Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Xianjun Yu and Version 2 by Lindsay Dong.
Pancreatic cancer is an aggressive malignancy with high mortality rates and poor prognoses. Despite rapid progress in the diagnosis and treatment of pancreatic cancer, the efficacy of current therapeutic strategies remains limited. Hence, better alternative therapeutic options for treating pancreatic cancer need to be urgently explored. Mesenchymal stromal cells (MSCs) have recently received much attention as a potential therapy for pancreatic cancer owing to their tumor-homing properties.
- pancreatic cancer
- mesenchymal stromal cells
- exosomes
- tumor-targeted therapy
1. Introduction
Pancreatic cancer, an aggressive human malignant tumor, is often termed a silent killer owing to its poor prognosis, and its incidence has been increasing over the years [1][2][3][1,2,3]. The mortality rate within one year after diagnosis is approximately 75%, and the 5-year survival rate is no more than 8% [4][5][6][4,5,6]. Pancreatic ductal adenocarcinoma (PDAC) accounts for 90% of all pancreatic tumors, and other subtypes include acinar carcinoma, pancreatoblastoma, and neuroendocrine neoplasms [3]. Approximately 50% of patients with PDAC display no symptoms during the early stage, and by the time a diagnosis is confirmed, they are in the late stage of PDAC [3][7][8][3,7,8]. Thus, most patients miss opportunities for radical surgical resection in the early stage and can only undergo radiotherapy and chemotherapy later. However, owing to the special extracellular matrix barrier of pancreatic cancer and resistance to chemotherapeutic drugs, some cancer cells cannot be killed [9][10][9,10]. Furthermore, approximately 40% of patients with PDAC experience tumor recurrence even after surgical resection and die within one year [11][12][13][11,12,13]. Despite the rapid progress in the diagnosis and therapy of pancreatic cancer, the efficacy of present therapeutic measures remains poor [7]. Therefore, identifying alternative treatment strategies for the better management of pancreatic cancer is an urgent requirement.
As a carrier of anti-tumor drugs, mesenchymal stromal cells (MSCs) can be genetically engineered to release various agents such as treatment proteins, suicide genes, and oncolytic viruses to decrease cancer growth and progression [14][15][14,15]. The application of MSCs as therapeutic biological carriers in cytotherapy has some distinct advantages, including low immunogenicity, tumor tropism, a massive expansion in vitro, and the ability to transfer various therapeutic agents [16][17][18][19][16,17,18,19]. Reportedly, MSCs can home to tumor locations and survive in the tumor microenvironment (TME) [16][20][21][16,20,21].
2. Tumor-Homing Properties of MSCs
MSCs are adult stem cells capable of multilineage differentiation and self-renewal [22][23][46,47]. MSCs exist in most tissues and are usually extracted from various sources, including bone marrow, umbilical cord, menstrual blood, placenta, adipose tissues, and muscles [19][24][25][26][19,48,49,50]. To date, MSCs have been shown to treat multiple diseases owing to their immunomodulatory and anti-inflammatory effects and tissue repair ability [18][27][28][18,51,52]. They thus have excellent application prospects in regenerative medicine. MSCs can accurately migrate to injured tissues and organs and play a key role in inhibiting inflammation, decreasing tissue fibrosis formation, and promoting regeneration, thereby indicating that MSCs can selectively migrate to certain sites in the body [29][30][53,54]. Moreover, MSCs have been found to selectively migrate to primary and metastatic tumor locations, thus revealing the tumor-homing capacity of MSCs [16][20][21][31][32][33][34][35][16,20,21,55,56,57,58,59]. However, despite reports that MSCs could migrate to tumor locations in various types of tumors, the potential mechanisms by which MSCs home to tumors are still unclear. MSCs express various chemokines and cell adhesion molecules that coordinate the mobilization of MSCs to the damage locations [15][36][37][38][39][40][15,60,61,62,63,64]. Recent research has found that the tumor-homing capacity of MSCs could be regulated by the cooperation of cytokines, chemokines, and adhesion molecules [15][41][42][43][44][45][15,24,65,66,67,68]. Hence, this observation indicates that the homing capabilities of MSCs could depend on the inflammatory microenvironment of the tumor. MSCs are involved in the initiation, development, progression, and metastasis of tumors [46][69]. They can directly affect tumor development through crosstalk with tumors or the release of soluble molecules [14]. Although MSCs are indicated to exhibit pro-tumor effects [47][70], they can also inhibit the growth of tumors by various mechanisms, such as inhibiting tumor cell proliferation and promoting tumor cell death [48][71]. Furthermore, owing to their tumor-homing properties, MSCs and their exosomes have been considered promising tools for the accurate and selective release of antitumor molecules, RNA, or anticancer drugs that aid in reducing tumor cell viability and invasive characteristics [16][17][49][50][51][16,17,72,73,74]. Therefore, MSCs may serve as a potential tumor-targeted therapeutic strategy. On infusing MSCs into rats, MSCs could home to pancreatic cancer sites to exert their anticancer effects [33][34][57,58]. However, the mechanism by which MSCs crosstalk with tumor cells has not yet been elucidated, and consequently, translational medicine progress has been limited. Thus, the underlying molecular mechanism by which MSCs crosstalk with tumors needs further exploration; this will aid in improving the effectiveness of MSC therapeutic potential.3. MSC Therapy for Pancreatic Cancer
3.1. Native MSCs
Naïve MSCs have some potential advantages for treatment, such as abundance, low immunogenicity, and ease of isolation and in vitro expansion. Hence, it is widely applied to various diseases including cancer. Cousin et al. found that native human adipose tissue-derived mesenchymal stromal cells (AD-MSCs) inhibit pancreatic cancer cell proliferation and promote tumor cell death by inhibiting the cell cycle at the G1 phase [52][75]. Doi et al. observed that native rat umbilical cord matrix-derived stem cells (UCMSCs) decrease the growth of pancreatic tumors in mouse peritoneal models and increase the overall survival time of mice [53][76].3.2. Genetically Engineered MSCs
Genetically modified MSCs are promising potential cancer therapies to further enhance the efficacy of MSCs to target tumor cells. These MSCs deliver anti-proliferative, pro-apoptotic, and anti-angiogenic molecules to target tumor cells [47][54][70,79]. These effects might depend on several mechanisms: MSCs preferentially migrate to locations of inflammation, ischemia, and malignancy; genetically modified MSCs only release therapeutic gene products in the special TME; transgenes encoding biologic agents might themselves exhibit targeted and differential effects in tumor cells [55][80]. Previous studies have shown that selective targeting of therapeutic gene expression by MSCs is feasible and effective in the treatment of various cancers [56][57][58][81,82,83]. TRAIL is a therapeutic protein that induces tumor cell death; however, pancreatic cancer cells present intrinsic resistance toward TRAIL by the expression of anti-apoptotic proteins like the X-linked inhibitor of apoptosis protein (XIAP) [59][60][85,86]. Inhibiting XIAP could promote TRAIL-induced apoptosis of pancreatic cancer cells [61][62][87,88]. Mohr et al. found that the TRAIL-modified mouse bone marrow MSCs (BM-MSCs) deliver soluble TRAIL that suppresses the metastatic growth of pancreatic cancers [63][89]. Moreover, TRAIL-transfected pancreas-derived MSCs can promote pancreatic cancer cell death [64][90]. In summary, combining MSCs with selective gene treatment results in enhanced therapeutic effects on inhibiting tumor growth; this might aid in developing new tools for pancreatic cancer treatment (Figure 1).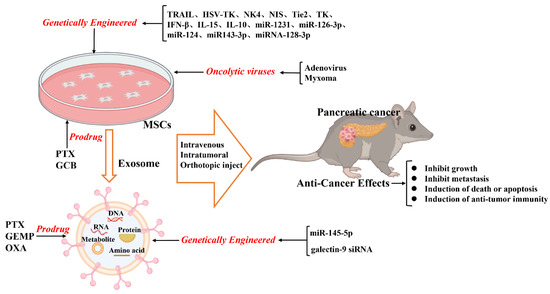
Figure 1. Schematic illustration of the current anticancer treatment based on mesenchymal stromal cells (MSCs) and MSC-derived exosomes.
3.3. Exosomes as a Vehicle for Therapy Delivery
Thus far, numerous studies have found that MSCs can secrete extracellular vesicles), including microvesicles (100–2000 nm in diameter) and exosomes (30–150 nm in diameter), which act as paracrine mediators between MSCs and target cells [65][66][67][99,100,101]. Exosomes can deliver cargo (nucleic acids, proteins, lipids, amino acids, and metabolites) from the originating cells to the target cells [67][68][69][101,102,103]. Compared with those of artificial nanocarriers, exosomes, as natural vesicles secreted by cells, have double lipid membranes, better biocompatibility, lower immunogenicity, stronger targeting specificity, deeper tissue permeability, and longer circulating half-life [67][70][71][72][73][101,104,105,106,107]. Based on these advantages, exosomes have been applied for engineering functional cargo loads, such as packaged nucleic acid, functional proteins, and other therapeutic molecules into exosomes [66][67][74][75][76][100,101,108,109,110].
Notably, exosomes have been shown to transfer microRNAs (miRNAs) to target cancer cell proliferation, differentiation, and metastasis [75][77][78][79][80][109,111,112,113,114]. For example, miRNA-100 carried by MSC-derived exosomes suppress tumor angiogenesis and breast cancer progression via the mTOR/HIF1A/VEGF pathway [81][115]. Additionally, Li et al. showed that engineered exosomes from UCMSCs enriched with miR-302a significantly inhibit endometrial cancer cell proliferation and migration by decreasing cyclin D1 expression and inhibiting the AKT pathway [82][116].
On modifying the normal fibroblast-like mesenchymal cell-derived exosomes to deliver short interfering RNA or short hairpin RNA to target oncogenic KrasG12D, tumor growth was found to be decreased in multiple mouse models of pancreatic cancer, thereby increasing the overall survival [83][118]. The study offers insight into the target therapeutic potential of exosomes in pancreatic cancer. Furthermore, infusing the exosomes derived from miRNA-engineered MSCs, which contain abundant MSC-sourced anti-tumorigenic miRNAs, can represent a potentially new therapeutic measure for pancreatic cancer. For example, the exosomes extracted from miR-1231-modified BM-MSCs with high levels of miR-1231 reduce the proliferation of pancreatic cancer cells [84][119]. Overexpressed miR-126-3p derived from BM-MSC exosomes inhibit the proliferation, invasion, and metastasis of pancreatic tumor cells and induce their apoptosis in vitro and in vivo by inhibiting the expression of ADAM9 [85][120]. Exosomes derived from miRNA-MSCs release miR-124 and miR143-3p in pancreatic tumor cells, inhibiting the proliferation of tumor cells [86][87][121,122]. The exosomes derived from miRNA-128-3p-modified UCMSCs can inhibit the proliferation, invasion, and migration of pancreatic cancer cells via the miRNA-128-3p/Galectin-3 axis [88][123].
3.4. MSC-Mediated Drug Delivery
MSCs can incorporate chemotherapeutic drugs in vitro, subsequently releasing the effective concentration of drugs in their conditioned medium to exert therapeutic effects [89][90][91][126,127,128]. Furthermore, tumor-homing properties of MSCs allow them to precisely deliver the drug to the tumor location; this has been widely studied as a targeted delivery agent of anti-cancer drugs [92][93][94][27,78,129].The proliferation of stromal fibroblasts and the deposition of extracellular matrix, which are the defining characteristics of PDAC, lead to a fibrotic state known as desmoplastic or reactive stroma [95][96][45,132]. Therefore, this could make it difficult to acquire an effective drug concentration by the common route of administration. Notably, after the MSCs were preconditioned to high doses of paclitaxel (PTX), they intracellularly accumulate the drug and then release it, thereby inhibiting pancreatic tumor cell proliferation [97][133]. Brini et al. demonstrated that PTX-loaded gingival interdental papilla MSCs can release a sufficient amount of PTX to inhibit the proliferation of pancreatic tumor cells [98][134]. Exosomes can be loaded with therapeutic drugs and then used to release them into the target cells [99][100][136,137]. The methods applied for directly loading drugs inside exosomes include incubation, electroporation, sonication, extrusion, freeze-thaw cycles, and saponin application. Presently, the most commonly applied methods are incubation and electroporation [80][100][114,137]. As drug carriers, exosomes are widely studied as therapeutic agents and can potentially be clinically applied. Recently, GEMP- and PTX-loaded exosomes revealed superiorities in homing and penetrating abilities that aided in inhibiting the growth of pancreatic tumors in vivo [101][138].3.5. Delivery of Oncolytic Viruses
The oncolytic virus has revealed promising results in the treatment of several cancers in various clinical trials [102][103][104][140,141,142]. It can directly cause oncolysis and spread to adjacent tumor cells to activate an anti-cancer immune response. Oncolytic viruses can replicate and selectively target tumor cells, but they cannot bind or effectively replicate in most normal cells. MSCs have been shown to protect viruses from immune clearance through a unique cell carrier tool before delivering them to metastatic tumor sites [105][106][107][143,144,145]. Although the tumor-homing ability of MSCs makes them a promising candidate for systemically delivering oncolytic viruses to tumor location, infection and particle production by MSCs remain areas of concern. The viruses genetically modified for improved delivery by MSCs are aimed at enhancing oncolysis and improving virus production in tumor cells [108][146].4. Challenges of MSCs in Treating Pancreatic Cancer
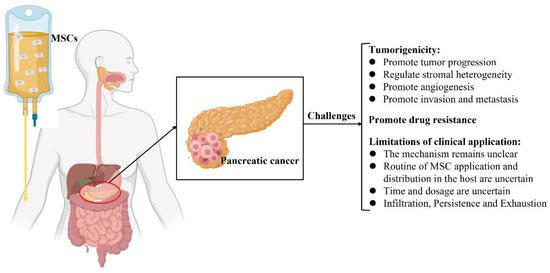
MSCs have been used as a therapeutic intervention for tumors; nevertheless, they are reportedly involved in tumor progression, including tumorigenesis, tumor growth, metastasis, and regulation of the TME [79][109][23,113]. Furthermore, the potential mechanisms by which MSCs crosstalk with tumor cells in the TME have not yet been elucidated [41][109][110][111][22,23,24,149]. Hence, the clinical application of MSCs in the treatment of pancreatic cancer remains controversial and challenging (Figure 2).
Figure 2. Schematic illustration of the current challenges of mesenchymal stromal cell treatment in pancreatic cancer.
4.1. Tumorigenicity
Numerous studies have shown that MSCs have inherent tumorigenicity properties [17][31][112][113][114][115]. MSCs possess the molecular potential to affect and direct several crucial processes, which are important for tumor development, as the cells contain an abundant source of various biochemical mediators [14][110]. MSCs have been successfully isolated from various types of tumor tissues, such as HCC, glioma, gastric cancer, breast cancer, ovarian cancer, prostate cancer, colon cancer, and pancreatic cancer, indicating that MSC is a distinct stromal cell type in the TME that participates in tumor development [17][116]. It consists of stromal cells that include tumor-associated fibroblasts, tumor endothelial cells, immune and inflammatory cells, and bone marrow-derived cells [117][118]. Interactions between tumor cells and the TME tremendously impact tumor development, metastasis, and drug resistance [119][120].
MSCs can reportedly modulate stromal heterogeneity in various solid tumors, including pancreatic cancer [113][121]. Furthermore, MSCs regulate specific secretory molecules in the TME and promote the progression and invasion of pancreatic cancer [114][122]. AD-MSCs migrate to pancreatic cancer locations to serve as a major source of a-SMA+ cells and promote tumor progression [31][32]. Nevertheless, the mechanism underlying the mobilization of these intricate molecules remains unclear. Notably, Ganguly et al. found that MUC5AC acts as a systemic carrier of tumor secretome and can alter stromal maturation in pancreatic cancer by mobilizing AD-MSCs via CD44 and CD29/ITGB1 clustering [123].
Schematic illustration of the current challenges of mesenchymal stromal cell treatment in pancreatic cancer.
4.1. Tumorigenicity
Numerous studies have shown that MSCs have inherent tumorigenicity properties [17,55,150,151,152,153]. MSCs possess the molecular potential to affect and direct several crucial processes, which are important for tumor development, as the cells contain an abundant source of various biochemical mediators [14,22]. MSCs have been successfully isolated from various types of tumor tissues, such as HCC, glioma, gastric cancer, breast cancer, ovarian cancer, prostate cancer, colon cancer, and pancreatic cancer, indicating that MSC is a distinct stromal cell type in the TME that participates in tumor development [17,154]. It consists of stromal cells that include tumor-associated fibroblasts, tumor endothelial cells, immune and inflammatory cells, and bone marrow-derived cells [155,156]. Interactions between tumor cells and the TME tremendously impact tumor development, metastasis, and drug resistance [157,158].
MSCs can reportedly modulate stromal heterogeneity in various solid tumors, including pancreatic cancer [151,159]. Furthermore, MSCs regulate specific secretory molecules in the TME and promote the progression and invasion of pancreatic cancer [152,160]. AD-MSCs migrate to pancreatic cancer locations to serve as a major source of a-SMA+ cells and promote tumor progression [55,56]. Nevertheless, the mechanism underlying the mobilization of these intricate molecules remains unclear. Notably, Ganguly et al. found that MUC5AC acts as a systemic carrier of tumor secretome and can alter stromal maturation in pancreatic cancer by mobilizing AD-MSCs via CD44 and CD29/ITGB1 clustering [161].
4.2. MSCs Promote Drug Resistance
MSCs have been revealed to induce and play an important role in the drug resistance of tumor cells in the TME [136,170]. Several potential mechanisms underlying this phenomenon might include promoting active drug sequestration, decreasing drug concentration, and delivering specific RNA, proteins, and functional small molecules into target cells to induce dysregulation of relevant signaling pathways. For example, Roodhart et al. found that endogenous MSCs are activated on treatment with platinum analogs and release some mediators to protect tumor cells against a range of chemotherapeutics. By a metabolomics method, the results showed that two distinct platinum-induced polyunsaturated fatty acids derived from MSCs, 12-oxo-5,8,10-heptadecatrienoic acid and hexadeca-4,7,10,13-tetraenoic acid [16:4 (n−3)], induce resistance to platinum-based chemotherapy [171]. Furthermore, MSC-derived exosomes induce drug resistance in various tumor cells. Wang et al. showed that BM-MSC-derived exosomes play key roles in drug resistance in multiple myeloma and induce their proliferation, migration, and survival [172].4.2. MSCs Promote Drug Resistance
4.3. Limitations of the Clinical Applications of MSCs
MSCs have been revealed to induce and play an important role in the drug resistance of tumor cells in the TME [99][124]. Several potential mechanisms underlying this phenomenon might include promoting active drug sequestration, decreasing drug concentration, and delivering specific RNA, proteins, and functional small molecules into target cells to induce dysregulation of relevant signaling pathways. For example, Roodhart et al. found that endogenous MSCs are activated on treatment with platinum analogs and release some mediators to protect tumor cells against a range of chemotherapeutics. By a metabolomics method, the results showed that two distinct platinum-induced polyunsaturated fatty acids derived from MSCs, 12-oxo-5,8,10-heptadecatrienoic acid and hexadeca-4,7,10,13-tetraenoic acid [16:4 (n−3)], induce resistance to platinum-based chemotherapy [125]. Furthermore, MSC-derived exosomes induce drug resistance in various tumor cells. Wang et al. showed that BM-MSC-derived exosomes play key roles in drug resistance in multiple myeloma and induce their proliferation, migration, and survival [126].
Numerous studies have reported the tumor-homing properties of MSCs; however, the migration and distribution of MSCs in the body are not yet clearly understood. In animal experiments, the most common method of infusion of MSCs is through the intravenous (IV) or intraperitoneal (i.p.) route [127][175]. Nevertheless, owing to their size and the small dimensions of the lung vessels, a large number of MSCs are temporarily distributed in the lung after IV administration [128][129][130][131][132][176,177,178,179,180]. Notably, three days after IV administration, most of the MSCs are recruited to the tumor locations in an orthotopic pancreatic cancer model of athymic nude mice; only some MSCs are observed in the lung [133][164]. 