Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Polymer Science
Wound care is a global health issue with a financial burden of up to US $96.8 billion annually in the USA alone. Chronic non-healing wounds which show delayed and incomplete healing are especially problematic. Natural products and their derivatives have long been a significant source of pharmaceuticals against AMR. Scrutinising the data of newly approved drugs has identified plants as one of the biggest and most important sources in the development of novel antibacterial drugs.
- antibacterial additives
- natural products
- polymer wound dressing
- endophytic fungi
1. Introduction
More than 3000 wound dressing types on the wound management market, different characteristics can be achieved based on the intrinsic properties of the polymers used in wound dressing preparation. These characteristics include their ability to absorb exudate, combat infection, relieve pain, promote autolytic debridement, or even provide and maintain a moist environment at the wound surface. However, there is no wound dressing that possesses all these properties. The type of wound dressing is selected based on the patient’s health status, wound type, location, depth, amount of exudate, wound adhesion, and economic considerations [10,11]. Hydrogels, foams, dermal patches, films, nanoparticles, hydrocolloids, nanofibers, and membranes are the main groups of dressings, and their description, characteristics, and polymers used to make them are summarised in Table 1 [10].
Table 1. Different types of wound dressings, their wound target, and polymer type.
| Variety | Description | Advantages | Disadvantages | Wound Type Application | Polymer | Ref. |
|---|---|---|---|---|---|---|
| Hydrogels | Water-absorbent cross-linked polymeric networks resulting from the reaction of monomers | Efficient flexibility, good ability in swelling and sustaining a significant amount of water, moisturizing, removal of necrotic tissue, good porosity, and monitoring the wound without removing the dressing | Inability to absorb enough exudates leading to bacterial proliferation, and low mechanical strength | Chemotherapy peels | Polyethylene oxide, polyvinyl pyrrolidine, Polyvinyl alcohol | [10,11,12,13,14] |
| Ulcers | ||||||
| Laser resurfacing | ||||||
| Average thickness wounds | ||||||
| Donor graft sites and artificial organ wounds | ||||||
| Hydrocolloids | Colloidal material (gel) constituted with elastomers and adhesives in the form of films or sheets | Excellent exudate absorption properties, transparency, enhanced angiogenesis, and formation of granulation tissue | Not permeable to gas, vapor, water, and bacteria, their debriding capability, skin maceration, and producing a foul smell | Chronic ulcers | Pectin, carboxymethylcellulose, gelatin, and cellulose | [10,13,15,16] |
| Burns | ||||||
| Average thickness wounds | ||||||
| Donor graft sites | ||||||
| Foams | A porous structure using capillary action as its mechanism to absorb fluids | Exudate absorbance, preventing bacteria invasion, maintaining sufficient moisture at the wound surface, being removed easily, protecting the skin around the wound, maintaining an efficient temperature, mechanical protection, being nontoxic, being cost-effective with a long shelf life | Drying out the wound in case of minimal or no exudate presence and maceration of the surrounding skin in case of exudate saturation in dressing | Chronic wounds | Polyurethane, silicone, silk fibroin | [13,17,18,19,20,21] |
| Burns | ||||||
| Mohs surgery and wounds | ||||||
| Laser resurfacing wounds | ||||||
| Films | Consists of adhesives, porous, and thin transparent polymers | The possibility of having a high mechanical strength, high water transmission rate, protecting the wound against bacterial infection | The possibility of having a low mechanical strength | Superficial wounds | Soy protein isolates, chitosan, polyvinyl alcohol | [13,22,23,24] |
| Laser wounds | ||||||
| Surgery defect sites | ||||||
| Skin tears | ||||||
| Dermal patches | Dressings consisted of a multilayered structure with an impermeable excipient-loaded film, drugs, and a release liner | Suitable for skin adhesion, not having a liquid reservoir, controlling the drug delivery rate | Needing flux moderation in case of loading with highly soluble drugs, and decrease in drug release rate with wear time, not suitable for most of the drugs | Hypertension | Poly(vinyl pyrrolidones), poly(vinyl alcohol) | [25,26,27,28,29,30] |
| Topical wounds | ||||||
| Fibers and nanofibers | Polymeric fibers produced with electrospinning process | Excellent mechanical properties, thermal stability, antimicrobial activity, biodegradability, control in water vapor transmission rate, oxygen permeability, fluid drainage ability, high porosity, and high surface area | Higher cost of production in some cases, hard to produce fibers with diameters less than 10 nm | Partial thickness burns | Polyurethane, collagen, silk fibroin, polycaprolactone, poly (lactic-co-glycolic acid), polyethylene oxide, etc. | [31,32,33,34,35,36,37,38,39,40] |
| Diabetic ulcers | ||||||
| Bone bleeding | ||||||
| Chronic infected wounds | ||||||
| Acute wounds | ||||||
| Venous ulcers | ||||||
| Pressure ulcers | ||||||
| Membranes | A thin semi-permeable barrier | Porous structure, transparency, excessive loss of water, the ability to contain an occlusive layer to impede microbial invasion | Cytotoxicity in some cases | Superficial wounds | Pectin, collagen, chitosan, chitin, alginate, zein, polycaprolactone, polyvinyl acetate, polyvinyl alcohol, polytetrafluoroethylene, cellulose, etc. | [41,42,43,44,45,46,47,48,49,50,51,52] |
| Frictional wounds | ||||||
| Skin-scratching wounds | ||||||
| Skin donor sites | ||||||
| Skin with external contamination | ||||||
| Polymer-drug conjugates | Polymer-based water-soluble nanocarriers conjugated with bioactive agents | Improving the water solubility of the hydrophobic drugs, enhancing the pharmacokinetic profile of the conjugated drug, extending the volume of distribution, and protecting the conjugated drug against degradation | Limitations to be applied on a large scale, low stability in vivo, short half-life, and immunogenicity | Diabetic wounds such as venous leg and lower limb ulcers | N-(2-hydroxypropyl) methacrylamide copolymer, polyglutamic acid, Poly(ethylene glycol), Polyamidoamine, hyaluronic acid, poly (vinyl ether-co-maleic anhydride), poly (vinyl pyrrolidone), etc. | [53,54,55,56,57,58,59,60,61] |
Studies show that the challenges of wound dressings linked to wound infection are significant. In most acute and chronic infections, a mixed population of both aerobic and anaerobic microorganisms is observed [62] and yet to be eliminated. This challenge emphasises the importance of strategies that target the most common bacteria on the wound surface. Data from recent studies on various wound infections (e.g., surgical incisions, burns, abscesses, and traumatic wounds) confirm the presence of Pseudomonas sp. and Staphylococcus aureus as the most common Gram-negative and Gram-positive bacteria, respectively, on wound surfaces with a share of 58.4% for Gram-negative and 41.6% for Gram-positive bacteria [63]. The percentage of each group of bacteria can be observed in detail in Figure 1.
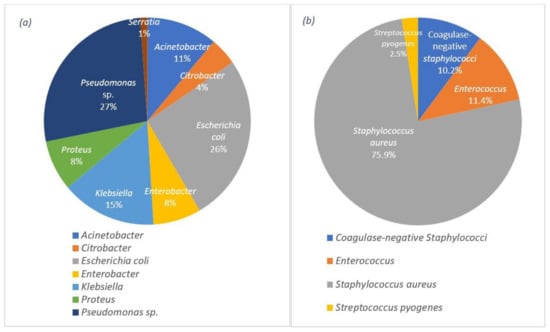
Figure 1. Distribution of the wound infections by: (a) the Gram-negative bacteria and (b) the Gram-positive bacteria.
There is a significant need for antibacterial wound dressings for controlling and reducing bacterial infections. One of the best approaches is wound dressings containing antibiotics as an additive. Numerous reports can be found on wound dressings containing antibiotic additives and their effects on wound dressings [64,65,66].
By using an efficient amount of antibiotics, suitable treatment of wound infections can be achieved. However, high amounts of antibiotics will cause systemic toxicity [67]. In order to overcome these detrimental effects, the antibacterial compounds and antibiotics are embedded in wound dressings for sustained and controlled drug release [10]. A lack of new antibiotics and antibacterial agents, as well as widespread distribution and misuse of these antibacterial compounds, has caused an increase in antimicrobial resistance (AMR). It has been proposed that AMR has the potential to kill ten million lives by 2050 worldwide, costing an estimated US $100 trillion [68]. This can result in the return to a pre-antibiotic era, with infections caused by multiple-resistant pathogens [31]. Thus, there is an urgent need for sustainable novel antibacterial additives to overcome this major clinical problem.
2. Bioactive Wound Dressings (Polymer + Additives)
2.1. Hydrogels
Hydrogel wound dressings containing natural antibacterial bio-additives have been used extensively for wound treatments. These additives are able to optimise the antibacterial properties against the unfavourable increase of bacterial proliferation in hydrogel wound dressings [11,71].
Plant-based antibacterial additives have been used in hydrogels to increase their activity against bacteria in infected wounds [71]. One of the most important class of additives in this group are the essential oils. The presence of essential oils as hydrophobic compounds in the hydrogel texture leads to good mechanical properties, degradability, improvement of the porous structure, and antioxidant properties [72]. Altaf et al. used a solution casting method to produce a polyvinyl alcohol/starch hydrogel membrane containing various concentrations of clove essential oil. The products resulted in excellent antibacterial activity, with a minimum inhibition zone of 34 ± 0.42 mm against S. aureus and 31 mm against E. coli [71]. The synthesis scheme has been demonstrated in Figure 2. Other essential oils used in hydrogels are lavender and tea tree oil [73]. Using these two essential oils in gellan gum hydrogels at 25% w/w resulted in an efficient zone of inhibition of 20 mm against S. aureus and 30 mm against E. coli in standard disc diffusion assays [73]. Several studies have used different essential oils in hydrogels, such as basil oil [74], tea tree oil [75], sweet fennel oil [76], rosemary essential oil, orange essential oil [77], and Thymus daenesis oil [78], to improve their antibacterial activity.
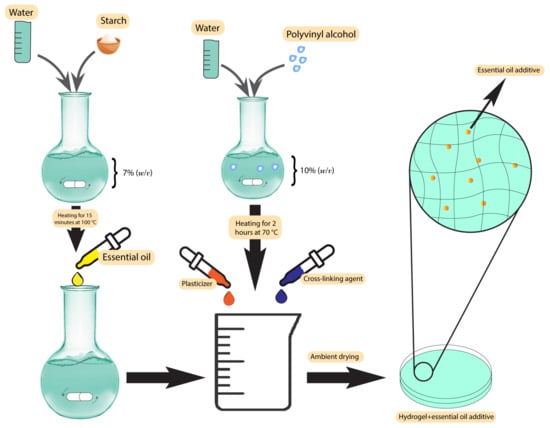
Figure 2. Synthesis scheme of polyvinyl alcohol/starch hydrogel membranes.
In addition to essential oils, plant extracts have been used as additives in hydrogel wound dressings. In a study by Shukla et al., a bioactive hydrogel dressing containing an ethanolic extract of Morus alba leaves was used against diabetic wounds. The apigenin derived from the extract was tailored with gellan gum-poly ethylene glycol-chitosan hydrogels and screened in vivo for its effectiveness. The results indicated that the apigenin additives caused effective stimulation of wound contraction and increase in the collagen content in diabetic as well as normal wound tissues, which leads to an accelerated wound healing process [79]. The antibacterial activity of Morus alba extracts against S. aureus has been previously investigated, resulting in a minimum inhibitory concentration (MIC) of 250 µg/mL [80].
2.2. Hydrocolloids
Hydrocolloids have been previously used along with natural antibacterial additives to improve their characteristics against wound bacteria and reduce the unpleasant odour [81]. These additives are the extracts of some pre-approved antibacterial plants, such as Centella asiatica (CA) and Phellodendri amurensis (PA) [82,83], which have been used in different studies against several bacteria. After loading CA plant extracts in alginate hydrocolloids using a hot melting method, Jin et al. showed excellent swelling, drug release, and mechanical properties compared with similar commercial products. Enhanced healing process in excision, infection, and abrasion wounds were observed in a rat wound model, which suggests that this extract is a potential candidate for the treatment of various wounds [82]. The preparation technique has been demonstrated in Figure 3. Antibacterial activity tests of the CA extracts at 100 µg/mL against P. aeruginosa, S. aureus, and E. coli resulted in zones of inhibition between 28–30 mm [83].
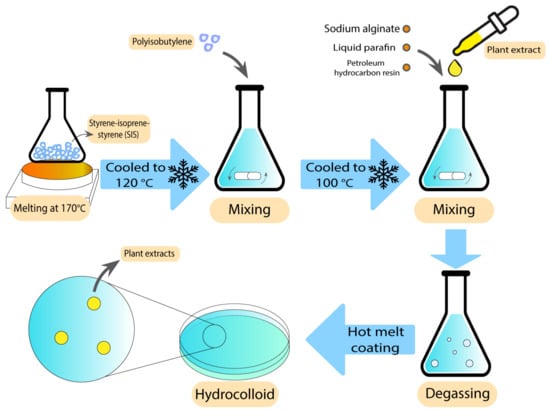
Figure 3. Preparation technique of alginate hydrocolloids using hot melt coating.
Another application of hydrocolloids containing CA extracts is skin treatment. Kuo et al. produced an anti-acne patch with gelatin/chitosan (GC) bilayer hydrocolloid patches. This anti-acne bilayer patch was loaded with Cortex PA and CA extracts. The results indicated that CA could reduce scar formation and improve the wound healing process. Water retention rate, weight loss rate, antibacterial activity, and in vitro cytotoxicity were tested as well. The results indicated that skin fibroblast cell viability was accelerated and the water retention of the patches was improved, which contributed to the exudate absorption [84].
2.3. Foams
Foams are another group of polymer wound dressings that have been previously used with additives to accelerate wound recovery. There are some reports on using plant-derived extracts as antibacterial agents in foam-based dressings. Nantaporn et al. prepared polyurethane foam sheets containing silver and asiaticoside (AS) (an extract derived from Centella asiatica plant) for healing dermal wounds. AS in a foam formulation played an essential role to increase the healing rate. The MIC of the additives against P. aeruginosa, S. aureus, E. coli, and B. subtilis were in a range of 0.4–3.1 ppm. However, the foam dressing released 4–5 ppm of the additive. The clear zones from disc diffusion assays were statistically larger than other tested formulations [21]. AS has been proved to be efficiently mixed with other polymers in different studies. Phaechamud et al. developed an absorbent chitosan-based dressing containing silver and asiaticoside as an additive. This dressing showed a successful controlled drug release along with angiogenic activity, indicating the potential to be further utilised as absorbents in medical wound dressings [85]. In what follows, the scheme of the preparation technique has been demonstrated in Figure 4.
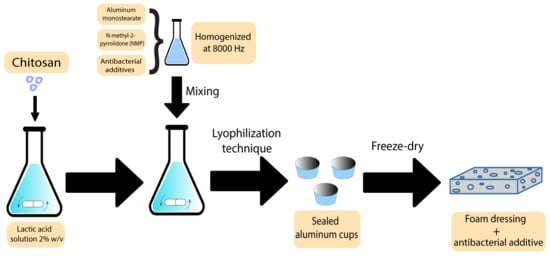
Figure 4. Preparation scheme of chitosan-based bioactive foams.
The other group of natural plant-based antibacterial additives used in foams is essential oils. The antibacterial activity of plant essential oils such as oregano and thyme has been proven previously, with MIC values of 0.0781 µL/mL [86] and 0.125 mg/mL [87], respectively. Adding these oils to a natural polymer such as sweet potato starch-based foam, along with their antibacterial activity, may lead to a lower degradation under the thermoforming temperature and higher mechanical resistance [88].
2.4. Films
Films have previously been used as bioactive wound dressings [10]. These types of wound dressings have been used with both plant extracts and essential oils. Some studies have shown the utilisation of different plants and plant extracts in film dressings. These plants are normally chosen based on their healing and antibacterial properties. Koga et al. developed an alginate film containing Aloe vera (Aloe barbadensis Miller) gel [89]. Aloe vera has already exhibited several pharmaceutical activities, such as the ability to promote the healing process as well as the ability to stimulate the proliferation of fibroblasts [90]. After characterising the different aspects of films containing Aloe vera, the results indicated adequate transparency, uniformity, mechanical tensile strength, and hydration capacity, which makes them an ideal candidate to be used as dressings. Furthermore, the films modulated the inflammatory phase, increased angiogenesis, and stimulated collagenesis, which leads to improved healing [89]. Figure 5 demonstrates the preparation process for these types of film.
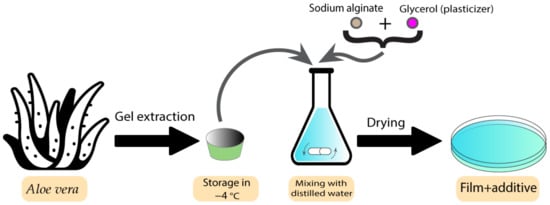
Figure 5. Preparation process of Aloe vera-containing alginate films.
The second group of additives used in film wound dressings are essential oils. Several types of essential oils have been used as an additive to optimise the antibacterial properties of film dressings. Clove, cinnamon, chamomile, thymol, lavender, tea tree, peppermint, Eucalyptus globulus juvenile, lemongrass, and lemon are some of the essential oils that have been used as antibacterial additives [91,92,93,94,95].
A combination of gelatin with clove essential oil (CEO) and hydrotalcite (HT) nanoparticles was prepared by Guilherme et al. as a wound dressing. In this study, CEO-containing films exhibited bactericidal activity against S. aureus and E. coli. HT was also hypothesised to relate positively to the antimicrobial performance of using films and enhance physical properties, which was lowered by the CEO [91].
One of the challenges in preparing films containing essential oils is choosing the proper oil to be used in the process. In this context, comparisons have been made between using each type of essential oil in a wound dressing environment. Liakos et al. used various types of essential oils such as lavender, tea tree, peppermint, Elicriso italic, cinnamon, Eucalyptus globulus, lemon, and lemongrass as an additive in sodium alginate matrixes. The produced films were tested for their antibacterial and anti-fungal properties. Among all the samples tested against E. coli, the cinnamon essential oils showed the largest inhibition zone of 12 mm, followed by lemongrass essential oil with an inhibition zone of 3 mm. The results of the antibacterial tests along with their stability indicates that films containing essential oils have the potential to be used as antibacterial wound-dressing materials [93].
2.5. Dermal Patches
The drugs used in these types of wound dressings should be penetrable to the skin, which makes most drugs unsuitable in this application. Solubility and diffusivity are two factors that determine the maximum skin penetration flux [96]. Some botanical-based additives have been used to improve the characteristics of dermal patches used in skin care and the prevention of mosquito bites [97]. In a study by Sroczyk et al., a polyimide patch was loaded with blackcurrant seed oil for atopic skin hydration studies. The application of these patches was against atopic dermatitis as a common disease among children. In this disease, gamma-linoleic acid is decreased, so the blackcurrant seed oil was used to restore the gamma-linoleic acid deficiencies. Based on the results, these patches adjust to skin movements, are stable with plant oils, and exchange air due to their high permeability, which makes them a good candidate to be used in skin care and treatment [97]. The process scheme has been demonstrated in Figure 6. There are different types of botanical-based oils with a high level of gamma-linoleic acid that can be used as additives instead of blackcurrant seed oil, such as Nigella sativa [98], borage [99], hempseed [100], and evening primrose [101].
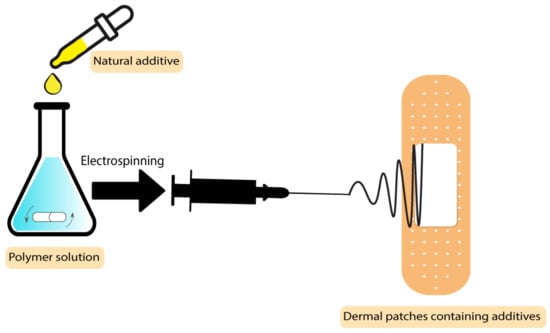
Figure 6. Process scheme of dermal patches containing natural additives.
As previously mentioned, another application of botanical-based skin patches is in the prevention of mosquito bites. In this case, essential oils as additives in patches act as insect repellents. Chattopadhyay et al. developed a patch from an optimised mixture of cinnamon, lemongrass, and eucalyptus essential oils embedded into ethylcellulose and polyvinylpyrrolidone polymer patches. These patches were shown to be safe and effective and to contain good physico-chemical properties at room temperature. The additives in this case are not only environmentally friendly but also make the patch more effective than the previous synthetic commercial products by providing complete protection for a longer time [102].
2.6. Fibers and Nanofibers-Based Electrospun Polymers
Bioactive agents added during nanofiber production have been shown to improve the wound healing process [10]. There are several strategies to tailor bioactive additives into the fibres, including emulsion electrospinning, blend electrospinning, co-axial electrospinning, and surface immobilization [103].
There are several studies indicating the use of natural botanical-based bio-additives such as plant extracts and essential oils in electrospun polymer wound dressings.
Plant extracts have been added to the polymer electrospun fibres based on the final properties required for the wound dressing. Numerous types of plant extracts have been used as an additive to nanofibers such as Azadirachta Indica [104], tumeric [105], Clerodendrum phlomidis [106], Gymnema sylvestre [107], Carica papaya [108], Aloe vera [109], Lawsonia inermis [110], Garcinia mangostana [111], mucilage [112], clove [113], Ataria multiflora [114], pomegranate [115], Achillea lyconica [116], corn [117], fenugreek [118], henna [119], and chamomile [120].
These extracts have been proved to be effective in diabetic wound dressings. In a study by Ranjbar-Mohammadi et al., curcumin extracted from turmeric was used as an antibacterial additive in polycaprolactone electrospun fibres. The experiments indicated that the wound dressing was active for the treatment of diabetic wounds. Exhibiting an MIC of 62.5 µg/mL against P. aeruginosa [121], curcumin showed a more accelerated wound healing process in comparison with the blank sample [105]. Another application of nanofibers containing plant extracts is skin tissue engineering. Henna leaf extract-loaded chitosan-based nanofibrous mats were used as a wound dressing by Yousefi et al. The final product displayed efficient antibacterial activity due to Lawsonia inermis (Henna) leaf extracts in mats (2 wt%), with zones of inhibition against S. aureus and E. coli of 18 mm and 25 mm, respectively. The presence of henna extract caused a reduction in the fibre diameter of the mats, which makes it favourable for wound healing applications due to increasing the surface area. Furthermore, the combined advantageous features including high biocompatibility, synergistic antibacterial activity, and acceleration of wound healing can be observed by using this additive in a mixture with polymer nanofibers [119].
The next group of botanical-based additives used in nanofiber polymer wound dressings is essential oils. Different types of essential oils have previously been used as additives in a mixture with polymer nanofibers targeting wound bacteria. These plants include lavender oil [122], thyme oil [123], cinnamon oil [124], and rosemary/oregano oil [125] that have shown antibacterial activity against the most common wound bacteria such as S. aureus, E. coli, and P. aeruginosa [122,123,124,125,126].
An improved wound healing device using encapsulation of cerium oxide (CeO2) and peppermint oil (PM oil) on polyethylene oxide/graphene oxide (PEO/GO) electrospun polymeric mats was shown by Suganya et al. This study involved testing against Gram-positive bacteria (S. aureus) and Gram-negative bacteria (E. coli) and evaluated in vitro cytotoxicity. The results indicated that the CeO2-PM oil-PEO/GO nanofibrous mats were less toxic to the L929 fibroblast cells. Furthermore, evaluations demonstrated that the incorporation of the plant-based bioactive agent and CeO2 in a nanofibrous mat accelerates re-epithelialization and collagen deposition, which makes the system an efficient potential candidate to be applied as wound dressings with skin infections [127]. The MIC values for peppermint essential oils are 3.1 µL/mL and 6.3 µL/mL against S. aureus and E. coli, respectively [128]. In what follows, the preparation technique of CeO2-PM oil-PEO/GO nanofibrous mats is demonstrated in Figure 7.
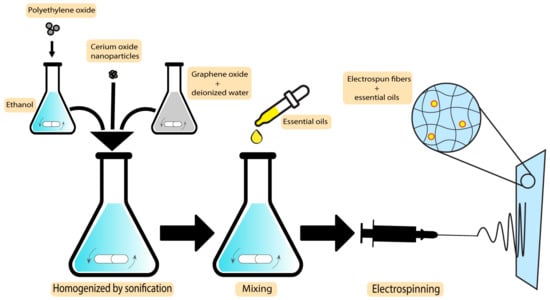
Figure 7. Preparation technique of CeO2-PM oil-PEO/GO nanofibrous mats.
2.7. Membranes
Another group of wound dressings that have been used in combination with plant-based natural additives are membranes. Both essential oils and plant extracts have shown the ability to optimise the characteristics of the final dressings. Egri et al. developed Hypericum perforatum oil-loaded polycaprolactone membranes to be used in wound dressing applications. After investigating the mechanical strength and antibacterial activity, the product exhibited sufficient elasticity and activity against S. aureus and E. coli, with inhibition zones of 8–13 mm and 10–12.2 mm, respectively. Not having the risk of adhering to the wound surface, not having apoptotic/necrotic effects, being biocompatible, and having a proliferative effect on cells are some of the advantageous features of the Hyperium perforatum-loaded membranes [129]. The preparation scheme of this membrane is demonstrated in Figure 8.
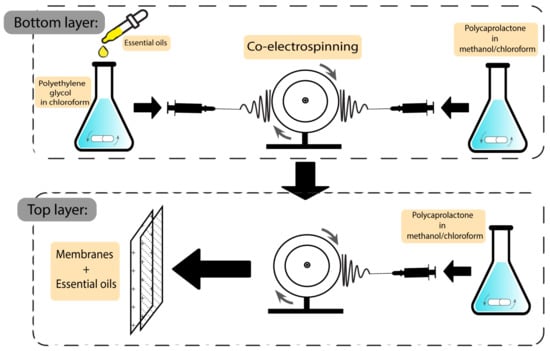
Figure 8. Preparation scheme of essential oil-based polycaprolactone membranes.
Another type of essential oil used in membranes is Artemisia argyi. The efficiency of this essential oil has previously been investigated against wound bacteria such as S. aureus, P. aeruginosa and E. coli, with MIC values of 16 µg/mL, 64 µg/mL, and 32 µg/mL, respectively [130]. Ting-Ting et al. fabricated Artemisia argyi oil-microcapsule (AAO-MC)/PVC fibrous membrane wound dressings and showed that the production process was enhanced using emulsification-internal gelation. The results showed excellent stability and a slow release of the oil. Furthermore, the produced membrane showed good water vapor transmission and high hydrophilicity as well as an excellent antibacterial rate of 94.3%, which is calculated by the difference between the colony counts of the blank specimen and the colony counts of culture medium that has been cultured with a bacterial solution for a specified time divided by the colony counts of the blank specimen [131].
Based on the targeted bacteria and the final characteristics, other types of essential oils may be used as additives, such as cabreuva (Myrocarpus fastigiatus) [132] and oregano [133]. The MIC values of pure oregano essential oil have been determined to be 0.25 mg/mL, 0.64 mg/mL, and 0.16 mg/mL against E. coli, P. aeruginosa, and S. aureus, respectively [134,135].
The addition of cabreuva essential oil to poly (vinyl alcohol) membranes proves its effectiveness against S. aureus. Its capacity to produce cell regeneration along with no detectable toxicity makes it a suitable dressing for superficial burns or minor wounds [132]. Oregano essential oils have been used with poly (L-lactide-co-caprolactone)/silk fibroin membranes as shown by Khan et al., showing a highly active membrane against both Gram-negative (E. coli) and Gram-positive (S. aureus) bacteria. The results indicated an accelerated healing process, boosted granulation, and also re-epithelialization, which confirms its potential to be used as a wound dressing [133].
2.8. Polymer-Drug Conjugates
Linkers used for the conjugation of drugs to polymers function to control the drug release in a pH specific manner and in the presence of enzymes depending on the chemistry of the linker employed [136]. For improving the therapeutic advantages of this type of wound dressing, moiety and solubilising units are also incorporated into polymer–drug conjugates [137,138]. Several studies indicate the use of plant extracts and essential oils conjugated with polymers. Some of the essential oils that have previously been used in polymer nanocarriers are thyme [139,140], peppermint oil [141], green tea oil [141], etc.
In a study by Shetta et al., peppermint and green tea essential oils were encapsulated into chitosan nanoparticles using the emulsification/ionic gelation method. The final product was tested against S. aureus and E. coli, showing minimum bactericidal concentration (MBC) values of 1.11 mg/mL and >2.72 mg/mL for peppermint oil and 0.57 mg/mL and 1.15 mg/mL for green tea, respectively, demonstrating their potential to be used in wound dressing applications [141]. Figure 9 demonstrates the preparation steps of this product.
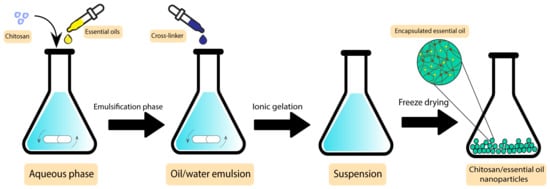
Figure 9. Preparation steps of chitosan/essential oil nanoparticles.
Another group of botanical-based antibacterial additives with the potential to be conjugated with polymers are plant extracts. Some of the utilised plant extracts conjugated with polymer wound dressings are polyphenolics and hydrolysable tannins from Hamamelis virginiana [142], seaweed extract [143], Mcrotyloma uniflorum [144], Aloe vera [145], and curcumin [146].
In a study by Yang et al., gallic acid was conjugated to a 2-hydroxy (ethyl methacrylate-co-2-diethylamino) methacrylate hydrogel. Gallic acid used in this study was extracted from an Indian plant called Terminalia bellinca, showing antioxidant and cytoprotective characteristics. The multifunctional hydrogel was used as a carrier for cell therapy and drug delivery applications. The results indicated that the product caused a faster recovery in affected tissues, which shows their significant potential to be used in medical applications [147].
2.9. Other Polymer Wound Dressings
Other types of polymer wound dressings including 3D-printed scaffolds, emulgels, and nanoemulgels have been used with various plant-based antibacterial additives previously. There are several studies indicating the use of essential oils and plant extracts in these types of wound dressings.
In a study by Ilhan et al., Satureja cuneifolia plant extracts were blended with sodium alginate and polyethylene 3D-printed scaffolds for treating diabetic ulcers. Disc diffusion testings against S. aureus demonstrated that the samples containing Satureja cuneifolia extracts (between 0.5 to 2 wt%) have an inhibition zone of 12–13 mm, which indicates their remarkable activity against Gram-positive bacteria. However, their activity against E. coli was reported to be in much higher concentrations (700 µg/mL) [148].
Emulgels and nanoemulgels have been used extensively with plant extracts and essential oils as an additive. Ocimum basilicum extracts [149], clove oil [150], rosemary oil [151], and piper betle oil [152] are some of these additives.
In a study by Razdan et al., clove oil-based nanoemulgels were used as a burn wound dressing. Levofloxacin nanoemulgels were combined with clove oil and were examined in vivo against P. aeruginosa biofilm-infected burn wounds. The product was tested against mice and the wound closure state was observed on the 1st, 3rd, 7th, 10th, and 15th day. The results indicated a faster reduction in wound size and a complete wound closure after 15 days in comparison with the samples without the additive, which were not completely closed in that period [150].
As mentioned before, one of the ways to improve wound dressing characteristics is to include bioactive additives. The role of natural antibacterial additives in polymer wound dressing groups were summarised before. In the following, different groups of plant-based natural products, as the source of novel antibacterial additives against the most common wound bacteria (S. aureus, E. coli, and P. aeruginosa), are discussed [69].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics15020644
This entry is offline, you can click here to edit this entry!
