You're using an outdated browser. Please upgrade to a modern browser for the best experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Meysam Firoozbahr | -- | 4244 | 2023-03-09 11:16:06 | | | |
| 2 | Dean Liu | -9 word(s) | 4235 | 2023-03-10 02:53:51 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Firoozbahr, M.; Kingshott, P.; Palombo, E.A.; Zaferanloo, B. Natural Antibacterial Additives in Bioactive Wound Dressings. Encyclopedia. Available online: https://encyclopedia.pub/entry/42016 (accessed on 02 January 2026).
Firoozbahr M, Kingshott P, Palombo EA, Zaferanloo B. Natural Antibacterial Additives in Bioactive Wound Dressings. Encyclopedia. Available at: https://encyclopedia.pub/entry/42016. Accessed January 02, 2026.
Firoozbahr, Meysam, Peter Kingshott, Enzo A. Palombo, Bita Zaferanloo. "Natural Antibacterial Additives in Bioactive Wound Dressings" Encyclopedia, https://encyclopedia.pub/entry/42016 (accessed January 02, 2026).
Firoozbahr, M., Kingshott, P., Palombo, E.A., & Zaferanloo, B. (2023, March 09). Natural Antibacterial Additives in Bioactive Wound Dressings. In Encyclopedia. https://encyclopedia.pub/entry/42016
Firoozbahr, Meysam, et al. "Natural Antibacterial Additives in Bioactive Wound Dressings." Encyclopedia. Web. 09 March, 2023.
Copy Citation
Wound care is a global health issue with a financial burden of up to US $96.8 billion annually in the USA alone. Chronic non-healing wounds which show delayed and incomplete healing are especially problematic. Natural products and their derivatives have long been a significant source of pharmaceuticals against AMR. Scrutinising the data of newly approved drugs has identified plants as one of the biggest and most important sources in the development of novel antibacterial drugs.
antibacterial additives
natural products
polymer wound dressing
endophytic fungi
1. Introduction
More than 3000 wound dressing types on the wound management market, different characteristics can be achieved based on the intrinsic properties of the polymers used in wound dressing preparation. These characteristics include their ability to absorb exudate, combat infection, relieve pain, promote autolytic debridement, or even provide and maintain a moist environment at the wound surface. However, there is no wound dressing that possesses all these properties. The type of wound dressing is selected based on the patient’s health status, wound type, location, depth, amount of exudate, wound adhesion, and economic considerations [1][2]. Hydrogels, foams, dermal patches, films, nanoparticles, hydrocolloids, nanofibers, and membranes are the main groups of dressings, and their description, characteristics, and polymers used to make them are summarised in Table 1 [1].
Table 1. Different types of wound dressings, their wound target, and polymer type.
| Variety | Description | Advantages | Disadvantages | Wound Type Application | Polymer | Ref. |
|---|---|---|---|---|---|---|
| Hydrogels | Water-absorbent cross-linked polymeric networks resulting from the reaction of monomers | Efficient flexibility, good ability in swelling and sustaining a significant amount of water, moisturizing, removal of necrotic tissue, good porosity, and monitoring the wound without removing the dressing | Inability to absorb enough exudates leading to bacterial proliferation, and low mechanical strength | Chemotherapy peels | Polyethylene oxide, polyvinyl pyrrolidine, Polyvinyl alcohol | [1][2][3][4][5] |
| Ulcers | ||||||
| Laser resurfacing | ||||||
| Average thickness wounds | ||||||
| Donor graft sites and artificial organ wounds | ||||||
| Hydrocolloids | Colloidal material (gel) constituted with elastomers and adhesives in the form of films or sheets | Excellent exudate absorption properties, transparency, enhanced angiogenesis, and formation of granulation tissue | Not permeable to gas, vapor, water, and bacteria, their debriding capability, skin maceration, and producing a foul smell | Chronic ulcers | Pectin, carboxymethylcellulose, gelatin, and cellulose | [1][4][6][7] |
| Burns | ||||||
| Average thickness wounds | ||||||
| Donor graft sites | ||||||
| Foams | A porous structure using capillary action as its mechanism to absorb fluids | Exudate absorbance, preventing bacteria invasion, maintaining sufficient moisture at the wound surface, being removed easily, protecting the skin around the wound, maintaining an efficient temperature, mechanical protection, being nontoxic, being cost-effective with a long shelf life | Drying out the wound in case of minimal or no exudate presence and maceration of the surrounding skin in case of exudate saturation in dressing | Chronic wounds | Polyurethane, silicone, silk fibroin | [4][8][9][10][11][12] |
| Burns | ||||||
| Mohs surgery and wounds | ||||||
| Laser resurfacing wounds | ||||||
| Films | Consists of adhesives, porous, and thin transparent polymers | The possibility of having a high mechanical strength, high water transmission rate, protecting the wound against bacterial infection | The possibility of having a low mechanical strength | Superficial wounds | Soy protein isolates, chitosan, polyvinyl alcohol | [4][13][14][15] |
| Laser wounds | ||||||
| Surgery defect sites | ||||||
| Skin tears | ||||||
| Dermal patches | Dressings consisted of a multilayered structure with an impermeable excipient-loaded film, drugs, and a release liner | Suitable for skin adhesion, not having a liquid reservoir, controlling the drug delivery rate | Needing flux moderation in case of loading with highly soluble drugs, and decrease in drug release rate with wear time, not suitable for most of the drugs | Hypertension | Poly(vinyl pyrrolidones), poly(vinyl alcohol) | [16][17][18][19][20][21] |
| Topical wounds | ||||||
| Fibers and nanofibers | Polymeric fibers produced with electrospinning process | Excellent mechanical properties, thermal stability, antimicrobial activity, biodegradability, control in water vapor transmission rate, oxygen permeability, fluid drainage ability, high porosity, and high surface area | Higher cost of production in some cases, hard to produce fibers with diameters less than 10 nm | Partial thickness burns | Polyurethane, collagen, silk fibroin, polycaprolactone, poly (lactic-co-glycolic acid), polyethylene oxide, etc. | [22][23][24][25][26][27][28][29][30][31] |
| Diabetic ulcers | ||||||
| Bone bleeding | ||||||
| Chronic infected wounds | ||||||
| Acute wounds | ||||||
| Venous ulcers | ||||||
| Pressure ulcers | ||||||
| Membranes | A thin semi-permeable barrier | Porous structure, transparency, excessive loss of water, the ability to contain an occlusive layer to impede microbial invasion | Cytotoxicity in some cases | Superficial wounds | Pectin, collagen, chitosan, chitin, alginate, zein, polycaprolactone, polyvinyl acetate, polyvinyl alcohol, polytetrafluoroethylene, cellulose, etc. | [32][33][34][35][36][37][38][39][40][41][42][43] |
| Frictional wounds | ||||||
| Skin-scratching wounds | ||||||
| Skin donor sites | ||||||
| Skin with external contamination | ||||||
| Polymer-drug conjugates | Polymer-based water-soluble nanocarriers conjugated with bioactive agents | Improving the water solubility of the hydrophobic drugs, enhancing the pharmacokinetic profile of the conjugated drug, extending the volume of distribution, and protecting the conjugated drug against degradation | Limitations to be applied on a large scale, low stability in vivo, short half-life, and immunogenicity | Diabetic wounds such as venous leg and lower limb ulcers | N-(2-hydroxypropyl) methacrylamide copolymer, polyglutamic acid, Poly(ethylene glycol), Polyamidoamine, hyaluronic acid, poly (vinyl ether-co-maleic anhydride), poly (vinyl pyrrolidone), etc. | [44][45][46][47][48][49][50][51][52] |
Studies show that the challenges of wound dressings linked to wound infection are significant. In most acute and chronic infections, a mixed population of both aerobic and anaerobic microorganisms is observed [53] and yet to be eliminated. This challenge emphasises the importance of strategies that target the most common bacteria on the wound surface. Data from recent studies on various wound infections (e.g., surgical incisions, burns, abscesses, and traumatic wounds) confirm the presence of Pseudomonas sp. and Staphylococcus aureus as the most common Gram-negative and Gram-positive bacteria, respectively, on wound surfaces with a share of 58.4% for Gram-negative and 41.6% for Gram-positive bacteria [54]. The percentage of each group of bacteria can be observed in detail in Figure 1.
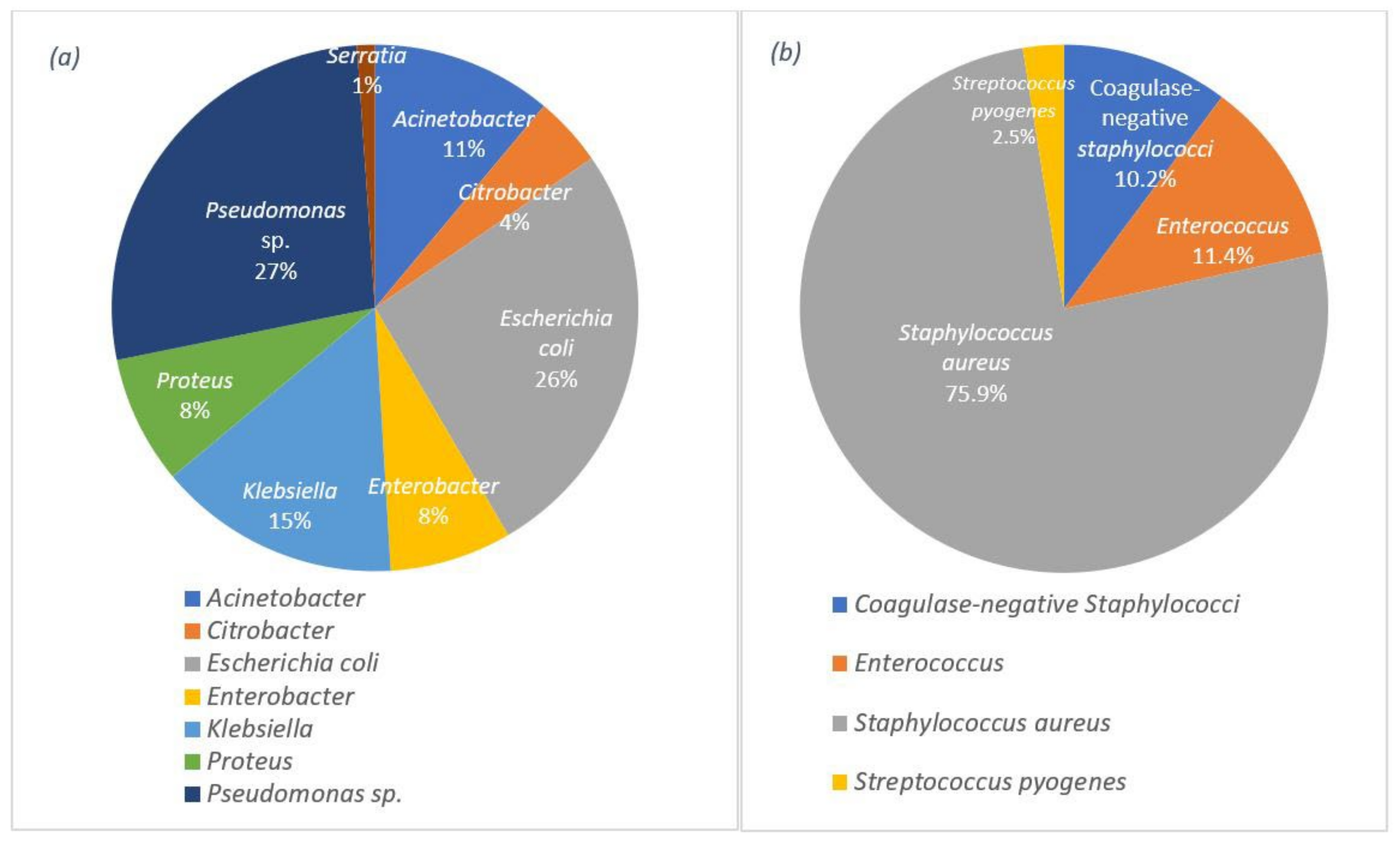
Figure 1. Distribution of the wound infections by: (a) the Gram-negative bacteria and (b) the Gram-positive bacteria.
There is a significant need for antibacterial wound dressings for controlling and reducing bacterial infections. One of the best approaches is wound dressings containing antibiotics as an additive. Numerous reports can be found on wound dressings containing antibiotic additives and their effects on wound dressings [55][56][57].
By using an efficient amount of antibiotics, suitable treatment of wound infections can be achieved. However, high amounts of antibiotics will cause systemic toxicity [58]. In order to overcome these detrimental effects, the antibacterial compounds and antibiotics are embedded in wound dressings for sustained and controlled drug release [1]. A lack of new antibiotics and antibacterial agents, as well as widespread distribution and misuse of these antibacterial compounds, has caused an increase in antimicrobial resistance (AMR). It has been proposed that AMR has the potential to kill ten million lives by 2050 worldwide, costing an estimated US $100 trillion [59]. This can result in the return to a pre-antibiotic era, with infections caused by multiple-resistant pathogens [22]. Thus, there is an urgent need for sustainable novel antibacterial additives to overcome this major clinical problem.
2. Bioactive Wound Dressings (Polymer + Additives)
2.1. Hydrogels
Hydrogel wound dressings containing natural antibacterial bio-additives have been used extensively for wound treatments. These additives are able to optimise the antibacterial properties against the unfavourable increase of bacterial proliferation in hydrogel wound dressings [2][60].
Plant-based antibacterial additives have been used in hydrogels to increase their activity against bacteria in infected wounds [60]. One of the most important class of additives in this group are the essential oils. The presence of essential oils as hydrophobic compounds in the hydrogel texture leads to good mechanical properties, degradability, improvement of the porous structure, and antioxidant properties [61]. Altaf et al. used a solution casting method to produce a polyvinyl alcohol/starch hydrogel membrane containing various concentrations of clove essential oil. The products resulted in excellent antibacterial activity, with a minimum inhibition zone of 34 ± 0.42 mm against S. aureus and 31 mm against E. coli [60]. The synthesis scheme has been demonstrated in Figure 2. Other essential oils used in hydrogels are lavender and tea tree oil [62]. Using these two essential oils in gellan gum hydrogels at 25% w/w resulted in an efficient zone of inhibition of 20 mm against S. aureus and 30 mm against E. coli in standard disc diffusion assays [62]. Several studies have used different essential oils in hydrogels, such as basil oil [63], tea tree oil [64], sweet fennel oil [65], rosemary essential oil, orange essential oil [66], and Thymus daenesis oil [67], to improve their antibacterial activity.
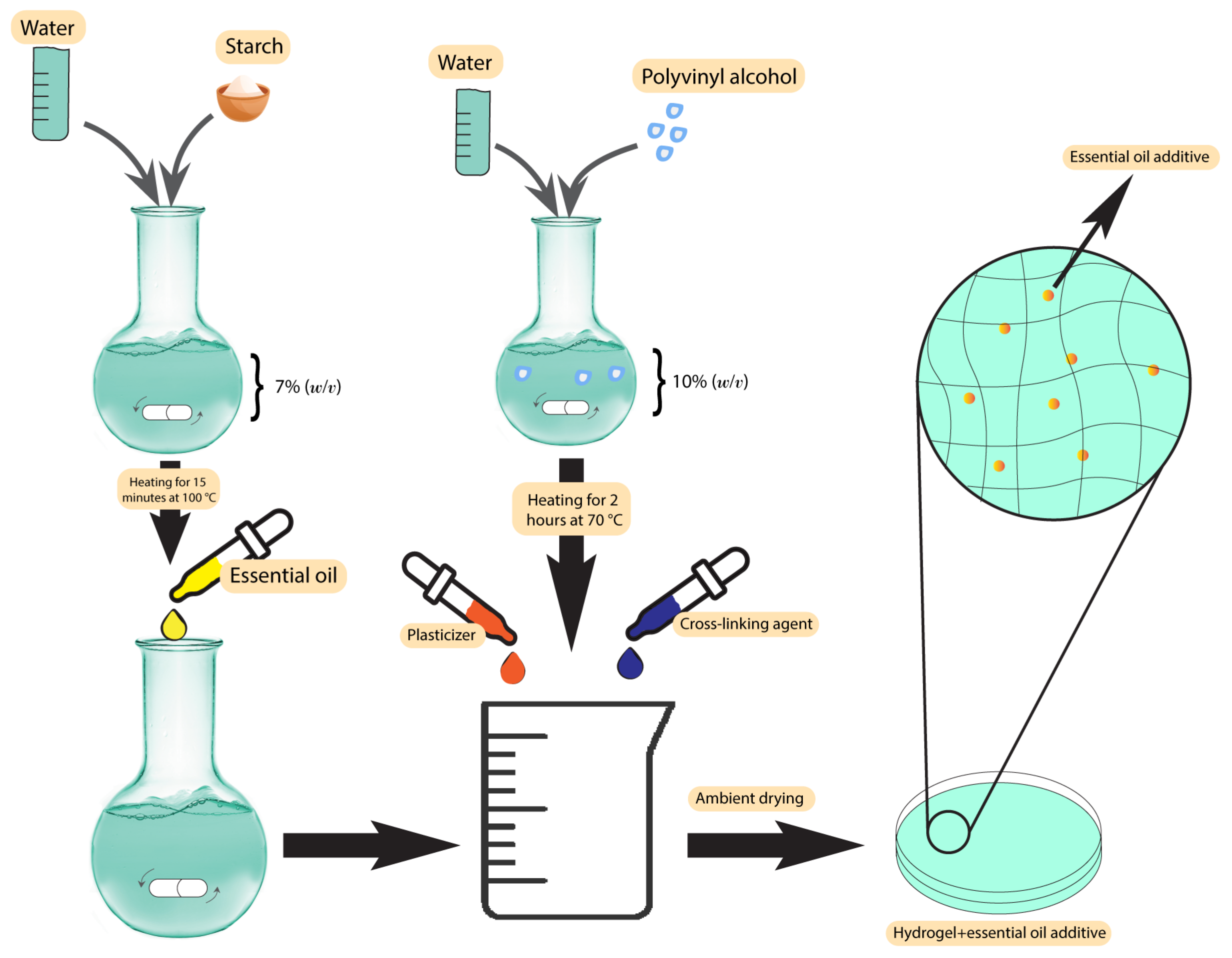
Figure 2. Synthesis scheme of polyvinyl alcohol/starch hydrogel membranes.
In addition to essential oils, plant extracts have been used as additives in hydrogel wound dressings. In a study by Shukla et al., a bioactive hydrogel dressing containing an ethanolic extract of Morus alba leaves was used against diabetic wounds. The apigenin derived from the extract was tailored with gellan gum-poly ethylene glycol-chitosan hydrogels and screened in vivo for its effectiveness. The results indicated that the apigenin additives caused effective stimulation of wound contraction and increase in the collagen content in diabetic as well as normal wound tissues, which leads to an accelerated wound healing process [68]. The antibacterial activity of Morus alba extracts against S. aureus has been previously investigated, resulting in a minimum inhibitory concentration (MIC) of 250 µg/mL [69].
2.2. Hydrocolloids
Hydrocolloids have been previously used along with natural antibacterial additives to improve their characteristics against wound bacteria and reduce the unpleasant odour [70]. These additives are the extracts of some pre-approved antibacterial plants, such as Centella asiatica (CA) and Phellodendri amurensis (PA) [71][72], which have been used in different studies against several bacteria. After loading CA plant extracts in alginate hydrocolloids using a hot melting method, Jin et al. showed excellent swelling, drug release, and mechanical properties compared with similar commercial products. Enhanced healing process in excision, infection, and abrasion wounds were observed in a rat wound model, which suggests that this extract is a potential candidate for the treatment of various wounds [71]. The preparation technique has been demonstrated in Figure 3. Antibacterial activity tests of the CA extracts at 100 µg/mL against P. aeruginosa, S. aureus, and E. coli resulted in zones of inhibition between 28–30 mm [72].
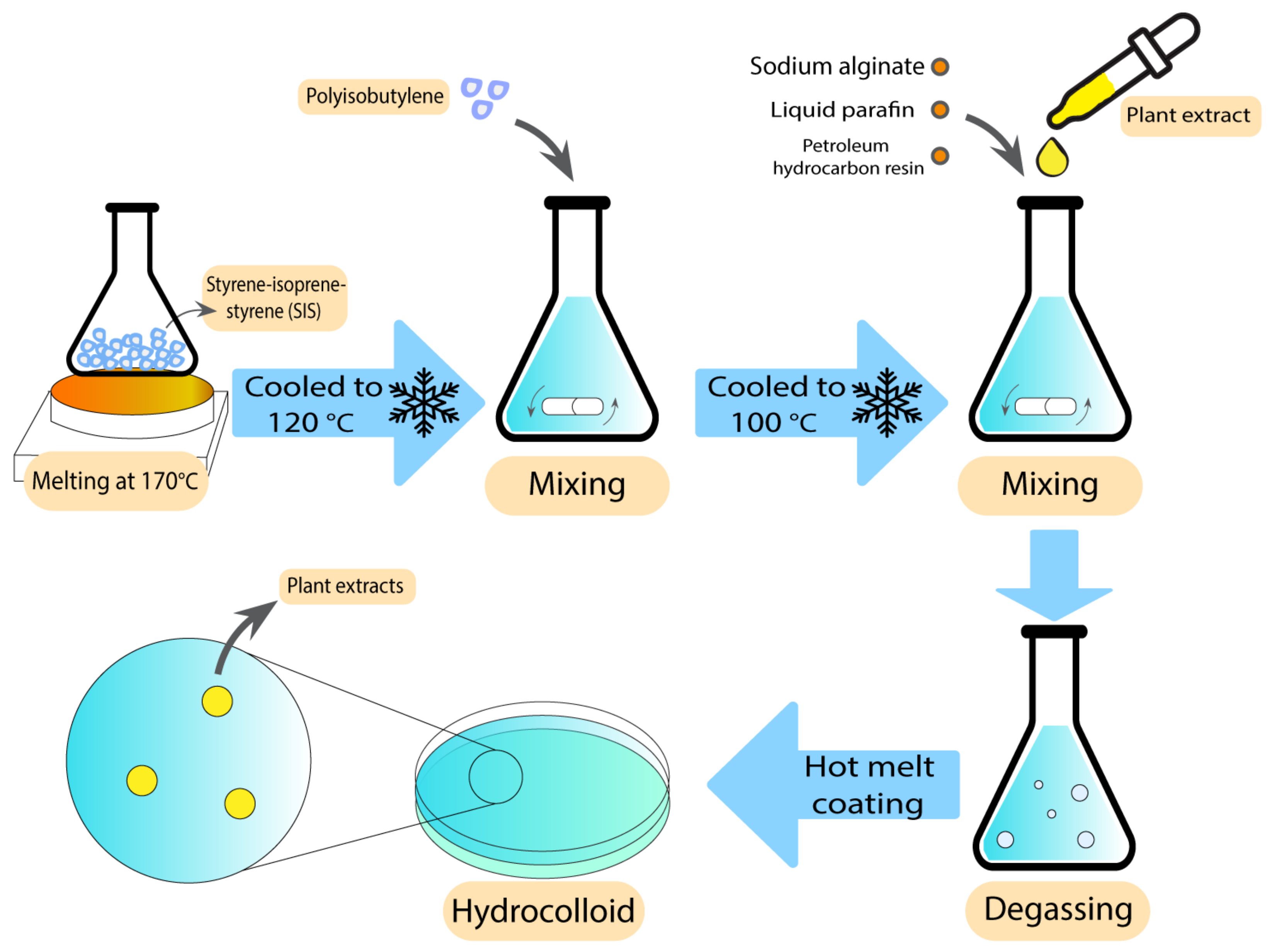
Figure 3. Preparation technique of alginate hydrocolloids using hot melt coating.
Another application of hydrocolloids containing CA extracts is skin treatment. Kuo et al. produced an anti-acne patch with gelatin/chitosan (GC) bilayer hydrocolloid patches. This anti-acne bilayer patch was loaded with Cortex PA and CA extracts. The results indicated that CA could reduce scar formation and improve the wound healing process. Water retention rate, weight loss rate, antibacterial activity, and in vitro cytotoxicity were tested as well. The results indicated that skin fibroblast cell viability was accelerated and the water retention of the patches was improved, which contributed to the exudate absorption [73].
2.3. Foams
Foams are another group of polymer wound dressings that have been previously used with additives to accelerate wound recovery. There are some reports on using plant-derived extracts as antibacterial agents in foam-based dressings. Nantaporn et al. prepared polyurethane foam sheets containing silver and asiaticoside (AS) (an extract derived from Centella asiatica plant) for healing dermal wounds. AS in a foam formulation played an essential role to increase the healing rate. The MIC of the additives against P. aeruginosa, S. aureus, E. coli, and B. subtilis were in a range of 0.4–3.1 ppm. However, the foam dressing released 4–5 ppm of the additive. The clear zones from disc diffusion assays were statistically larger than other tested formulations [12]. AS has been proved to be efficiently mixed with other polymers in different studies. Phaechamud et al. developed an absorbent chitosan-based dressing containing silver and asiaticoside as an additive. This dressing showed a successful controlled drug release along with angiogenic activity, indicating the potential to be further utilised as absorbents in medical wound dressings [74]. In what follows, the scheme of the preparation technique has been demonstrated in Figure 4.
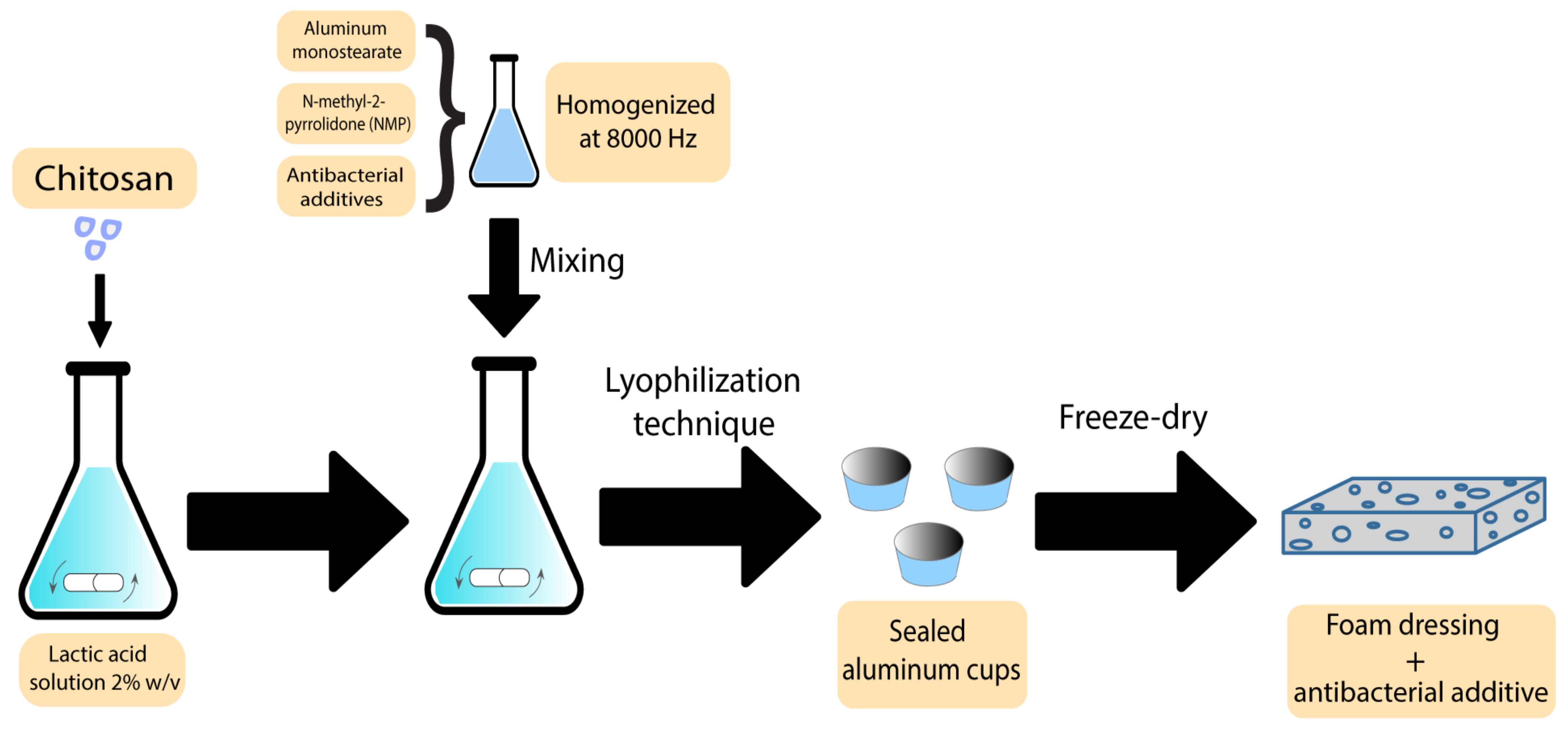
Figure 4. Preparation scheme of chitosan-based bioactive foams.
The other group of natural plant-based antibacterial additives used in foams is essential oils. The antibacterial activity of plant essential oils such as oregano and thyme has been proven previously, with MIC values of 0.0781 µL/mL [75] and 0.125 mg/mL [76], respectively. Adding these oils to a natural polymer such as sweet potato starch-based foam, along with their antibacterial activity, may lead to a lower degradation under the thermoforming temperature and higher mechanical resistance [77].
2.4. Films
Films have previously been used as bioactive wound dressings [1]. These types of wound dressings have been used with both plant extracts and essential oils. Some studies have shown the utilisation of different plants and plant extracts in film dressings. These plants are normally chosen based on their healing and antibacterial properties. Koga et al. developed an alginate film containing Aloe vera (Aloe barbadensis Miller) gel [78]. Aloe vera has already exhibited several pharmaceutical activities, such as the ability to promote the healing process as well as the ability to stimulate the proliferation of fibroblasts [79]. After characterising the different aspects of films containing Aloe vera, the results indicated adequate transparency, uniformity, mechanical tensile strength, and hydration capacity, which makes them an ideal candidate to be used as dressings. Furthermore, the films modulated the inflammatory phase, increased angiogenesis, and stimulated collagenesis, which leads to improved healing [78]. Figure 5 demonstrates the preparation process for these types of film.
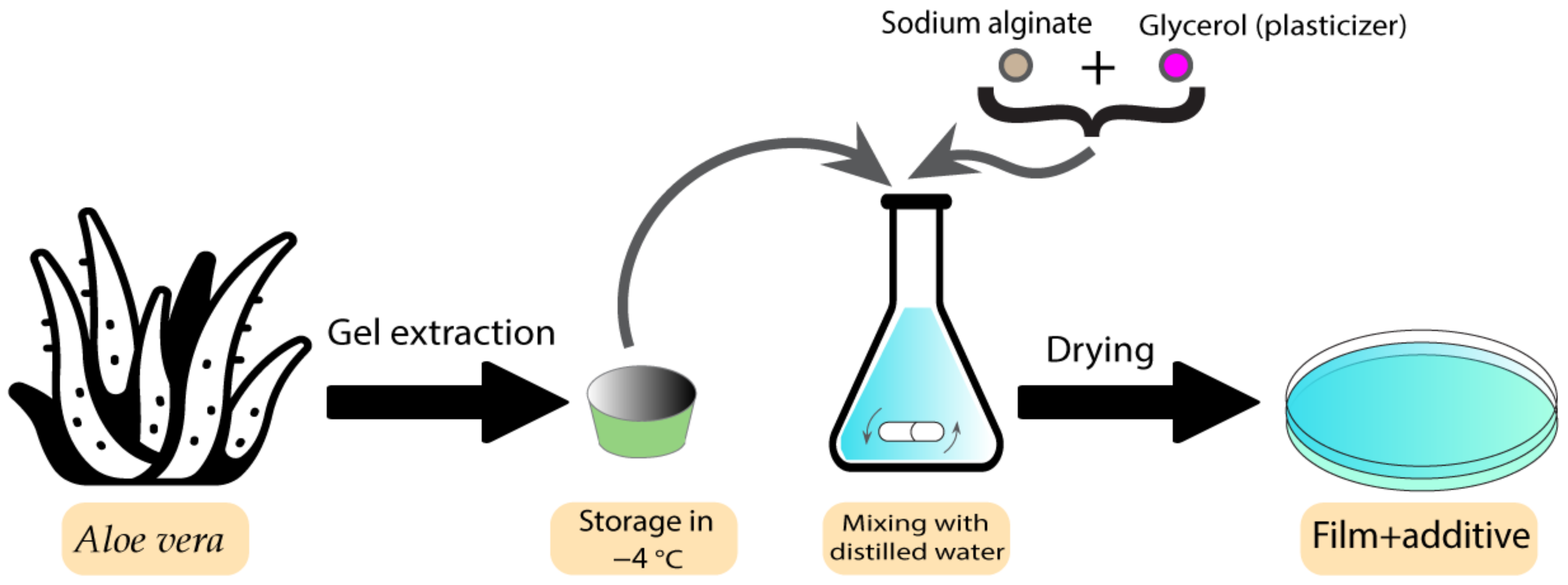
Figure 5. Preparation process of Aloe vera-containing alginate films.
The second group of additives used in film wound dressings are essential oils. Several types of essential oils have been used as an additive to optimise the antibacterial properties of film dressings. Clove, cinnamon, chamomile, thymol, lavender, tea tree, peppermint, Eucalyptus globulus juvenile, lemongrass, and lemon are some of the essential oils that have been used as antibacterial additives [80][81][82][83][84].
A combination of gelatin with clove essential oil (CEO) and hydrotalcite (HT) nanoparticles was prepared by Guilherme et al. as a wound dressing. CEO-containing films exhibited bactericidal activity against S. aureus and E. coli. HT was also hypothesised to relate positively to the antimicrobial performance of using films and enhance physical properties, which was lowered by the CEO [80].
One of the challenges in preparing films containing essential oils is choosing the proper oil to be used in the process. In this context, comparisons have been made between using each type of essential oil in a wound dressing environment. Liakos et al. used various types of essential oils such as lavender, tea tree, peppermint, Elicriso italic, cinnamon, Eucalyptus globulus, lemon, and lemongrass as an additive in sodium alginate matrixes. The produced films were tested for their antibacterial and anti-fungal properties. Among all the samples tested against E. coli, the cinnamon essential oils showed the largest inhibition zone of 12 mm, followed by lemongrass essential oil with an inhibition zone of 3 mm. The results of the antibacterial tests along with their stability indicates that films containing essential oils have the potential to be used as antibacterial wound-dressing materials [82].
2.5. Dermal Patches
The drugs used in these types of wound dressings should be penetrable to the skin, which makes most drugs unsuitable in this application. Solubility and diffusivity are two factors that determine the maximum skin penetration flux [85]. Some botanical-based additives have been used to improve the characteristics of dermal patches used in skin care and the prevention of mosquito bites [86]. In a study by Sroczyk et al., a polyimide patch was loaded with blackcurrant seed oil for atopic skin hydration studies. The application of these patches was against atopic dermatitis as a common disease among children. In this disease, gamma-linoleic acid is decreased, so the blackcurrant seed oil was used to restore the gamma-linoleic acid deficiencies. Based on the results, these patches adjust to skin movements, are stable with plant oils, and exchange air due to their high permeability, which makes them a good candidate to be used in skin care and treatment [86]. The process scheme has been demonstrated in Figure 6. There are different types of botanical-based oils with a high level of gamma-linoleic acid that can be used as additives instead of blackcurrant seed oil, such as Nigella sativa [87], borage [88], hempseed [89], and evening primrose [90].
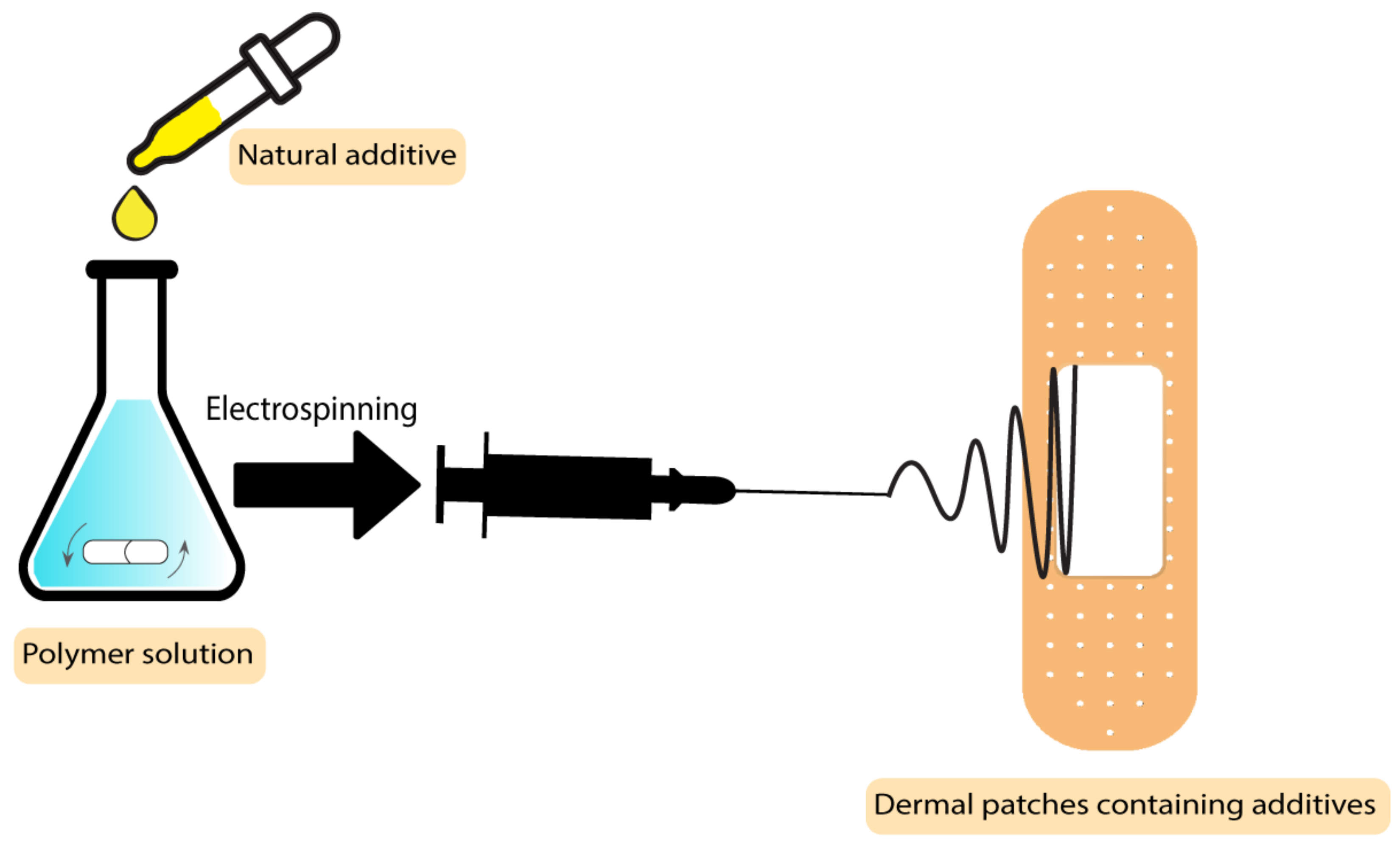
Figure 6. Process scheme of dermal patches containing natural additives.
As previously mentioned, another application of botanical-based skin patches is in the prevention of mosquito bites. In this case, essential oils as additives in patches act as insect repellents. Chattopadhyay et al. developed a patch from an optimised mixture of cinnamon, lemongrass, and eucalyptus essential oils embedded into ethylcellulose and polyvinylpyrrolidone polymer patches. These patches were shown to be safe and effective and to contain good physico-chemical properties at room temperature. The additives in this case are not only environmentally friendly but also make the patch more effective than the previous synthetic commercial products by providing complete protection for a longer time [91].
2.6. Fibers and Nanofibers-Based Electrospun Polymers
Bioactive agents added during nanofiber production have been shown to improve the wound healing process [1]. There are several strategies to tailor bioactive additives into the fibres, including emulsion electrospinning, blend electrospinning, co-axial electrospinning, and surface immobilization [92].
There are several studies indicating the use of natural botanical-based bio-additives such as plant extracts and essential oils in electrospun polymer wound dressings.
Plant extracts have been added to the polymer electrospun fibres based on the final properties required for the wound dressing. Numerous types of plant extracts have been used as an additive to nanofibers such as Azadirachta Indica [93], tumeric [94], Clerodendrum phlomidis [95], Gymnema sylvestre [96], Carica papaya [97], Aloe vera [98], Lawsonia inermis [99], Garcinia mangostana [100], mucilage [101], clove [102], Ataria multiflora [103], pomegranate [104], Achillea lyconica [105], corn [106], fenugreek [107], henna [108], and chamomile [109].
These extracts have been proved to be effective in diabetic wound dressings. In a study by Ranjbar-Mohammadi et al., curcumin extracted from turmeric was used as an antibacterial additive in polycaprolactone electrospun fibres. The experiments indicated that the wound dressing was active for the treatment of diabetic wounds. Exhibiting an MIC of 62.5 µg/mL against P. aeruginosa [110], curcumin showed a more accelerated wound healing process in comparison with the blank sample [94]. Another application of nanofibers containing plant extracts is skin tissue engineering. Henna leaf extract-loaded chitosan-based nanofibrous mats were used as a wound dressing by Yousefi et al. The final product displayed efficient antibacterial activity due to Lawsonia inermis (Henna) leaf extracts in mats (2 wt%), with zones of inhibition against S. aureus and E. coli of 18 mm and 25 mm, respectively. The presence of henna extract caused a reduction in the fibre diameter of the mats, which makes it favourable for wound healing applications due to increasing the surface area. Furthermore, the combined advantageous features including high biocompatibility, synergistic antibacterial activity, and acceleration of wound healing can be observed by using this additive in a mixture with polymer nanofibers [108].
The next group of botanical-based additives used in nanofiber polymer wound dressings is essential oils. Different types of essential oils have previously been used as additives in a mixture with polymer nanofibers targeting wound bacteria. These plants include lavender oil [111], thyme oil [112], cinnamon oil [113], and rosemary/oregano oil [114] that have shown antibacterial activity against the most common wound bacteria such as S. aureus, E. coli, and P. aeruginosa [111][112][113][114][115].
An improved wound healing device using encapsulation of cerium oxide (CeO2) and peppermint oil (PM oil) on polyethylene oxide/graphene oxide (PEO/GO) electrospun polymeric mats was shown by Suganya et al. The study involved testing against Gram-positive bacteria (S. aureus) and Gram-negative bacteria (E. coli) and evaluated in vitro cytotoxicity. The results indicated that the CeO2-PM oil-PEO/GO nanofibrous mats were less toxic to the L929 fibroblast cells. Furthermore, evaluations demonstrated that the incorporation of the plant-based bioactive agent and CeO2 in a nanofibrous mat accelerates re-epithelialization and collagen deposition, which makes the system an efficient potential candidate to be applied as wound dressings with skin infections [116]. The MIC values for peppermint essential oils are 3.1 µL/mL and 6.3 µL/mL against S. aureus and E. coli, respectively [117]. In what follows, the preparation technique of CeO2-PM oil-PEO/GO nanofibrous mats is demonstrated in Figure 7.
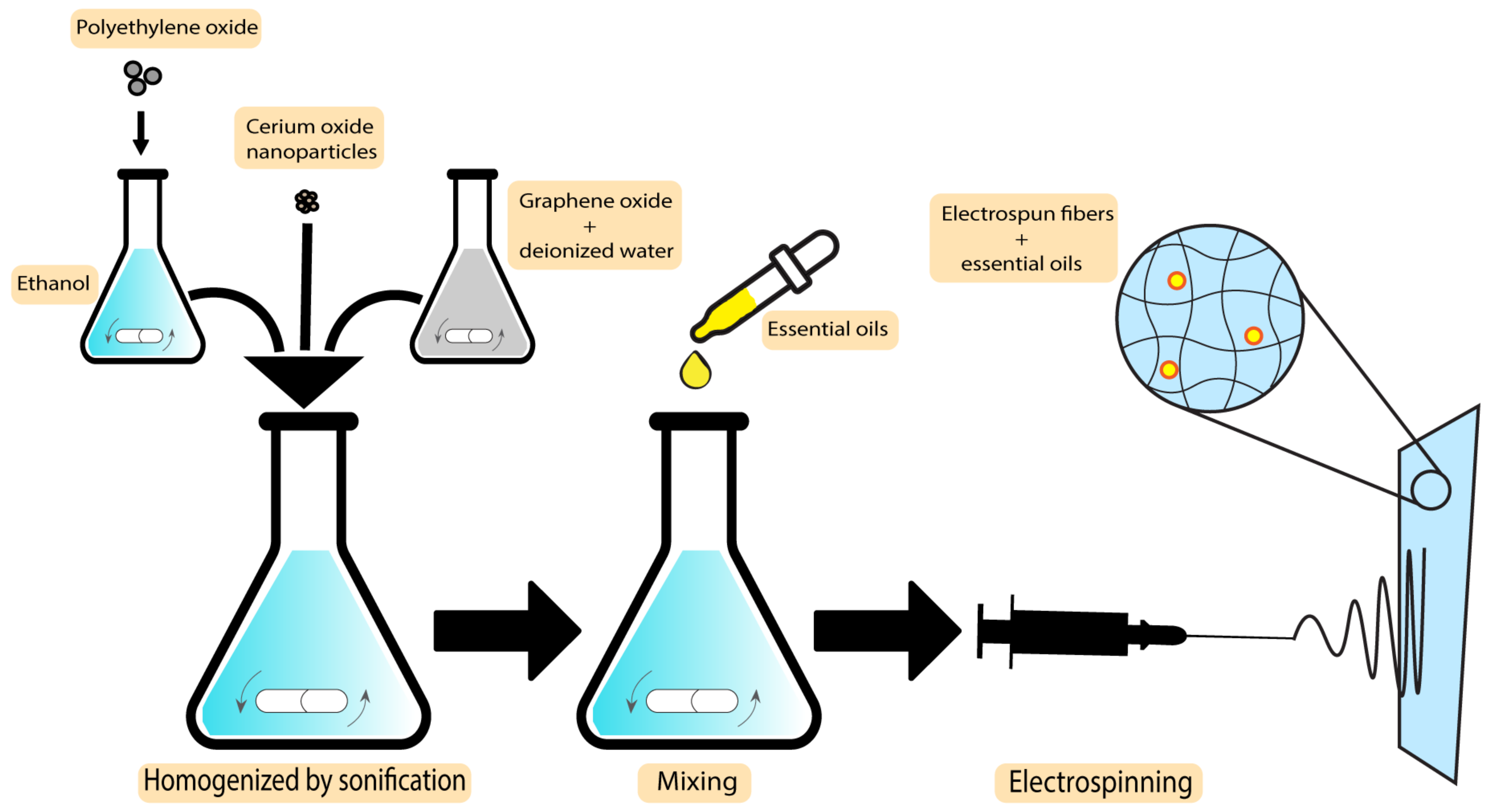
Figure 7. Preparation technique of CeO2-PM oil-PEO/GO nanofibrous mats.
2.7. Membranes
Another group of wound dressings that have been used in combination with plant-based natural additives are membranes. Both essential oils and plant extracts have shown the ability to optimise the characteristics of the final dressings. Egri et al. developed Hypericum perforatum oil-loaded polycaprolactone membranes to be used in wound dressing applications. After investigating the mechanical strength and antibacterial activity, the product exhibited sufficient elasticity and activity against S. aureus and E. coli, with inhibition zones of 8–13 mm and 10–12.2 mm, respectively. Not having the risk of adhering to the wound surface, not having apoptotic/necrotic effects, being biocompatible, and having a proliferative effect on cells are some of the advantageous features of the Hyperium perforatum-loaded membranes [118]. The preparation scheme of this membrane is demonstrated in Figure 8.
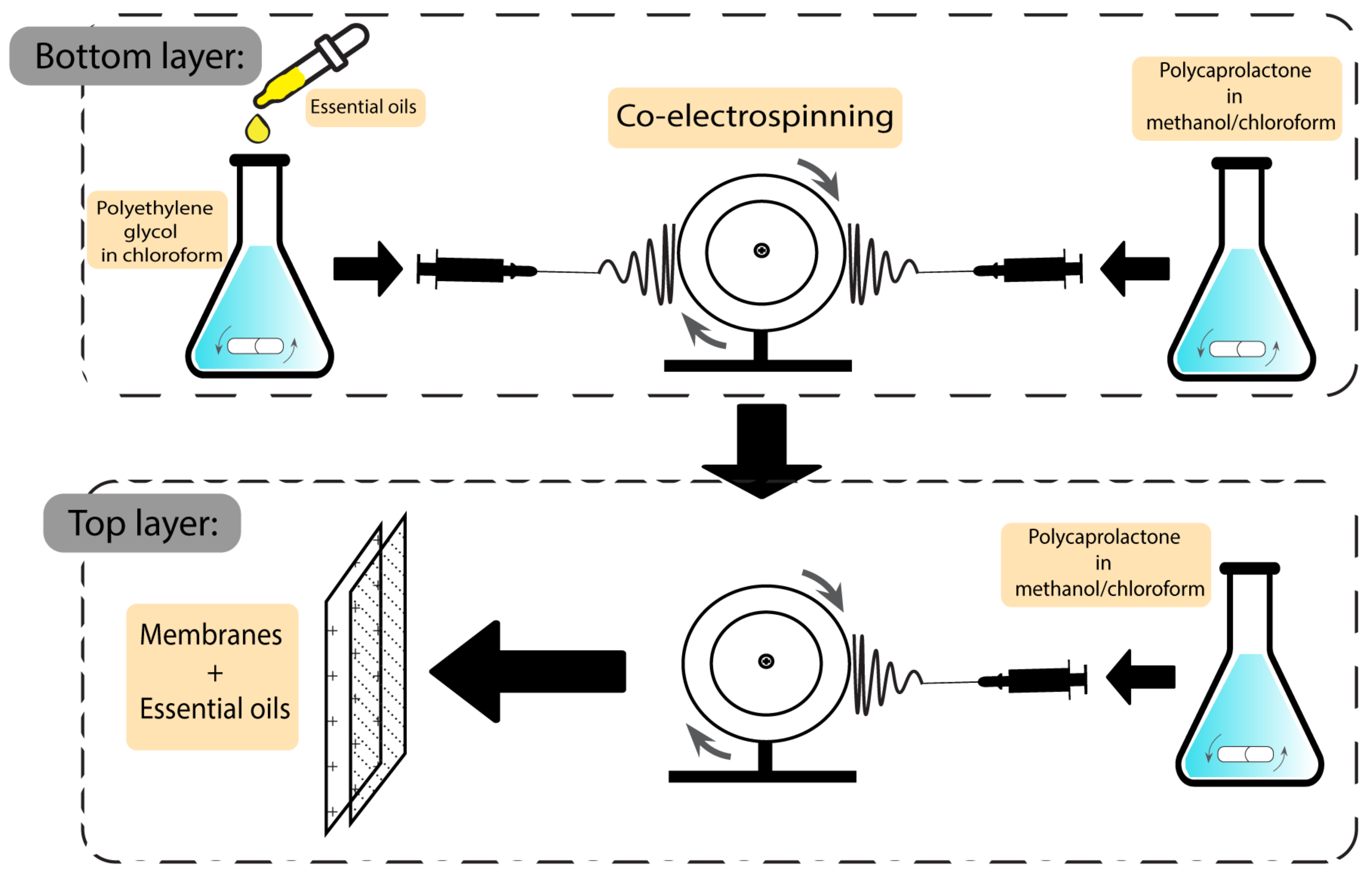
Figure 8. Preparation scheme of essential oil-based polycaprolactone membranes.
Another type of essential oil used in membranes is Artemisia argyi. The efficiency of this essential oil has previously been investigated against wound bacteria such as S. aureus, P. aeruginosa and E. coli, with MIC values of 16 µg/mL, 64 µg/mL, and 32 µg/mL, respectively [119]. Ting-Ting et al. fabricated Artemisia argyi oil-microcapsule (AAO-MC)/PVC fibrous membrane wound dressings and showed that the production process was enhanced using emulsification-internal gelation. The results showed excellent stability and a slow release of the oil. Furthermore, the produced membrane showed good water vapor transmission and high hydrophilicity as well as an excellent antibacterial rate of 94.3%, which is calculated by the difference between the colony counts of the blank specimen and the colony counts of culture medium that has been cultured with a bacterial solution for a specified time divided by the colony counts of the blank specimen [120].
Based on the targeted bacteria and the final characteristics, other types of essential oils may be used as additives, such as cabreuva (Myrocarpus fastigiatus) [121] and oregano [122]. The MIC values of pure oregano essential oil have been determined to be 0.25 mg/mL, 0.64 mg/mL, and 0.16 mg/mL against E. coli, P. aeruginosa, and S. aureus, respectively [123][124].
The addition of cabreuva essential oil to poly (vinyl alcohol) membranes proves its effectiveness against S. aureus. Its capacity to produce cell regeneration along with no detectable toxicity makes it a suitable dressing for superficial burns or minor wounds [121]. Oregano essential oils have been used with poly (L-lactide-co-caprolactone)/silk fibroin membranes as shown by Khan et al., showing a highly active membrane against both Gram-negative (E. coli) and Gram-positive (S. aureus) bacteria. The results indicated an accelerated healing process, boosted granulation, and also re-epithelialization, which confirms its potential to be used as a wound dressing [122].
2.8. Polymer-Drug Conjugates
Linkers used for the conjugation of drugs to polymers function to control the drug release in a pH specific manner and in the presence of enzymes depending on the chemistry of the linker employed [125]. For improving the therapeutic advantages of this type of wound dressing, moiety and solubilising units are also incorporated into polymer–drug conjugates [126][127]. Several studies indicate the use of plant extracts and essential oils conjugated with polymers. Some of the essential oils that have previously been used in polymer nanocarriers are thyme [128][129], peppermint oil [130], green tea oil [130], etc.
In a study by Shetta et al., peppermint and green tea essential oils were encapsulated into chitosan nanoparticles using the emulsification/ionic gelation method. The final product was tested against S. aureus and E. coli, showing minimum bactericidal concentration (MBC) values of 1.11 mg/mL and >2.72 mg/mL for peppermint oil and 0.57 mg/mL and 1.15 mg/mL for green tea, respectively, demonstrating their potential to be used in wound dressing applications [130]. Figure 9 demonstrates the preparation steps of this product.
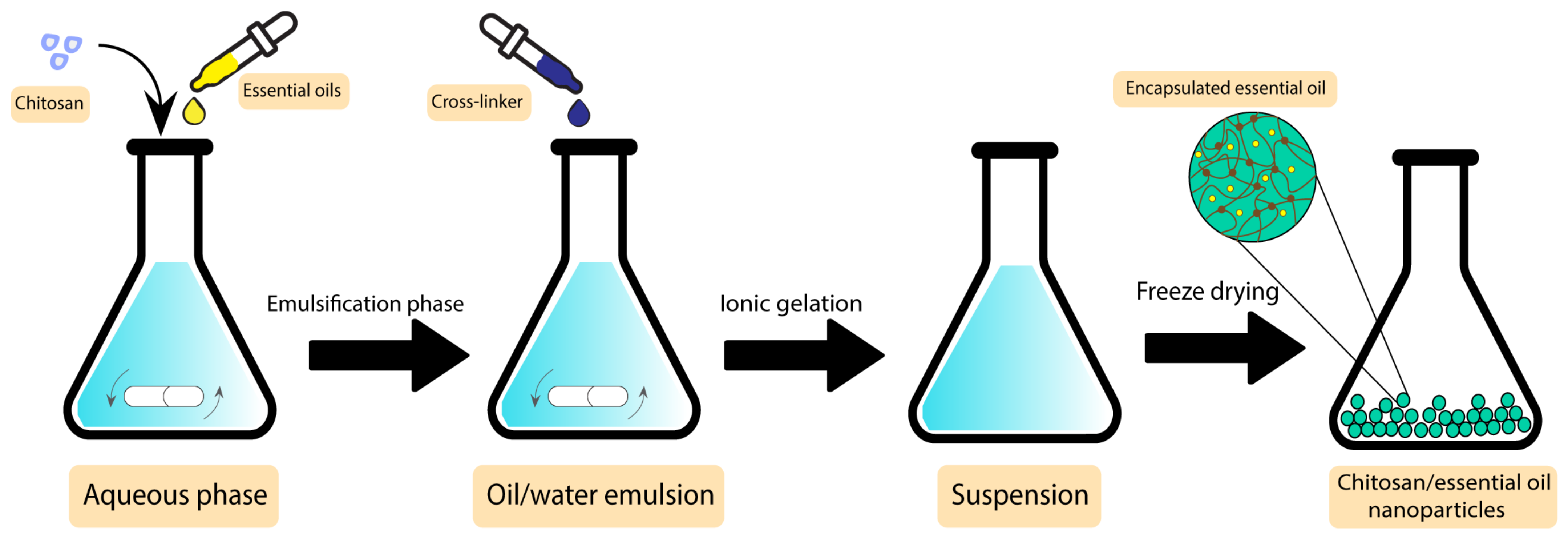
Figure 9. Preparation steps of chitosan/essential oil nanoparticles.
Another group of botanical-based antibacterial additives with the potential to be conjugated with polymers are plant extracts. Some of the utilised plant extracts conjugated with polymer wound dressings are polyphenolics and hydrolysable tannins from Hamamelis virginiana [131], seaweed extract [132], Mcrotyloma uniflorum [133], Aloe vera [134], and curcumin [135].
In a study by Yang et al., gallic acid was conjugated to a 2-hydroxy (ethyl methacrylate-co-2-diethylamino) methacrylate hydrogel. Gallic acid was extracted from an Indian plant called Terminalia bellinca, showing antioxidant and cytoprotective characteristics. The multifunctional hydrogel was used as a carrier for cell therapy and drug delivery applications. The results indicated that the product caused a faster recovery in affected tissues, which shows their significant potential to be used in medical applications [136].
2.9. Other Polymer Wound Dressings
Other types of polymer wound dressings including 3D-printed scaffolds, emulgels, and nanoemulgels have been used with various plant-based antibacterial additives previously. There are several studies indicating the use of essential oils and plant extracts in these types of wound dressings.
In a study by Ilhan et al., Satureja cuneifolia plant extracts were blended with sodium alginate and polyethylene 3D-printed scaffolds for treating diabetic ulcers. Disc diffusion testings against S. aureus demonstrated that the samples containing Satureja cuneifolia extracts (between 0.5 to 2 wt%) have an inhibition zone of 12–13 mm, which indicates their remarkable activity against Gram-positive bacteria. However, their activity against E. coli was reported to be in much higher concentrations (700 µg/mL) [137].
Emulgels and nanoemulgels have been used extensively with plant extracts and essential oils as an additive. Ocimum basilicum extracts [138], clove oil [139], rosemary oil [140], and piper betle oil [141] are some of these additives.
In a study by Razdan et al., clove oil-based nanoemulgels were used as a burn wound dressing. Levofloxacin nanoemulgels were combined with clove oil and were examined in vivo against P. aeruginosa biofilm-infected burn wounds. The product was tested against mice and the wound closure state was observed on the 1st, 3rd, 7th, 10th, and 15th day. The results indicated a faster reduction in wound size and a complete wound closure after 15 days in comparison with the samples without the additive, which were not completely closed in that period [139].
As mentioned before, one of the ways to improve wound dressing characteristics is to include bioactive additives. The role of natural antibacterial additives in polymer wound dressing groups were summarised before. In the following, different groups of plant-based natural products, as the source of novel antibacterial additives against the most common wound bacteria (S. aureus, E. coli, and P. aeruginosa), are discussed [142].
References
- Aderibigbe, B.A. Chapter 6—Efficacy of Polymer-Based Wound Dressings in Chronic Wounds. In Modeling and Control of Drug Delivery Systems; Azar, A.T., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 79–110.
- Sikka, M.P.; Midha, V.K. 16—The role of biopolymers and biodegradable polymeric dressings in managing chronic wounds. In Advanced Textiles for Wound Care, 2nd ed.; Rajendran, S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 463–488.
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121.
- Kamoun, E.A.; Kenawy, E.-R.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233.
- Zeng, D.; Shen, S.; Fan, D. Molecular design, synthesis strategies and recent advances of hydrogels for wound dressing applications. Chin. J. Chem. Eng. 2021, 30, 308–320.
- Agarwal, A.; McAnulty, J.F.; Schurr, M.J.; Murphy, C.J.; Abbott, N.L. 8—Polymeric materials for chronic wound and burn dressings. In Advanced Wound Repair Therapies; Farrar, D., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 186–208.
- Leveriza-Oh, M.; Phillips, T. Chapter 32—Dressings and postoperative carea. In Lower Extremity Soft Tissue & Cutaneous Plastic Surgery, 2nd ed.; Dockery, G.D., Crawford, M.E., Eds.; W.B. Saunders: Oxford, UK, 2012; pp. 471–488.
- Nielsen, J.; Fogh, K. Clinical utility of foam dressings in wound management: A review. Chronic Wound Care Manag. Res. 2015, 2, 31–38.
- Kirwan, H.; Pignataro, R. Chapter 2—The Skin and Wound Healing. In Pathology and Intervention in Musculoskeletal Rehabilitation, 2nd ed.; Magee, D.J., Zachazewski, J.E., Quillen, W.S., Manske, R.C., Eds.; W.B. Saunders: Oxford, UK, 2016; pp. 25–62.
- Weller, C. 4—Interactive dressings and their role in moist wound management. In Advanced Textiles for Wound Care; Rajendran, S., Ed.; Woodhead Publishing: Cambridge, UK, 2009; pp. 97–113.
- Chaganti, P.; Gordon, I.; Chao, J.H.; Zehtabchi, S. A systematic review of foam dressings for partial thickness burns. Am. J. Emerg. Med. 2019, 37, 1184–1190.
- Namviriyachote, N.; Lipipun, V.; Akkhawattanangkul, Y.; Charoonrut, P.; Ritthidej, G.C. Development of polyurethane foam dressing containing silver and asiaticoside for healing of dermal wound. Asian J. Pharm. Sci. 2019, 14, 63–77.
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 182.
- Peles, Z.; Zilberman, M. Novel soy protein wound dressings with controlled antibiotic release: Mechanical and physical properties. Acta Biomater. 2012, 8, 209–217.
- Srivastava, C.M.; Purwar, R.; Kannaujia, R.; Sharma, D. Flexible silk fibroin films for wound dressing. Fibers Polym. 2015, 16, 1020–1030.
- Pastore, M.N.; Kalia, Y.N.; Horstmann, M.; Roberts, M.S. Transdermal patches: History, development and pharmacology. Br. J. Pharmacol. 2015, 172, 2179–2209.
- Levin, A. The Clinical Epidemiology of Cardiovascular Diseases in Chronic Kidney Disease: Clinical Epidemiology of Cardiovascular Disease in Chronic Kidney Disease Prior to Dialysis. Semin. Dial. 2003, 16, 101–105.
- Cilurzo, F.; Musazzi, U.M.; Franzé, S.; Fedele, G.; Minghetti, P. Design of in vitro skin permeation studies according to the EMA guideline on quality of transdermal patches. Eur. J. Pharm. Sci. 2018, 125, 86–92.
- Auda, S.H.; Mahrous, G.M.; Ibrahim, M.A.; Shazly, G.A.; Salem-Bekhit, M.M. Novel chlorhexidine dermal patches, preparation characterization and antimicrobial evaluation. Polym. Bull. 2017, 74, 3995–4007.
- Suhaeri, M.; Noh, M.; Moon, J.; Kim, I.; Oh, S.; Ha, S.; Lee, J.; Park, K. Novel skin patch combining human fibroblast-derived matrix and ciprofloxacin for infected wound healing. Theranostics 2018, 8, 5025–5038.
- Nilani, P.; Pranavi, A.; Duraisamy, B.; Damodaran, P.; Subhashini, V.; Elango, K. Formulation and evaluation of wound healing dermal patch. Afr. J. Pharm. Pharmacol. 2011, 5, 1252–1257.
- Guglielmi, P.; Pontecorvi, V.; Rotondi, G. Natural compounds and extracts as novel antimicrobial agents. Expert Opin. Ther. Pat. 2020, 30, 949–962.
- Khil, M.S.; Cha, D.I.; Kim, H.Y.; Kim, I.S.; Bhattarai, N. Electrospun Nanofibrous Polyurethane Membrane as Wound Dressing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 67, 675–679.
- Rho, K.S.; Jeong, L.; Lee, G.; Seo, B.; Park, Y.J.; Hong, S.; Roh, S.; Cho, J.J.; Park, W.H.; Min, B. Electrospinning of collagen nanofibers: Effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials 2006, 27, 1452–1461.
- Ju, H.W.; Lee, O.J.; Lee, J.M.; Moon, B.M.; Park, H.J.; Park, Y.R.; Lee, M.C.; Kim, S.H.; Chao, J.R.; Ki, C.S.; et al. Wound healing effect of electrospun silk fibroin nanomatrix in burn-model. Int. J. Biol. Macromol. 2016, 85, 29–39.
- Lin, J.; Li, C.; Zhao, Y.; Hu, J.; Zhang, L. Co-electrospun Nanofibrous Membranes of Collagen and Zein for Wound Healing. ACS Appl. Mater. Interfaces 2012, 4, 1050–1057.
- Venugopal, J.R.; Zhang, Y.; Ramakrishna, S. In Vitro Culture of Human Dermal Fibroblasts on Electrospun Polycaprolactone Collagen Nanofibrous Membrane. Artif. Organs 2006, 30, 440–446.
- Coelho, D.S.; Veleirinho, B.; Alberti, T.; Maestri, A.; Yunes, R.; Dias, P.F.; Maraschin, M. Electrospinning technology: Designing nanofibers toward wound healing application. In Nanomaterials-Toxicity, Human Health and Environment; Intechopen: London, UK, 2018; pp. 1–19.
- Shi, X.; Zhou, W.; Ma, D.; Ma, Q.; Bridges, D.; Ma, Y.; Hu, A. Electrospinning of Nanofibers and Their Applications for Energy Devices. J. Nanomater. 2015, 2015, 140716.
- Azimi, B.; Maleki, H.; Zavagna, L.; De la Ossa, J.G.; Linari, S.; Lazzeri, A.; Danti, S. Bio-Based Electrospun Fibers for Wound Healing. J. Funct. Biomater. 2020, 11, 67.
- Abrigo, M.; Kingshott, P.; McArthur, S. Electrospun Polystyrene Fiber Diameter Influencing Bacterial Attachment, Proliferation, and Growth. ACS Appl. Mater. Interfaces 2015, 7, 7644–7652.
- Genevro, G.M.; Gomes Neto, R.J.; de Paulo, L.A.; Lopes, P.S.; de Moraes, M.A.; Beppu, M.M. Glucomannan asymmetric membranes for wound dressing. J. Mater. Res. 2019, 34, 481–489.
- Sahana, T.G.; Rekha, P. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867.
- López-Mata, M.A.; Gastelum-Cabrera, M.; Valbuena-Gregorio, E.; Zamudio-Flores, P.B.; Burruel-Ibarra, S.E.; Morales-Figueroa, G.G.; Quihui-Cota, L.; Juárez-Onofre, J.E. Physicochemical properties of novel pectin/Aloe gel membranes. Iran. Polym. J. 2018, 27, 545–553.
- Azad, A.K.; Sermsintham, N.; Chandrkrachang, S.; Stevens, W.F. Chitosan membrane as a wound-healing dressing: Characterization and clinical application. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 69B, 216–222.
- Chattopadhyay, S.; Raines, R. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833.
- Pires, A.L.R.; de Azevedo Motta, L.; Dias, A.M.A.; de Sousa, H.C.; Moraes, Â.M.; Braga, M.E.M. Towards wound dressings with improved properties: Effects of poly(dimethylsiloxane) on chitosan-alginate films loaded with thymol and beta-carotene. Mater. Sci. Eng. C 2018, 93, 595–605.
- Bueno, C.Z.; Moraes, A.J. Development of porous lamellar chitosan-alginate membranes: Effect of different surfactants on biomaterial properties. Appl. Polym. Sci. 2011, 122, 624.
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923.
- Kimna, C.; Tamburaci, S.; Tihminlioglu, F. Novel zein-based multilayer wound dressing membranes with controlled release of gentamicin. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2057–2070.
- Popa, G.-M.L.; Truşcă, R.D.; Ilie, C.-I.; Țiplea, R.E.; Ficai, D.; Oprea, O.; Stoica-Guzun, A.; Ficai, A.; Dițu, L.-M. Antibacterial Activity of Bacterial Cellulose Loaded with Bacitracin and Amoxicillin: In Vitro Studies. Molecules 2020, 25, 4069.
- Cheng, C.-F.; Wu, K.; Chen, Y.; Hung, S. Bacterial adhesion to antibiotic-loaded guided tissue regeneration membranes—A scanning electron microscopy study. J. Formos. Med. Assoc. 2015, 114, 35–45.
- Lei, J.; Sun, L.; Li, P.; Zhu, C.; Lin, Z.; Mackey, V.; Coy, D.H.; He, Q. The wound dressings and their applications in wound healing and management. Health Sci. J. 2019, 13, 1–8.
- Pasut, G.; Veronese, F. Polymer–drug conjugation, recent achievements and general strategies. Prog. Polym. Sci. 2007, 32, 933–961.
- Rohini, N.A.; Joseph, A.; Mukerji, A. Polymeric prodrugs: Recent achievements and general strategies. J. Antivir. Antiretrovir. 2013, 2, S15.
- Abuchowski, A.; van Es, T.; Palczuk, N.C.; Davis, F.F. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J. Biol. Chem. 1977, 252, 3578–3581.
- Abuchowski, A.; McCoy, J.R.; Palczuk, N.C.; van Es, T.; Davis, F.F. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J. Biol. Chem. 1977, 252, 3582–3586.
- Schoemaker, N.E.; van Kesteren, C.; Rosing, H.; Jansen, S.; Swart, M.; Lieverst, J.; Fraier, D.; Breda, M.; Pellizzoni, C.; Spinelli, R.; et al. A phase I and pharmacokinetic study of MAG-CPT, a water-soluble polymer conjugate of camptothecin. Br. J. Cancer 2002, 87, 608–614.
- Kim, C.J.; Lee, Y.S.; Lee, K.H.; Jeong, B.; Kim, T.W.; Kang, T.H.; Kim, H.S.; Park, J. Effect of topical Paclitaxel using PEG/PLGA polymer on the animal model of cervical cancer. Korean J. Gynecol. Oncol. 2008, 19, 68–74.
- Maeda, H. SMANCS and polymer-conjugated macromolecular drugs: Advantages in cancer chemotherapy. Adv. Drug Deliv. Rev. 2001, 46, 169–185.
- Balasubramaniam, M.P.; Murugan, P.; Chenthamara, D.; Ramakrishnan, S.G.; Salim, A.; Lin, F.; Robert, B.; Subramaniam, S. Synthesis of chitosan-ferulic acid conjugated poly(vinyl alcohol) polymer film for an improved wound healing. Mater. Today Commun. 2020, 25, 101510.
- Hardwicke, J.T.; Hart, J.; Bell, A.; Duncan, R.; Thomas, D.W.; Moseley, R. The effect of dextrin–rhEGF on the healing of full-thickness, excisional wounds in the (db/db) diabetic mouse. J. Control. Release 2011, 152, 411–417.
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269.
- Alam, M.M.; Islam, M.N.; Hossain Hawlader, M.D.; Ahmed, S.; Wahab, A.; Islam, M.; Uddin, K.M.R.; Hossain, A. Prevalence of multidrug resistance bacterial isolates from infected wound patients in Dhaka, Bangladesh: A cross-sectional study. Int. J. Surg. Open 2021, 28, 56–62.
- Unalan, I.; Slavik, B.; Buettner, A.; Goldmann, W.H.; Frank, G.; Boccaccini, A.R. Physical and Antibacterial Properties of Peppermint Essential Oil Loaded Poly (ε-caprolactone) (PCL) Electrospun Fiber Mats for Wound Healing. Front. Bioeng. Biotechnol. 2019, 7, 346.
- Javanbakht, S.; Nabi, M.; Shadi, M.; Amini, M.M.; Shaabani, A. Carboxymethyl cellulose/ nanocomposite hydrogel films as a potential antibacterial wound dressing. Int. J. Biol. Macromol. 2021, 188, 811–819.
- Zhong, Y.; Xiao, H.; Seidi, F.; Jin, Y. Natural Polymer-Based Antimicrobial Hydrogels without Synthetic Antibiotics as Wound Dressings. Biomacromolecules 2020, 21, 2983–3006.
- Negut, I.; Grumezescu, V.; Grumezescu, A. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392.
- Jasovský, D.; Littmann, J.; Zorzet, A.; Cars, O. Antimicrobial resistance—A threat to the world’s sustainable development. Upsala J. Med. Sci. 2016, 121, 159–164.
- Altaf, F.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Akram, M.A.; Safdar, A.; Butt, M.S.; Noor, T.; Sher, F. Synthesis and Characterization of PVA/Starch Hydrogel Membranes Incorporating Essential Oils Aimed to be Used in Wound Dressing Applications. J. Polym. Environ. 2021, 29, 156–174.
- Syed, I.; Garg, S.; Sarkar, P. 5—Entrapment of essential oils in hydrogels for biomedical applications. In Polymeric Gels; Pal, K., Banerjee, I., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 125–141.
- Mahmood, H.; Khan, I.U.; Asif, M.; Khan, R.U.; Asghar, S.; Khalid, I.; Khalid, S.H.; Irfan, M.; Rehman, F.; Shahzad, Y.; et al. In vitro and in vivo evaluation of gellan gum hydrogel films: Assessing the co impact of therapeutic oils and ofloxacin on wound healing. Int. J. Biol. Macromol. 2021, 166, 483–495.
- Chinnaiyan, S.K.; Pandiyan, R.; Natesan, S.; Chindam, S.; Gouti, A.K.; Sugumaran, A. Fabrication of basil oil Nanoemulsion loaded gellan gum hydrogel—Evaluation of its antibacterial and anti-biofilm potential. J. Drug Deliv. Sci. Technol. 2022, 68, 103129.
- Ghosh, B.; Bhattacharya, D.; Mukhopadhyay, M. A hydrogel sheet mask with tea tree essential oil entrapment and targeted dose delivery capability. Mater. Today Proc. 2022, 57, 77–83.
- Barradas, T.N.; Senna, J.P.; Cardoso, S.A.; Nicoli, S.; Padula, C.; Santi, P.; Rossi, F.; de Holanda e Silva, K.G.; Mansur, C.R.E. Hydrogel-thickened nanoemulsions based on essential oils for topical delivery of psoralen: Permeation and stability studies. Eur. J. Pharm. Biopharm. 2017, 116, 38–50.
- Goudoulas, T.B.; Vanderhaeghen, S.; Germann, N. Micro-dispersed essential oils loaded gelatin hydrogels with antibacterial activity. LWT 2022, 154, 112797.
- Shabkhiz, M.A.; Khalil Pirouzifard, M.; Pirsa, S.; Mahdavinia, G.R. Alginate hydrogel beads containing Thymus daenensis essential oils/Glycyrrhizic acid loaded in β-cyclodextrin. Investigation of structural, antioxidant/antimicrobial properties and release assessment. J. Mol. Liq. 2021, 344, 117738.
- Shukla, R.; Kashaw, S.K.; Jain, A.P.; Lodhi, S. Fabrication of Apigenin loaded gellan gum–chitosan hydrogels (GGCH-HGs) for effective diabetic wound healing. Int. J. Biol. Macromol. 2016, 91, 1110–1119.
- Aelenei, P.; Luca, S.V.; Horhogea, C.E.; Rimbu, C.M.; Dimitriu, G.; Macovei, I.; Silion, M.; Aprotosoaie, A.C.; Miron, A. Morus alba leaf extract: Metabolite profiling and interactions with antibiotics against Staphylococcus spp. including MRSA. Phytochem. Lett. 2019, 31, 217–224.
- Akhmetova, A.; Saliev, T.; Allan, I.U.; Illsley, M.J.; Nurgozhin, T.; Mikhalovsky, S. A Comprehensive Review of Topical Odor-Controlling Treatment Options for Chronic Wounds. J. Wound Ostomy Cont. Nurs. 2016, 43, 598–609.
- Jin, S.G.; Kim, K.S.; Yousaf, A.M.; Kim, D.W.; Jang, S.W.; Son, M.; Kim, Y.H.; Yong, C.S.; Kim, J.O.; Choi, H. Mechanical properties and in vivo healing evaluation of a novel Centella asiatica-loaded hydrocolloid wound dressing. Int. J. Pharm. 2015, 490, 240–247.
- Thangavel, A.; Ayyanar, M.; Justin Koilpillai, Y.; Sekar, T. Phytochemical Screening and Antibacterial activity of leaf and callus extracts of Centella asiatica. Bangladesh J. Pharmacol. 2011, 6, 55–60.
- Kuo, C.-W.; Chiu, Y.-F.; Wu, M.-H.; Li, M.-H.; Wu, C.-N.; Chen, W.-S.; Huang, C.-H. Gelatin/Chitosan Bilayer Patches Loaded with Cortex Phellodendron amurense/Centella asiatica Extracts for Anti-Acne Application. Polymers 2021, 13, 579.
- Phaechamud, T.; Yodkhum, K.; Charoenteeraboon, J.; Tabata, Y. Chitosan-aluminum monostearate composite sponge dressing containing asiaticoside for wound healing and angiogenesis promotion in chronic wound. Mater. Sci. Eng. C 2015, 50, 210–225.
- Oh, J.; Kim, H.; Beuchat, L.R.; Ryu, J.-H. Inhibition of Staphylococcus aureus on a laboratory medium and black peppercorns by individual and combinations of essential oil vapors. Food Control 2022, 132, 108487.
- He, Q.; Zhang, L.; Yang, Z.; Ding, T.; Ye, X.; Liu, D.; Guo, M. Antibacterial mechanisms of thyme essential oil nanoemulsions against Escherichia coli O157:H7 and Staphylococcus aureus: Alterations in membrane compositions and characteristics. Innov. Food Sci. Emerg. Technol. 2022, 75, 102902.
- Cruz-Tirado, J.P.; Barros Ferreira, R.S.; Lizárraga, E.; Tapia-Blácido, D.R.; Silva, N.C.C.; Angelats-Silva, L.; Siche, R. Bioactive Andean sweet potato starch-based foam incorporated with oregano or thyme essential oil. Food Packag. Shelf Life 2020, 23, 100457.
- Koga, A.Y.; Pereira, A.V.; Lipinski, L.C.; Oliveira, M.R.P. Evaluation of wound healing effect of alginate films containing Aloe vera (Aloe barbadensis Miller) gel. J. Biomater. Appl. 2018, 32, 1212–1221.
- Atiba, A.; Nishimura, M.; Kakinuma, S.; Hiraoka, T.; Goryo, M.; Shimada, Y.; Ueno, H.; Uzuka, Y. Aloe vera oral administration accelerates acute radiation-delayed wound healing by stimulating transforming growth factor-β and fibroblast growth factor production. Am. J. Surg. 2011, 201, 809–818.
- Guilherme, E.D.; de Souza, C.W.; Bernardo, M.P.; Zenke, M.; Mattoso, L.H.; Moreira, F.K. Antimicrobially active gelatin/-LDH composite films based on clove essential oil for skin wound healing. Mater. Today Commun. 2021, 27, 102169.
- Razavi, M.S.; Golmohammadi, A.; Nematollahzadeh, A.; Rovera, C.; Farris, S. Cinnamon Essential Oil Encapsulated into a Fish Gelatin-Bacterial Cellulose Nanocrystals Complex and Active Films Thereof. Food Biophys. 2021, 17, 38–46.
- Liakos, I.; Rizzello, L.; Scurr, D.J.; Pompa, P.P.; Bayer, I.S.; Athanassiou, A. All-natural composite wound dressing films of essential oils encapsulated in sodium alginate with antimicrobial properties. Int. J. Pharm. 2014, 463, 137–145.
- Kavoosi, G.; Dadfar, S.M.; Purfard, A.M. Mechanical, physical, antioxidant, and antimicrobial properties of gelatin films incorporated with thymol for potential use as nano wound dressing. J. Food Sci. 2013, 78, E244–E250.
- Otoni, C.G.; Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; Soares, N.F.F.; Mattoso, L.H.C. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194.
- Roberts, M.S. Solute-Vehicle-Skin Interactions in Percutaneous Absorption: The Principles and the People. Ski. Pharmacol. Physiol. 2013, 26, 356–370.
- Sroczyk, E.A.; Berniak, K.; Jaszczur, M.; Stachewicz, U. Topical electrospun patches loaded with oil for effective gamma linoleic acid transport and skin hydration towards atopic dermatitis skincare. Chem. Eng. J. 2022, 429, 132256.
- Tuter, M.; Secundo, F.; Riva, S.; Aksoy, H.A.; Ustun, G. Partial purification of Nigella sativa L. Seed lipase and its application in transesterification reactions. J. Am. Oil Chem. Soc. 2003, 80, 43–48.
- Foster, R.H.; Hardy, G.; Alany, R. Borage oil in the treatment of atopic dermatitis. Nutrition 2010, 26, 708–718.
- Callaway, J.C. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72.
- Yoon, S.; Lee, J.; Lee, S. The Therapeutic Effect of Evening Primrose Oil in Atopic Dermatitis Patients with Dry Scaly Skin Lesions Is Associated with the Normalization of Serum Gamma-Interferon Levels. Ski. Pharmacol. Physiol. 2002, 15, 20–25.
- Chattopadhyay, P.; Dhiman, S.; Borah, S.; Rabha, B.; Chaurasia, A.K.; Veer, V. Essential oil based polymeric patch development and evaluating its repellent activity against mosquitoes. Acta Trop. 2015, 147, 45–53.
- Shan, Y.-H.; Peng, L.-H.; Liu, X.; Chen, X.; Xiong, J.; Gao, J.-Q. Silk fibroin/gelatin electrospun nanofibrous dressing functionalized with astragaloside IV induces healing and anti-scar effects on burn wound. Int. J. Pharm. 2015, 479, 291–301.
- Safaee-Ardakani, M.R.; Hatamian-Zarmi, A.; Sadat, S.M.; Mokhtari-Hosseini, Z.B.; Ebrahimi-Hosseinzadeh, B.; Rashidiani, J.; Kooshki, H. Electrospun Schizophyllan/polyvinyl alcohol blend nanofibrous scaffold as potential wound healing. Int. J. Biol. Macromol. 2019, 127, 27–38.
- Ranjbar-Mohammadi, M.; Rabbani, S.; Bahrami, S.H.; Joghataei, M.; Moayer, F. Antibacterial performance and in vivo diabetic wound healing of curcumin loaded gum tragacanth/poly (ε-caprolactone) electrospun nanofibers. Mater. Sci. Eng. C 2016, 69, 1183–1191.
- Faraji, S.; Nowroozi, N.; Nouralishahi, A.; Shayeh, J.S. Electrospun poly-caprolactone/graphene oxide/quercetin nanofibrous scaffold for wound dressing: Evaluation of biological and structural properties. Life Sci. 2020, 257, 118062.
- Ramalingam, R.; Dhand, C.; Leung, C.M.; Ong, S.T.; Annamalai, S.K.; Kamruddin, M.; Verma, N.K.; Ramakrishna, S.; Lakshminarayanan, R.; Arunachalam, K.D. Antimicrobial properties and biocompatibility of electrospun poly-ε-caprolactone fibrous mats containing Gymnema sylvestre leaf extract. Mater. Sci. Eng. C 2019, 98, 503–514.
- Amiri, N.; Ajami, S.; Shahroodi, A.; Jannatabadi, N.; Darban, S.A.; Bazzaz, B.S.F.; Pishavar, E.; Kalalinia, F.; Movaffagh, J. Teicoplanin-loaded chitosan-PEO nanofibers for local antibiotic delivery and wound healing. Int. J. Biol. Macromol. 2020, 162, 645–656.
- Abdel-Mohsen, A.; Abdel-Rahman, R.; Kubena, I.; Kobera, L.; Spotz, Z.; Zboncak, M.; Prikryl, R.; Brus, J.; Jancar, J. Chitosan-glucan complex hollow fibers reinforced collagen wound dressing embedded with aloe vera. Part I: Preparation and characterization. Carbohydr. Polym. 2020, 230, 115708.
- Dong, W.-H.; Liu, J.-X.; Mou, X.-J.; Liu, G.-S.; Huang, X.-W.; Yan, X.; Ning, X.; Russell, S.J.; Long, Y.-Z. Performance of polyvinyl pyrrolidone-isatis root antibacterial wound dressings produced in situ by handheld electrospinner. Colloids Surf. B Biointerfaces 2020, 188, 110766.
- Ajmal, G.; Bonde, G.V.; Mittal, P.; Khan, G.; Pandey, V.K.; Bakade, B.V.; Mishra, B. Biomimetic PCL-gelatin based nanofibers loaded with ciprofloxacin hydrochloride and quercetin: A potential antibacterial and anti-oxidant dressing material for accelerated healing of a full thickness wound. Int. J. Pharm. 2019, 567, 118480.
- Urena-Saborio, H.; Alfaro-Viquez, E.; Esquivel-Alvarado, D.; Madrigal-Carballo, S.; Gunasekaran, S. Electrospun plant mucilage nanofibers as biocompatible scaffolds for cell proliferation. Int. J. Biol. Macromol. 2018, 115, 1218–1224.
- Jafari, A.; Amirsadeghi, A.; Hassanajili, S.; Azarpira, N. Bioactive antibacterial bilayer PCL/gelatin nanofibrous scaffold promotes full-thickness wound healing. Int. J. Pharm. 2020, 583, 119413.
- Ardekani, N.T.; Khorram, M.; Zomorodian, K.; Yazdanpanah, S.; Veisi, H.; Veisi, H. Evaluation of electrospun poly (vinyl alcohol)-based nanofiber mats incorporated with Zataria multiflora essential oil as potential wound dressing. Int. J. Biol. Macromol. 2019, 125, 743–750.
- Abou Zekry, S.S.; Abdellatif, A.; Azzazy, H.M. Fabrication of pomegranate/honey nanofibers for use as antibacterial wound dressings. Wound Med. 2020, 28, 100181.
- Zhou, Y.; Yang, H.; Liu, X.; Mao, J.; Gu, S.; Xu, W. Electrospinning of carboxyethyl chitosan/poly(vinyl alcohol)/silk fibroin nanoparticles for wound dressings. Int. J. Biol. Macromol. 2013, 53, 88–92.
- Varshney, N.; Sahi, A.K.; Poddar, S.; Mahto, S.K. Soy protein isolate supplemented silk fibroin nanofibers for skin tissue regeneration: Fabrication and characterization. Int. J. Biol. Macromol. 2020, 160, 112–127.
- Selvaraj, S.; Fathima, N.N. Fenugreek Incorporated Silk Fibroin Nanofibers A Potential Antioxidant Scaffold for Enhanced Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 5916–5926.
- Yousefi, I.; Pakravan, M.; Rahimi, H.; Bahador, A.; Farshadzadeh, Z.; Haririan, I. An investigation of electrospun Henna leaves extract-loaded chitosan based nanofibrous mats for skin tissue engineering. Mater. Sci. Eng. C 2017, 75, 433–444.
- Charernsriwilaiwat, N.; Rojanarata, T.; Ngawhirunpat, T.; Sukma, M.; Opanasopit, P. Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. Int. J. Pharm. 2013, 452, 333–343.
- Adamczak, A.; Ożarowski, M.; Karpiński, T. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153.
- Özlem, E. Production of lavender oil loaded antibacterial polymeric membranes. Cumhur. Sci. J. 2020, 41, 160–168.
- Chomachayi, M.D.; Solouk, A.; Akbari, S.; Sadeghi, D.; Mirahmadi, F.; Mirzadeh, H. Electrospun nanofibers comprising of silk fibroin/gelatin for drug delivery applications: Thyme essential oil and doxycycline monohydrate release study. J. Biomed. Mater. Res. Part A 2018, 106, 1092–1103.
- Son, B.C.; Park, C.; Kim, C. Fabrication of antimicrobial nanofiber air filter using activated carbon and cinnamon essential oil. J. Nanosci. Nanotechnol. 2020, 20, 4376–4380.
- Liakos, I.L.; Holban, A.M.; Carzino, R.; Lauciello, S.; Grumezescu, A.M. Electrospun fiber pads of cellulose acetate and essential oils with antimicrobial activity. Nanomaterials 2017, 7, 84.
- Rieger, K.A.; Schiffman, J. Electrospinning an essential oil: Cinnamaldehyde enhances the antimicrobial efficacy of chitosan/poly(ethylene oxide) nanofibers. Carbohydr. Polym. 2014, 113, 561–568.
- Bharathi, B.S.; Stalin, T. Cerium oxide and peppermint oil loaded polyethylene oxide/graphene oxide electrospun nanofibrous mats as antibacterial wound dressings. Mater. Today Commun. 2019, 21, 100664.
- Bazargani, M.M.; Rohloff, J. Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control 2016, 61, 156–164.
- Eğri, Ö.; Erdemir, N. Production of Hypericum perforatum oil-loaded membranes for wound dressing material and in vitro tests. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1404–1415.
- Xiang, F.; Bai, J.; Tan, X.; Chen, T.; Yang, W.; He, F. Antimicrobial activities and mechanism of the essential oil from Artemisia argyi Levl. et Van. var. argyi cv. Qiai. Ind. Crops Prod. 2018, 125, 582–587.
- Li, T.-T.; Li, J.; Zhang, Y.; Huo, J.-L.; Liu, S.; Shiu, B.-C.; Lin, J.-H.; Lou, C.-W. A study on artemisia argyi oil/sodium alginate/PVA nanofibrous membranes: Micro-structure, breathability, moisture permeability, and antibacterial efficacy. J. Mater. Res. Technol. 2020, 9, 13450–13458.
- Lamarra, J.; Bucci, P.; Giannuzzi, L.; Montanari, J.; Rivero, S.; Pinotti, A. Biomaterial-based dressings as vehicle for chitosan-encapsulated cabreuva essential oil: Cytotoxicity and regenerative activity. React. Funct. Polym. 2020, 156, 104728.
- Khan, A.u.R.; Huang, K.; Jinzhong, Z.; Zhu, T.; Morsi, Y.; Aldalbahi, A.; El-Newehy, M.; Yan, X.; Mo, X. PLCL/Silk fibroin based antibacterial nano wound dressing encapsulating oregano essential oil: Fabrication, characterization and biological evaluation. Colloids Surf. B: Biointerfaces 2020, 196, 111352.
- Özkalp, B.; Sevgi, F.; Özcan, M.; Özcan, M. The antibacterial activity of essential oil of oregano (Origanum vulgare L.). J. Food Agric. Environ. 2010, 8, 6–8.
- Lu, M.; Dai, T.; Murray, C.K.; Wu, M.X. Bactericidal Property of Oregano Oil Against Multidrug-Resistant Clinical Isolates. Front. Microbiol. 2018, 9, 2329.
- Greco, F.; Vicent, M. Combination therapy: Opportunities and challenges for polymer–drug conjugates as anticancer nanomedicines. Adv. Drug Deliv. Rev. 2009, 61, 1203–1213.
- Aderibigbe, B.A. Design and therapeutic efficacy of polymer based drug delivery systems for antimalarials. Polym. Sci. Res. Adv. Pract. Appl. Educ. Asp. 2016, 188–198.
- Sanchis, J.; Canal, F.; Lucas, R.; Vicent, M.J. Polymer–drug conjugates for novel molecular targets. Nanomedicine 2010, 5, 915–935.
- Trifković, K.T.; Milašinović, N.Z.; Djordjević, V.B.; Krušić, M.T.K.; Knežević-Jugović, Z.D.; Nedović, V.A.; Bugarski, B.M. Chitosan microbeads for encapsulation of thyme (Thymus serpyllum L.) polyphenols. Carbohydr. Polym. 2014, 111, 901–907.
- Sotelo-Boyás, M.; Correa-Pacheco, Z.; Bautista-Baños, S.; Gómez y Gómez, Y. Release study and inhibitory activity of thyme essential oil-loaded chitosan nanoparticles and nanocapsules against foodborne bacteria. Int. J. Biol. Macromol. 2017, 103, 409–414.
- Shetta, A.; Kegere, J.; Mamdouh, W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: Encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2019, 126, 731–742.
- Francesko, A.; Fernandes, M.; Rocasalbas, G.; Gautier, S.; Tzanov, T. Polymers in Wound Repair. In Advanced Polymers in Medicine; Springer: Cham, Switzerland, 2015; pp. 401–431.
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792.
- Muthukumar, T.; Senthil, R.; Sastry, T. Synthesis and characterization of biosheet impregnated with Macrotyloma uniflorum extract for burn/wound dressings. Colloids Surf B Biointerfaces 2013, 102, 694–699.
- Silva, S.S.; Caridade, S.G.; Mano, J.F.; Reis, R.L. Effect of crosslinking in chitosan/aloe vera-based membranes for biomedical applications. Carbohydr Polym 2013, 98, 581–588.
- Agarwal, R.; Alam, S.; Gupta, B. Preparation of Curcumin Loaded Poly(Vinyl Alcohol)-Poly(Ethylene Oxide)-Carboxymethyl Cellulose Membranes for Wound Care Application. J. Biomater. Tissue Eng. 2013, 3, 273–283.
- Yang, J.Y.; Singh, D.; Singh, D.; Lee, E.; Choi, S.; Han, S.S.; Park, S.J. Terminalia bellirica Extracts Loaded on Stimuli Responsive HEMA-DEA Hydrogel for Enhanced Growth and Proliferation of Mesenchymal Stem Cells. J. Biomater. Tissue Eng. 2014, 4, 37–45.
- Ilhan, E.; Cesur, S.; Guler, E.; Topal, F.; Albayrak, D.; Guncu, M.M.; Cam, M.E.; Taskin, T.; Sasmazel, H.T.; Aksu, B.; et al. Development of Satureja cuneifolia-loaded sodium alginate/polyethylene glycol scaffolds produced by 3D-printing technology as a diabetic wound dressing material. Int. J. Biol. Macromol. 2020, 161, 1040–1054.
- Ali Khan, B.; Ullah, S.; Khan, M.K.; Alshahrani, S.M.; Braga, V.A. Formulation and evaluation of Ocimum basilicum-based emulgel for wound healing using animal model. Saudi Pharm. J. 2020, 28, 1842–1850.
- Razdan, K.; Kanta, S.; Chaudhary, E.; Kumari, S.; Rahi, D.K.; Yadav, A.K.; Sinha, V.R. Levofloxacin loaded clove oil nanoscale emulgel promotes wound healing in Pseudomonas aeruginosa biofilm infected burn wound in mice. Colloids Surf. B Biointerfaces 2023, 222, 113113.
- Eid, A.M.; Jaradat, N.; Issa, L.; Abu-Hasan, A.; Salah, N.; Dalal, M.; Mousa, A.; Zarour, A. Evaluation of anticancer, antimicrobial, and antioxidant activities of rosemary (Rosmarinus Officinalis) essential oil and its Nanoemulgel. Eur. J. Integr. Med. 2022, 55, 102175.
- Ting, T.C.; Amat Rahim, N.F.; Che Zaudin, N.A.; Abdullah, N.H.; Mohamad, M.; Shoparwe, N.F.; Mhd Ramle, S.F.; Aimi, Z.; Abdul Hamid, Z.A.; Yusof, A.H. Development and Characterization of Nanoemulgel Containing Piper betle Essential Oil as Active Ingredient. IOP Conf. Ser. Earth Environ. Sci. 2020, 596, 012032.
- Newman, D.J.; Cragg, G. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Entry Collection:
Biopharmaceuticals Technology
Revisions:
2 times
(View History)
Update Date:
10 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




