E.; Sitia, G.; Mazzoleni, S.; Moi, D.; Venneri, M.A.; Indraccolo, S.; et al. Tumor-Targeted Interferon-α Delivery by Tie2-Expressing Monocytes Inhibits Tumor Growth and Metastasis. Cancer Cell 2008, 14, 299–311.
- Niess, H.; Bao, Q.; Conrad, C.; Zischek, C.; Notohamiprodjo, M.; Schwab, F.; Schwarz, B.; Huss, R.; Jauch, K.-W.; Nelson, P.J.; et al. Selective Targeting of Genetically Engineered Mesenchymal Stem Cells to Tumor Stroma Microenvironments Using Tissue-Specific Suicide Gene Expression Suppresses Growth of Hepatocellular Carcinoma. Ann. Surg. 2011, 254, 767–774, discussion 774–765.
- Mueller, L.P.; Lützkendorf, J.; Widder, M.; Nerger, K.; Caysa, H.; Mueller, T.D. TRAIL-transduced multipotent mesenchymal stromal cells (TRAIL-MSC) overcome TRAIL resistance in selected CRC cell lines in vitro and in vivo. Cancer Gene Ther. 2011, 18, 229–239.
- Vogler, M.; Dürr, K.; Jovanovic, M.; Debatin, K.-M.; Fulda, S. Regulation of TRAIL-induced apoptosis by XIAP in pancreatic carcinoma cells. Oncogene 2007, 26, 248–257.
- Niidome, T.; Huang, L. Gene Therapy Progress and Prospects: Nonviral vectors. Gene Ther. 2002, 9, 1647–1652.
- Vogler, M.; Walczak, H.; Stadel, D.; Haas, T.L.; Genze, F.; Jovanovic, M.; Bhanot, U.; Hasel, C.; Möller, P.; Gschwend, J.E.; et al. Small Molecule XIAP Inhibitors Enhance TRAIL-Induced Apoptosis and Antitumor Activity in Preclinical Models of Pancreatic Carcinoma. Cancer Res. 2009, 69, 2425–2434.
- Vogler, M.; Walczak, H.; Stadel, D.; Haas, T.L.; Genze, F.; Jovanovic, M.; Gschwend, J.E.; Simmet, T.; Debatin, K.-M.; Fulda, S. Targeting XIAP Bypasses Bcl-2–Mediated Resistance to TRAIL and Cooperates with TRAIL to Suppress Pancreatic Cancer Growth In vitro and In vivo. Cancer Res. 2008, 68, 7956–7965.
- Mohr, A.; Albarenque, S.M.; Deedigan, L.; Yu, R.; Reidy, M.; Fulda, S.; Zwacka, R.M. Targeting of XIAP Combined with Systemic Mesenchymal Stem Cell-Mediated Delivery of sTRAIL Ligand Inhibits Metastatic Growth of Pancreatic Carcinoma Cells. STEM CELLS 2010, 28, 2109–2120.
- Moniri, M.R.; Sun, X.-Y.; Rayat, J.; Dai, D.; Ao, Z.; He, Z.; Verchere, C.B.; Dai, L.-J.; Warnock, G.L. TRAIL-engineered pancreas-derived mesenchymal stem cells: Characterization and cytotoxic effects on pancreatic cancer cells. Cancer Gene Ther. 2012, 19, 652–658.
- Heldring, N.; Mäger, I.; Wood, M.J.; Le Blanc, K.; Andaloussi, S.E. Therapeutic Potential of Multipotent Mesenchymal Stromal Cells and Their Extracellular Vesicles. Hum. Gene Ther. 2015, 26, 506–517.
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63.
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977.
- Marote, A.; Teixeira, F.G.; Pinheiro, B.; Salgado, A.J. MSCs-Derived Exosomes: Cell-Secreted Nanovesicles with Regenerative Potential. Front. Pharmacol. 2016, 7, 231.
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852.
- Wang, J.; Li, G.; Tu, C.; Chen, X.; Yang, B.; Huo, Y.; Li, Y.; Chen, A.-Z.; Lan, P.; Zhang, Y.S.; et al. High-throughput single-cell analysis of exosome mediated dual drug delivery, in vivo fate and synergistic tumor therapy. Nanoscale 2020, 12, 13742–13756.
- Malhotra, H.; Sheokand, N.; Kumar, S.; Chauhan, A.S.; Kumar, M.; Jakhar, P.; Boradia, V.M.; Raje, C.I.; Raje, M. Exosomes: Tunable Nano Vehicles for Macromolecular Delivery of Transferrin and Lactoferrin to Specific Intracellular Compartment. J. Biomed. Nanotechnol. 2016, 12, 1101–1114.
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345.
- Shao, J.; Zaro, J.; Shen, Y. Advances in Exosome-Based Drug Delivery and Tumor Targeting: From Tissue Distribution to Intracellular Fate. Int. J. Nanomed. 2020, 15, 9355–9371.
- Yaghoubi, Y.; Movassaghpour, A.; Zamani, M.; Talebi, M.; Mehdizadeh, A.; Yousefi, M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019, 233, 116733.
- Xunian, Z.; Kalluri, R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020, 111, 3100–3110.
- Mendt, M.; Rezvani, K.; Shpall, E. Mesenchymal stem cell-derived exosomes for clinical use. Bone Marrow Transplant. 2019, 54, 789–792.
- Ohno, S.-I.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically Injected Exosomes Targeted to EGFR Deliver Antitumor MicroRNA to Breast Cancer Cells. Mol. Ther. 2013, 21, 185–191.
- Shibayama, Y.; Kondo, T.; Ohya, H.; Fujisawa, S.-I.; Teshima, T.; Iseki, K. Upregulation of microRNA-126-5p is associated with drug resistance to cytarabine and poor prognosis in AML patients. Oncol. Rep. 2015, 33, 2176–2182.
- Zhao, L.-X.; Zhang, K.; Shen, B.-B.; Li, J.-N. Mesenchymal stem cell-derived exosomes for gastrointestinal cancer. World J. Gastrointest. Oncol. 2021, 13, 1981–1996.
- Zhang, F.; Guo, J.; Zhang, Z.; Qian, Y.; Wang, G.; Duan, M.; Zhao, H.; Yang, Z.; Jiang, X. Mesenchymal stem cell-derived exosome: A tumor regulator and carrier for targeted tumor therapy. Cancer Lett. 2021, 526, 29–40.
- Pakravan, K.; Babashah, S.; Sadeghizadeh, M.; Mowla, S.; Mossahebi-Mohammadi, M.; Ataei, F.; Dana, N.; Javan, M. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1alpha/VEGF signaling axis in breast cancer cells. Cell Oncol. 2017, 40, 457–470.
- Li, X.; Liu, L.L.; Yao, J.L.; Wang, K.; Ai, H. Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles Inhibit Endometrial Cancer Cell Proliferation and Migration through Delivery of Exogenous miR-302a. Stem Cells Int. 2019, 2019, 8108576.
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503.
- Shang, S.; Wang, J.; Chen, S.; Tian, R.; Zeng, H.; Wang, L.; Xia, M.; Zhu, H.; Zuo, C. Exosomal miRNA-1231 derived from bone marrow mesenchymal stem cells inhibits the activity of pancreatic cancer. Cancer Med. 2019, 8, 7728–7740.
- Wu, D.-M.; Wen, X.; Han, X.-R.; Wang, S.; Wang, Y.-J.; Shen, M.; Fan, S.-H.; Zhang, Z.-F.; Shan, Q.; Li, M.-Q.; et al. RETRACTED: Bone Marrow Mesenchymal Stem Cell-Derived Exosomal MicroRNA-126-3p Inhibits Pancreatic Cancer Development by Targeting ADAM9. Mol. Ther. Nucleic Acids 2019, 16, 229–245.
- Xu, Y.; Liu, N.; Wei, Y.; Zhou, D.; Lin, R.; Wang, X.; Shi, B. Anticancer effects of miR-124 delivered by BM-MSC derived exosomes on cell proliferation, epithelial mesenchymal transition, and chemotherapy sensitivity of pancreatic cancer cells. Aging 2020, 12, 19660–19676.
- Wang, B.; Xu, Y.; Wei, Y.; Lv, L.; Liu, N.; Lin, R.; Wang, X.; Shi, B. Human Mesenchymal Stem Cell-Derived Exosomal microRNA-143 Promotes Apoptosis and Suppresses Cell Growth in Pancreatic Cancer via Target Gene Regulation. Front. Genet. 2021, 12, 581694.
- Xie, X.; Ji, J.; Chen, X.; Xu, W.; Chen, H.; Zhu, S.; Wu, J.; Wu, Y.; Sun, Y.; Sai, W.Z. Human umbilical cord mesenchymal stem cell-derived exosomes carrying hsa-miRNA-128-3p suppress pancreatic ductal cell carcinoma by inhibiting Galectin-3. Clin. Transl. Oncol. 2022, 24, 517–531.
- Pessina, A.; Bonomi, A.; Coccè, V.; Invernici, G.; Navone, S.; Cavicchini, L.; Sisto, F.; Ferrari, M.; Viganò, L.; Locatelli, A.; et al. Mesenchymal Stromal Cells Primed with Paclitaxel Provide a New Approach for Cancer Therapy. PLoS ONE 2011, 6, e28321.
- Bonomi, A.; Coccè, V.; Cavicchini, L.; Sisto, F.; Dossena, M.; Balzarini, P.; Portolani, N.; Ciusani, E.; Parati, E.; Alessandri, G.; et al. Adipose Tissue-Derived Stromal Cells Primed in Vitro with Paclitaxel Acquire Anti-Tumor Activity. Int. J. Immunopathol. Pharmacol. 2013, 26, 33–41.
- Pessina, A.; Cocce, V.; Bonomi, A.; Cavicchini, L.; Sisto, F.; Ferrari, M.; Ciusani, E.; Navone, S.; Marfia, G.; Parati, E.; et al. Human skin-derived fibroblasts acquire in vitro anti-tumor potential after priming with Paclitaxel. Anticancer Agents Med. Chem. 2013, 13, 523–530.
- Lin, W.; Huang, L.; Li, Y.; Fang, B.; Li, G.; Chen, L.; Xu, L. Mesenchymal Stem Cells and Cancer: Clinical Challenges and Opportunities. BioMed Res. Int. 2019, 2019, 2820853.
- Chulpanova, D.S.; Kitaeva, K.V.; Tazetdinova, L.G.; James, V.; Rizvanov, A.A.; Solovyeva, V.V. Application of Mesenchymal Stem Cells for Therapeutic Agent Delivery in Anti-tumor Treatment. Front. Pharmacol. 2018, 9, 259.
- Babajani, A.; Soltani, P.; Jamshidi, E.; Farjoo, M.H.; Niknejad, H. Recent Advances on Drug-Loaded Mesenchymal Stem Cells With Anti-neoplastic Agents for Targeted Treatment of Cancer. Front. Bioeng. Biotechnol. 2020, 8, 748.
- Henze, J.; Tacke, F.; Hardt, O.; Alves, F.; Al Rawashdeh, W. Enhancing the Efficacy of CAR T Cells in the Tumor Microenvironment of Pancreatic Cancer. Cancers 2020, 12, 1389.
- Varghese, A.M. Chimeric antigen receptor (CAR) T and other T cell strategies for pancreas adenocarcinoma. Chin. Clin. Oncol. 2017, 6, 66.
- Bonomi, A.; Silini, A.; Vertua, E.; Signoroni, P.B.; Coccè, V.; Cavicchini, L.; Sisto, F.; Alessandri, G.; Pessina, A.; Parolini, O. Human amniotic mesenchymal stromal cells (hAMSCs) as potential vehicles for drug delivery in cancer therapy: An in vitro study. Stem Cell Res. Ther. 2015, 6, 155.
- Brini, A.; Cocce, V.; Ferreira, L.; Giannasi, C.; Cossellu, G.; Gianni, A.; Angiero, F.; Bonomi, A.; Pascucci, L.; Falchetti, M. Cell-mediated drug delivery by gingival interdental papilla mesenchymal stromal cells (GinPa-MSCs) loaded with paclitaxel. Expert Opin. Drug Deliv. 2016, 13, 789–798.
- Eltoukhy, H.S.; Sinha, G.; Moore, C.A.; Sandiford, O.A.; Rameshwar, P. Immune modulation by a cellular network of mesenchymal stem cells and breast cancer cell subsets: Implication for cancer therapy. Cell. Immunol. 2018, 326, 33–41.
- Haney, M.; Klyachko, N.; Zhao, Y.; Gupta, R.; Plotnikova, E.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30.
- Zhou, Y.; Zhou, W.; Chen, X.; Wang, Q.; Li, C.; Chen, Q.; Zhang, Y.; Lu, Y.; Ding, X.; Jiang, C. Bone marrow mesenchymal stem cells-derived exosomes for penetrating and targeted chemotherapy of pancreatic cancer. Acta Pharm. Sin. B 2020, 10, 1563–1575.
- Jiang, M.; Liu, Z.; Xiang, Y.; Ma, H.; Liu, S.; Liu, Y.; Zheng, D. Synergistic antitumor effect of AAV-mediated TRAIL expression combined with cisplatin on head and neck squamous cell carcinoma. BMC Cancer 2011, 11, 54.
- Breitbach, C.J.; Burke, J.; Jonker, D.; Stephenson, J.; Haas, A.R.; Chow, L.Q.M.; Nieva, J.; Hwang, T.-H.; Moon, A.; Patt, R.; et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature 2011, 477, 99–102.
- Porter, D.L.; Levine, B.L.; Kalos, M.; Bagg, A.; June, C.H. Chimeric Antigen Receptor–Modified T Cells in Chronic Lymphoid Leukemia. N. Engl. J. Med. 2011, 365, 725–733.
- Mader, E.K.; Maeyama, Y.; Lin, Y.; Butler, G.W.; Russell, H.M.; Galanis, E.; Russell, S.J.; Dietz, A.B.; Peng, K.-W. Mesenchymal Stem Cell Carriers Protect Oncolytic Measles Viruses from Antibody Neutralization in an Orthotopic Ovarian Cancer Therapy Model. Clin. Cancer Res. 2009, 15, 7246–7255.
- Dembinski, J.; Spaeth, E.L.; Fueyo, J.; Gomez-Manzano, C.; Studeny, M.; Andreeff, M.; Marini, F.C. Reduction of nontarget infection and systemic toxicity by targeted delivery of conditionally replicating viruses transported in mesenchymal stem cells. Cancer Gene Ther. 2010, 17, 289–297.
- Shimizu, Y.; Gumin, J.; Gao, F.; Hossain, A.; Shpall, E.J.; Kondo, A.; Kerrigan, B.C.P.; Yang, J.; Ledbetter, D.; Fueyo, J.; et al. Characterization of patient-derived bone marrow human mesenchymal stem cells as oncolytic virus carriers for the treatment of glioblastoma. J. Neurosurg. 2022, 136, 757–767.
- Hammer, K.; Kazcorowski, A.; Liu, L.; Behr, M.; Schemmer, P.; Herr, I.; Nettelbeck, D.M. Engineered adenoviruses combine enhanced oncolysis with improved virus production by mesenchymal stromal carrier cells. Int. J. Cancer 2015, 137, 978–990.
- Moniri, M.R.; Dai, L.-J.; Warnock, G.L. The challenge of pancreatic cancer therapy and novel treatment strategy using engineered mesenchymal stem cells. Cancer Gene Ther. 2014, 21, 12–23.
- Błogowski, W.; Bodnarczuk, T.; Starzyńska, T. Concise Review: Pancreatic Cancer and Bone Marrow-Derived Stem Cells. STEM CELLS Transl. Med. 2016, 5, 938–945.
- Whiteside, T.L. Exosome and mesenchymal stem cell cross-talk in the tumor microenvironment. Semin. Immunol. 2018, 35, 69–79.
- Harrell, C.R.; Volarevic, A.; Djonov, V.G.; Jovicic, N.; Volarevic, V. Mesenchymal Stem Cell: A Friend or Foe in Anti-Tumor Immunity. Int. J. Mol. Sci. 2021, 22, 12429.
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladanyi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–1503.
- Saito, K.; Sakaguchi, M.; Maruyama, S.; Iioka, H.; Putranto, E.W.; Sumardika, I.W.; Tomonobu, N.; Kawasaki, T.; Homma, K.; Kondo, E. Stromal mesenchymal stem cells facilitate pancreatic cancer progression by regulating specific secretory molecules through mutual cellular interaction. J. Cancer 2018, 9, 2916–2929.
- Corsten, M.F.; Shah, K. Therapeutic stem-cells for cancer treatment: Hopes and hurdles in tactical warfare. Lancet Oncol. 2008, 9, 376–384.
- Waghray, M.; Yalamanchili, M.; Dziubinski, M.; Zeinali, M.; Erkkinen, M.; Yang, H.; Schradle, K.A.; Urs, S.; Di Magliano, M.P.; Welling, T.H.; et al. GM-CSF Mediates Mesenchymal–Epithelial Cross-talk in Pancreatic Cancer. Cancer Discov. 2016, 6, 886–899.
- Belli, C.; Trapani, D.; Viale, G.; D’Amico, P.; Duso, B.A.; Della Vigna, P.; Orsi, F.; Curigliano, G. Targeting the microenvironment in solid tumors. Cancer Treat. Rev. 2018, 65, 22–32.
- Quante, M.; Tu, S.P.; Tomita, H.; Gonda, T.; Wang, S.S.; Takashi, S.; Baik, G.H.; Shibata, W.; DiPrete, B.; Betz, K.S.; et al. Bone Marrow-Derived Myofibroblasts Contribute to the Mesenchymal Stem Cell Niche and Promote Tumor Growth. Cancer Cell 2011, 19, 257–272.
- Catalano, V.; Turdo, A.; Di Franco, S.; Dieli, F.; Todaro, M.; Stassi, G. Tumor and its microenvironment: A synergistic interplay. Semin. Cancer Biol. 2013, 23, 522–532.
- Giannoni, E.; Bianchini, F.; Masieri, L.; Serni, S.; Torre, E.; Calorini, L.; Chiarugi, P. Reciprocal Activation of Prostate Cancer Cells and Cancer-Associated Fibroblasts Stimulates Epithelial-Mesenchymal Transition and Cancer Stemness. Cancer Res. 2010, 70, 6945–6956.
- Kidd, S.; Spaeth, E.; Watson, K.; Burks, J.; Lu, H.; Klopp, A.; Andreeff, M.; Marini, F.C. Origins of the Tumor Microenvironment: Quantitative Assessment of Adipose-Derived and Bone Marrow–Derived Stroma. PLoS ONE 2012, 7, e30563.
- Zhou, H.; Su, X.; Fu, X.; Wu, G.; Luo, K.; Fang, Z.; Yu, F.; Liu, H.; Hu, H.; Chen, L. Mesenchymal stem cells promote pancreatic adenocarcinoma cells invasion by transforming growth factor-beta1 induced epithelial-mesenchymal transition. Oncotarget 2016, 7, 41294–41305.
- Ganguly, K.; Cox, J.; Ghersi, D.; Grandgenett, P.; Hollingsworth, M.; Jain, M.; Kumar, S.; Batra, S. Mucin 5AC-Mediated CD44/ITGB1 Clustering Mobilizes Adipose-Derived Mesenchymal Stem Cells to Modulate Pancreatic Cancer Stromal Heterogeneity. Gastroenterology 2022, 162, 2032–2046.e2012.
- Seebach, C.; Schultheiss, J.; Wilhelm, K.; Frank, J.; Henrich, D. Comparison of six bone-graft substitutes regarding to cell seeding efficiency, metabolism and growth behaviour of human mesenchymal stem cells (MSC) in vitro. Injury 2010, 41, 731–738.
- Roodhart, J.M.; Daenen, L.G.; Stigter, E.C.; Prins, H.-J.; Gerrits, J.; Houthuijzen, J.M.; Gerritsen, M.G.; Schipper, H.S.; Backer, M.J.; van Amersfoort, M.; et al. Mesenchymal Stem Cells Induce Resistance to Chemotherapy through the Release of Platinum-Induced Fatty Acids. Cancer Cell 2011, 20, 370–383.
- Wang, J.; Hendrix, A.; Hernot, S.; Lemaire, M.; De Bruyne, E.; Van Valckenborgh, E.; Lahoutte, T.; De Wever, O.; Vanderkerken, K.; Menu, E. Bone marrow stromal cell–derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood 2014, 124, 555–566.
- Bao, Q.; Zhao, Y.; Niess, H.; Conrad, C.; Schwarz, B.; Jauch, K.-W.; Huss, R.; Nelson, P.J.; Bruns, C.J. Mesenchymal Stem Cell-Based Tumor-Targeted Gene Therapy in Gastrointestinal Cancer. Stem Cells Dev. 2012, 21, 2355–2363.
- Barbash, I.; Chouraqui, P.; Baron, J.; Feinberg, M.; Etzion, S.; Tessone, A.; Miller, L.; Guetta, E.; Zipori, D.; Kedes, L. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: Feasibility, cell migration, and body distribution. Circulation 2003, 108, 863–868.
- Kraitchman, D.L.; Tatsumi, M.; Gilson, W.D.; Ishimori, T.; Kedziorek, D.; Walczak, P.; Segars, W.P.; Chen, H.H.; Fritzges, D.; Izbudak, I.; et al. Dynamic Imaging of Allogeneic Mesenchymal Stem Cells Trafficking to Myocardial Infarction. Circulation 2005, 112, 1451–1461.
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S., Jr. Pulmonary Passage is a Major Obstacle for Intravenous Stem Cell Delivery: The Pulmonary First-Pass Effect. Stem Cells Dev. 2009, 18, 683–692.
- Assis, A.C.M.; Carvalho, J.; Jacoby, B.A.; Ferreira, R.L.B.; Castanheira, P.; Diniz, S.O.F.; Cardoso, V.N.; Goes, A.M.; Ferreira, A.J. Time-Dependent Migration of Systemically Delivered Bone Marrow Mesenchymal Stem Cells to the Infarcted Heart. Cell Transplant. 2010, 19, 219–230.
- Lee, R.H.; Yoon, N.; Reneau, J.C.; Prockop, D.J. Preactivation of Human MSCs with TNF-α Enhances Tumor-Suppressive Activity. Cell Stem Cell 2012, 11, 825–835.
- Beckermann, B.M.; Kallifatidis, G.; Groth, A.; Frommhold, D.; Apel, A.; Mattern, J.; Salnikov, A.V.; Moldenhauer, G.; Wagner, W.; Diehlmann, A.; et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br. J. Cancer 2008, 99, 622–631.
- Miloradovic, D.; Miloradovic, D.; Markovic, B.S.; Acovic, A.; Harrell, C.R.; Djonov, V.; Arsenijevic, N.; Volarevic, V. The Effects of Mesenchymal Stem Cells on Antimelanoma Immunity Depend on the Timing of Their Administration. Stem Cells Int. 2020, 2020, 8842659.
- Allen, M.; Louise Jones, J. Jekyll and Hyde: The role of the microenvironment on the progression of cancer. J. Pathol. 2011, 223, 163–177.
- D’Aloia, M.M.; Zizzari, I.G.; Sacchetti, B.; Pierelli, L.; Alimandi, M. CAR-T cells: The long and winding road to solid tumors. Cell Death Dis. 2018, 9, 282.
More
Information
Subjects:
Surgery
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
829
Revisions:
2 times
(View History)
Update Date:
15 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




