
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anna Lenart-Boroń | -- | 3820 | 2023-03-06 11:53:10 | | | |
| 2 | Lindsay Dong | Meta information modification | 3820 | 2023-03-07 07:31:41 | | |
Video Upload Options
Environmental aquatic pollution with antibiotics is a global challenge that affects even pristine mountain environments. Monitoring the concentration of antibiotics in water is critical to water resource management. The pollution is strongly related to anthropopressure resulting from intensive tourism. An important aspect of the threat to the environment is water containing antibiotics at sub-inhibitory concentrations, which affects bacterial populations. Antibiotics are ecological factors driving microbial evolution by changing the bacterial community composition, inhibiting or promoting their ecological functions, and enriching and maintaining drug resistance. Modern methods of wastewater treatment are crucial in reducing the supply of antibiotics to aquatic environments and enhancing the possibility of economic and safe reuse of wastewater for technical purposes.
1. Introduction
2. Sources of Antibiotics in Mountain Rivers
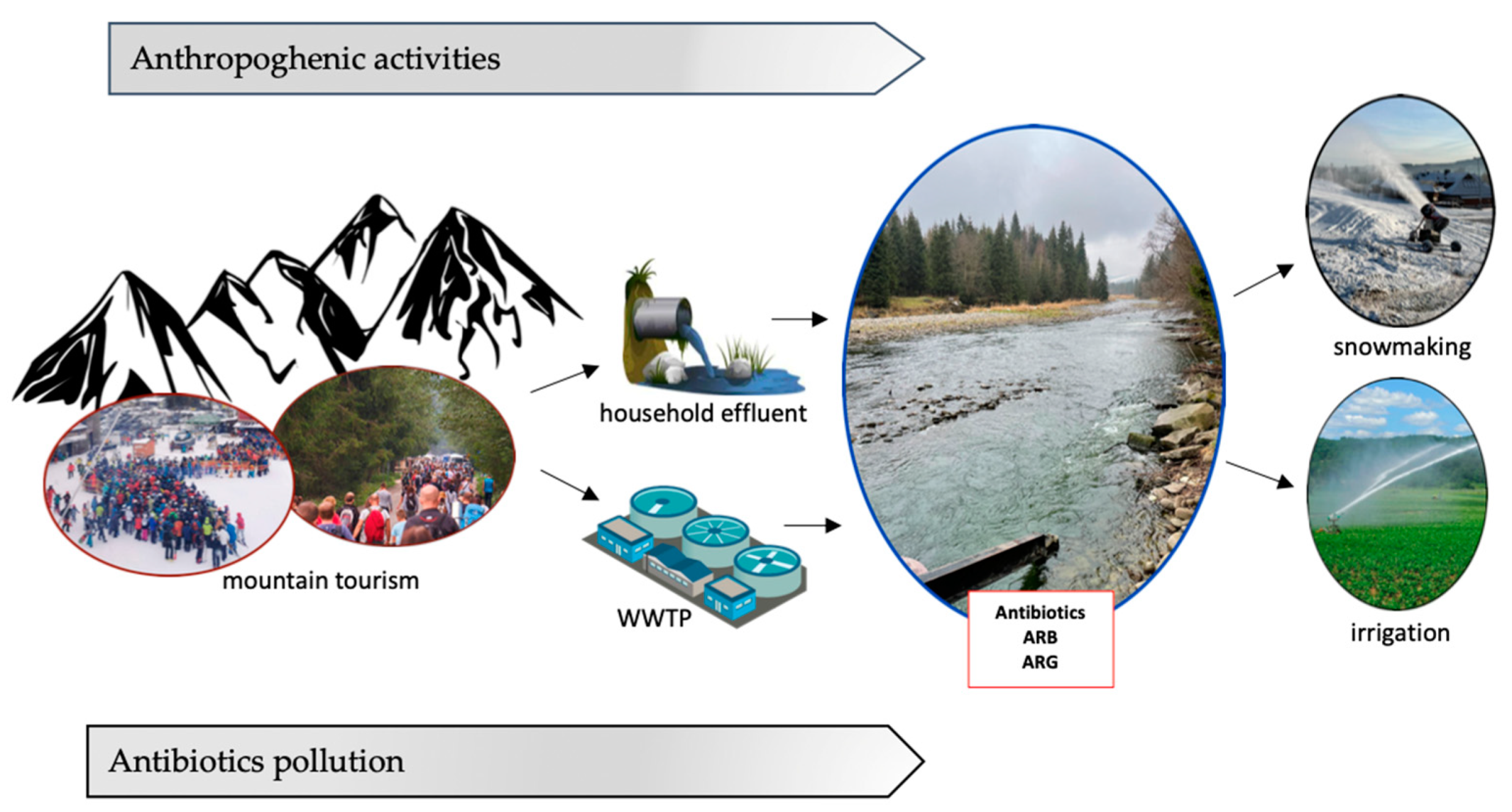
The quality of water in mountain regions is shaped by many natural and anthropogenic factors. In the most pristine regions, including national parks and natural valuable protected areas, the natural factors include variable weather conditions, surface runoff, soil leaching, and snowmelt water [6][7]. Along the course of rivers, water pollution increases, which is strongly related to the influence of anthropopressure-related factors, including illegal discharge of sewage from households (human and animals feces), surface runoff carrying natural fertilizers from agricultural fields, and wastewater inflow from WWTPs increased by tourist traffic-related sewage inflow [6][8].
In many cases, anthropogenic pressure in mountain regions is related to tourist traffic contributing to increased wastewater inflow, thus contributing to the presence of antibiotics and bacterial contaminants in mountain rivers [9]. Constantly developing winter tourism can pollute the environment and affect the ecology of microorganisms in mountain aquatic ecosystems in various ways. Mountain hiking is one of the causes of the pollution of rivers with antimicrobial agents. Mountain areas attract tourists due to their unspoiled nature, hiking trails for mountain trekking, and areas for active recreation. One of the reasons for the growing number of tourists is the constant development of winter sports centers with snow-covered ski slopes, which also offer activities outside the winter season, such as downhill skiing, as well as facilities offering thermal pools with geothermal water (thermal spas).
The metabolism rate of these compounds varies for humans and animals depending on the antibiotic class. These pharmaceuticals may reach aquatic environments by effluents from WWTPs to rivers and groundwater, as well as leachate from unsealed sewage systems and manure and/or sewage storage tanks [10]. During the wastewater treatment process, bacteria are continuously mixed with sub-inhibitory concentrations of antibiotics, which creates suitable conditions for the development of drug resistance, and then released with antibiotic residues into water environments [11]. The removal efficiency of antibiotics and antibiotic resistance determinants from wastewater by conventional WWTPs is insufficient. WWTPs are not specifically designed to completely reduce levels of antibiotics and ARGs. Removal of different antibiotics occurs in different steps of the wastewater treatment process, and the effectiveness of their removal varies among antibiotics [5]. Removal efficiency depends on the physical and chemical properties of antibiotics and on the treatment process conditions.
Another significant source of antimicrobials in rivers, including mountain rivers, is their use in animal husbandry for therapeutic and preventive purposes. Mountain agriculture is dominated by livestock production based on grazing. Veterinary antibiotic usage is related to the treatment of infective diseases in animals. The use of antimicrobial agents in animal husbandry ensures the welfare and health of the animals. However, the use of antibiotics is extended to the whole livestock flock in order to limit pathogen spread, thus uninfected animals also take doses of antibiotics [12]. This is referred to as metaphylaxis—short-term antibiotic treatment of animal groups without disease symptoms that had contact with infected animals [13]. This action involves observation of a livestock flock and administration of high doses of antibiotics before clinical symptoms occur in order to counteract the effects of infection. In contrast, antibiotics can also be used for disease prevention (prophylaxis). This includes antibiotic administration in water and food for farm animals in low doses for longer periods of time. During this period, the risk of infection still exists [14]. Metaphylaxis and prophylaxis are common practices in livestock and poultry production to prevent whole livestock mortality and minimize losses, but they have boosted antibiotic consumption. From an epidemiological point of view, the preventive administration of antibiotics increases the risk of drug-resistant bacteria development in the livestock herd and significantly influences the contamination of the environment with antibiotics and drug resistance determinants. In addition, selection for antibiotic-resistant strains can be widespread in the environment via animal feces, thereby enhancing environmental drug resistance [15]. Residues of antibiotics and ARB are usually found in livestock and poultry manure and in waste from livestock companies, resulting in persistent environmental pollution [16]. Animal manure studies have proven the presence of various classes of antibiotics excreted in feces, for instance: enrofloxacin in broiler chicken feces (74% of orally applied enrofloxacin was excreted as the parent compound) [17], oxytetracyclines present in dairy cow feces (20% of injected oxytetracycline was detected in manure samples) [18], and sulfonamides in pig excreta (excretions of four sulfonamides reached 36–87%) [19]. Stored animal manure often reaches soil and surface water with runoff water after rain or due to leaks in manure tanks. Livestock manure is also used as a fertilizer to enrich the soil before growing crops. Mountain areas, in addition to their environmental and cultural functions, also have an agricultural function as they have abundant arable fields, meadows, and pastures. In sustainable and organic farming, the use of manure as a source of organic matter to improve soil quality is a common practice. However, manure is also a source of antibiotic residues, which can adsorb on soil particles, enter plant tissues, and end up in the food chain. There is a risk of enhanced antimicrobial resistance as a result of consumption of vegetables grown on manure [20][21]. Manure widely applied to agricultural lands as fertilizer has enriched the abundance of some ARGs (ermA, ermB, blaOXA-1, qnrS, and oqxA) in agricultural soil [22]. Antibiotic residues and drug resistance determinants in soil fertilized with manure enter rivers with surface runoff, thus polluting the aquatic environment. Active forms of antibiotics occurring in manure can act as a selective pressure and contribute to dissemination of antimicrobial resistance. Livestock animals are a constant link in the spread of ARGs and antibiotics in the aquatic environment because they are continuously exposed to large amounts of antibiotics. Livestock farming can be one of the main sources of antibiotics in rivers due to the excretion of incompletely metabolized antibiotics in animal feces and their further dissemination into the environment [16].
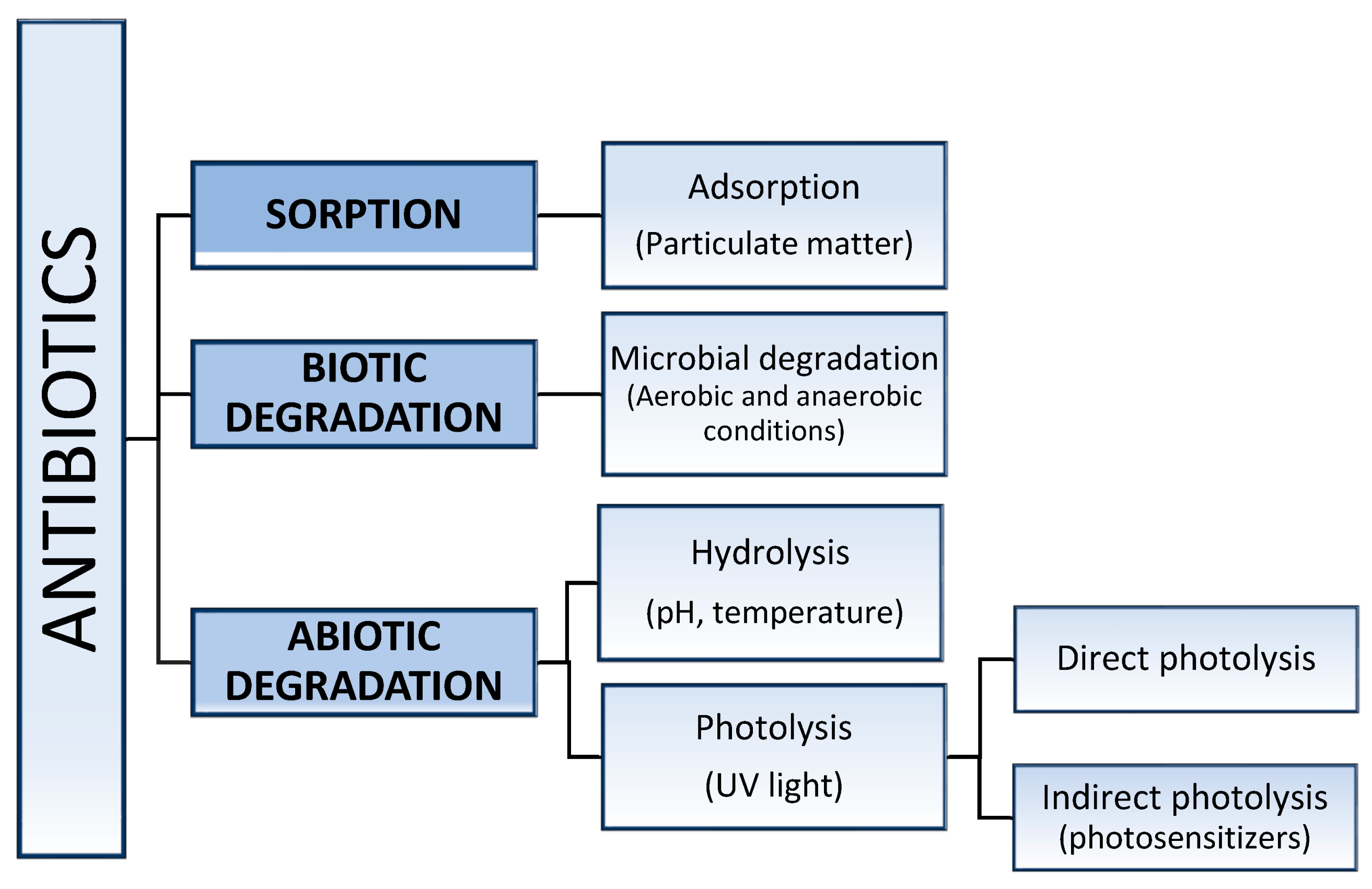
3. Stability of Antimicrobial Agents in Water Environments
4. Effect of Sub-Inhibitory Concentrations of Antibiotics on Bacterial Populations
5. Antibiotic Removal Processes from Water and Wastewater
6. Conclusions
Antibiotics polluting the environment are recognized as emerging micropollutants affecting microbial populations. Water is the main dissemination pathway of antibiotics and drug resistance determinants between various environmental compartments. The rate of antibiotics entering the aquatic environment is higher than their rate of elimination. Long-term exposure to sub-inhibitory concentrations of antibiotics (ng/L-μg/L) in waters is the main driver of changes in the genomes of microorganisms, thus resulting in the emergence of drug resistance and exchange of drug resistance genes by HGT. Antibiotics (acting as signaling molecules) are the ecological factor driving the evolution of bacteria by interfering with their ecological functions and compositions of bacterial communities. This causes the reduction of bacterial biodiversity responsible for the proper occurrence of biological processes in ecosystems. Antibiotics at low concentrations and bioavailability are capable of modifying bacterial communities and affect transcriptional regulation, thereby causing drug resistant mutations. Microorganisms evolve in response to emerging factors, such as antibiotics, in their environment. This is particularly evident in sensitive environments such as pristine mountain ecosystems where rivers can be exposed to strong anthropogenic factors closely related to tourism, agriculture, and animal husbandry.
Contamination of mountain waters with antibiotics is already present in the upper river courses of high-mountain national parks under protection. Mountain shelters, which are not equipped with sewage systems, are also sources of antibiotic contamination. There is a conflict between maintaining the pristine mountain environment and the continuous development of mountain tourism. The main threat to public health is the development of drug resistance and possible transfer of ARGs from environmental strains to clinical strains. With the continuous supply of sub-inhibitory concentrations of antibiotics in the environment affecting changes in the genomes of microorganisms, there may be a risk of a link between environmental and clinical drug resistance. On the other hand, changes in the biodiversity and composition of microbial populations that are responsible for important ecological functions in the ecosystem pose a threat to the environment. For this reason, monitoring the contamination of surface waters with antimicrobial agents is an important aspect. Contamination of surface waters with antibiotics is particularly harmful in the mountain environment. This is due to the fact that mountain water supplies are a valuable natural resource found mostly in pristine and protected areas where they give rise to rivers and constitute a reservoir of drinking water in every country. Protected environments, which are a valuable source of biodiversity, should be taken care of. Antibiotics are a type of micropollutant that is not routinely tested. Therefore, monitoring concentrations of antibiotics in waters is crucial for maintaining the quality of water resources for human use and the microbiological biodiversity within water ecosystems.
References
- Meek, R.W.; Vyas, H.; Piddock, L.J.V. Nonmedical Uses of Antibiotics: Time to Restrict Their Use? PLOS Biol. 2015, 13, e1002266.
- Van, T.T.H.; Yidana, Z.; Smooker, P.M.; Coloe, P.J. Antibiotic Use in Food Animals Worldwide, with a Focus on Africa: Pluses and Minuses. J. Glob. Antimicrob. Resist. 2020, 20, 170–177.
- Du, L.; Liu, W. Occurrence, Fate, and Ecotoxicity of Antibiotics in Agro-Ecosystems. A Review. Agron. Sustain. Dev. 2012, 32, 309–327.
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Ștefan, M.G.; Loghin, F. Antibiotics in the Environment: Causes and Consequences. Med. Pharm. Rep. 2020, 93, 231.
- Chow, L.K.M.; Ghaly, T.M.; Gillings, M.R. A Survey of Sub-Inhibitory Concentrations of Antibiotics in the Environment. J. Environ. Sci. 2021, 99, 21–27.
- Lenart-Boroń, A.; Wolanin, A.; Jelonkiewicz, Ł.; Chmielewska-Błotnicka, D.; Żelazny, M. Spatiotemporal Variability in Microbiological Water Quality of the Białka River and Its Relation to the Selected Physicochemical Parameters of Water. Water. Air. Soil Pollut. 2016, 227, 22.
- Mokhtar, M.B.; Aris, A.Z.; Abdullah, M.H.; Yusoff, M.K.; Abdullah, M.P.; Idris, A.R.; Raja Uzir, R.I. A Pristine Environment and Water Quality in Perspective: Maliau Basin, Borneo’s Mysterious World. Water Environ. J. 2009, 23, 219–228.
- Bojarczuk, A.; Jelonkiewicz, Ł.; Lenart-Boroń, A. The Effect of Anthropogenic and Natural Factors on the Prevalence of Physicochemical Parameters of Water and Bacterial Water Quality Indicators along the River Białka, Southern Poland. Environ. Sci. Pollut. Res. 2018, 25, 10102–10114.
- Lenart-Boroń, A.; Prajsnar, J.; Guzik, M.; Boroń, P.; Chmiel, M. How Much of Antibiotics Can Enter Surface Water with Treated Wastewater and How It Affects the Resistance of Waterborne Bacteria: A Case Study of the Białka River Sewage Treatment Plant. Environ. Res. 2020, 191, 110037.
- Carvalho, I.T.; Santos, L. Antibiotics in the Aquatic Environments: A Review of the European Scenario. Environ. Int. 2016, 94, 736–757.
- Pulicharla, R.; Brar, S.K.; Drogui, P.; Verma, M.; Surampalli, R.Y. Removal Processes of Antibiotics in Waters and Wastewaters: Crucial Link to Physical-Chemical Properties and Degradation. J. Hazard. Toxic Radioact. Waste 2015, 19, 04015008.
- Economou, V.; Gousia, P. Agriculture and Food Animals as a Source of Antimicrobial-Resistant Bacteria. Infect. Drug Resist. 2015, 8, 49–91.
- Baptiste, K.E. Do Antimicrobial Mass Medications Work? A Systematic Review and Meta-Analysis of Randomized Clinical Trials Investigating Antimicrobial Prophylaxis or Metaphylaxis against Naturally Occurring Bovine Respiratory Disease. Pathog. Dis. 2017, 29, ftx083.
- Hosain, M.Z.; Kabir, S.M.L.; Kamal, M.M. Antimicrobial Uses for Livestock Production in Developing Countries. Vet. World 2021, 14, 210–221.
- Spielmeyer, A. Occurrence and Fate of Antibiotics in Manure during Manure Treatments: A Short Review. Sustain. Chem. Pharm. 2018, 9, 76–86.
- Zhang, X.; Li, Y.; Liu, B.; Wang, J.; Feng, C.; Gao, M.; Wang, L. Prevalence of Veterinary Antibiotics and Antibiotic-Resistant Escherichia Coli in the Surface Water of a Livestock Production Region in Northern China. PLoS ONE 2014, 9, e111026.
- Slana, M.; Pahor, V.; Cvitkovič Maričič, L.; Sollner-Dolenc, M. Excretion Pattern of Enrofloxacin after Oral Treatment of Chicken Broilers. J. Vet. Pharmacol. Ther. 2014, 37, 611–614.
- Ince, B.; Coban, H.; Turker, G.; Ertekin, E.; Ince, O. Effect of Oxytetracycline on Biogas Production and Active Microbial Populations during Batch Anaerobic Digestion of Cow Manure. Bioprocess Biosyst. Eng. 2013, 36, 541–546.
- Qiu, J.; Zhao, T.; Liu, Q.; He, J.; He, D.; Wu, G.; Li, Y.; Jiang, C.; Xu, Z. Residual Veterinary Antibiotics in Pig Excreta after Oral Administration of Sulfonamides. Environ. Geochem. Health 2016, 38, 549–556.
- Wei, R.; He, T.; Zhang, S.; Zhu, L.; Shang, B.; Li, Z.; Wang, R. Occurrence of Seventeen Veterinary Antibiotics and Resistant Bacterias in Manure-Fertilized Vegetable Farm Soil in Four Provinces of China. Chemosphere 2019, 215, 234–240.
- Kumar, K.; Gupta, S.C.; Baidoo, S.K.; Chander, Y.; Rosen, C.J. Antibiotic Uptake by Plants from Soil Fertilized with Animal Manure. J. Environ. Qual. 2005, 34, 2082–2085.
- Laconi, A.; Mughini-Gras, L.; Tolosi, R.; Grilli, G.; Trocino, A.; Carraro, L.; Di Cesare, F.; Cagnardi, P.; Piccirillo, A. Microbial Community Composition and Antimicrobial Resistance in Agricultural Soils Fertilized with Livestock Manure from Conventional Farming in Northern Italy. Sci. Total Environ. 2021, 760, 143404.
- Jalloul, G.; Keniar, I.; Tehrani, A.; Boyadjian, C. Antibiotics Contaminated Irrigation Water: An Overview on Its Impact on Edible Crops and Visible Light Active Titania as Potential Photocatalysts for Irrigation Water Treatment. Front. Environ. Sci. 2021, 9, 767963.
- Pan, M.; Chu, L.M. Fate of Antibiotics in Soil and Their Uptake by Edible Crops. Sci. Total Environ. 2017, 599–600, 500–512.
- Yang, Q.; Gao, Y.; Ke, J.; Show, P.L.; Ge, Y.; Liu, Y.; Guo, R.; Chen, J. Antibiotics: An Overview on the Environmental Occurrence, Toxicity, Degradation, and Removal Methods. Bioengineered 2021, 12, 7376–7416.
- Mitchell, S.M. PH and Temperature Effects on the Hydrolysis of Three β-Lactam Antibiotics: Ampicillin, Cefalotin and Cefoxitin. Sci. Total Environ. 2014, 9, 547–555.
- Xuan, R.; Arisi, L.; Wang, Q.; Yates, S.R.; Biswas, K.C. Hydrolysis and Photolysis of Oxytetracycline in Aqueous Solution. J. Environ. Sci. Health Part B 2009, 45, 73–81.
- Fang, L.; Zhou, Y.; Huang, Z.; Yang, G.; Li, T.; Song, C.; Chen, J. Dynamic Elimination of Enrofloxacin Under Varying Temperature and PH in Aquaculture Water: An Orthogonal Study. Bull. Environ. Contam. Toxicol. 2021, 106, 866–872.
- Jiang, M.; Wang, L.; Ji, R. Biotic and Abiotic Degradation of Four Cephalosporin Antibiotics in a Lake Surface Water and Sediment. Chemosphere 2010, 80, 1399–1405.
- Reis, A.C.; Kolvenbach, B.A.; Nunes, O.C.; Corvini, P.F.X. Biodegradation of Antibiotics: The New Resistance Determinants—Part I. New Biotechnol. 2020, 54, 34–51.
- Andreozzi, R.; Raffaele, M.; Nicklas, P. Pharmaceuticals in STP Effluents and Their Solar Photodegradation in Aquatic Environment. Chemosphere 2003, 50, 1319–1330.
- Jiao, S.; Zheng, S.; Yin, D.; Wang, L.; Chen, L. Aqueous Photolysis of Tetracycline and Toxicity of Photolytic Products to Luminescent Bacteria. Chemosphere 2008, 73, 377–382.
- Wei, C.; Li, X.; Xie, Y.; Wang, X. Direct Photo Transformation of Tetracycline and Sulfanomide Group Antibiotics in Surface Water: Kinetics, Toxicity and Site Modeling. Sci. Total Environ. 2019, 686, 1–9.
- Pils, J.R.V.; Laird, D.A. Sorption of Tetracycline and Chlortetracycline on K- and Ca-Saturated Soil Clays, Humic Substances, and Clay−Humic Complexes. Environ. Sci. Technol. 2007, 41, 1928–1933.
- Kwon, J.-W. Environmental Impact Assessment of Veterinary Drug on Fish Aquaculture for Food Safety: Environmental Impact Assessment on Veterinary Drugs for Aquaculture. Drug Test. Anal. 2016, 8, 556–564.
- Li, J.; Zhang, K.; Zhang, H. Adsorption of Antibiotics on Microplastics. Environ. Pollut. 2018, 237, 460–467.
- Wang, S.; Xue, N.; Li, W.; Zhang, D.; Pan, X.; Luo, Y. Selectively Enrichment of Antibiotics and ARGs by Microplastics in River, Estuary and Marine Waters. Sci. Total Environ. 2020, 708, 134594.
- McCormick, A.R.; Hoellein, T.J.; London, M.G.; Hittie, J.; Scott, J.W.; Kelly, J.J. Microplastic in Surface Waters of Urban Rivers: Concentration, Sources, and Associated Bacterial Assemblages. Ecosphere 2016, 7, e01556.
- Ding, C.; He, J. Effect of Antibiotics in the Environment on Microbial Populations. Appl. Microbiol. Biotechnol. 2010, 87, 925–941.
- Klaver, A.L.; Matthews, R.A. Effects of Oxytetracycline on Nitrification in a Model Aquatic System. Aquaculture 1994, 123, 237–247.
- Halling-Sørensen, B. Inhibition of Aerobic Growth and Nitrification of Bacteria in Sewage Sludge by Antibacterial Agents. Arch. Environ. Contam. Toxicol. 2001, 40, 451–460.
- Özel Duygan, B.D.; Gaille, C.; Fenner, K.; van der Meer, J.R. Assessing Antibiotics Biodegradation and Effects at Sub-Inhibitory Concentrations by Quantitative Microbial Community Deconvolution. Front. Environ. Sci. 2021, 9, 737247.
- Kümmerer, K. Antibiotics in the Aquatic Environment—A Review—Part II. Chemosphere 2009, 75, 435–441.
- Lenart-Boroń, A.M.; Boroń, P.M.; Prajsnar, J.A.; Guzik, M.W.; Żelazny, M.S.; Pufelska, M.D.; Chmiel, M.J. COVID-19 Lockdown Shows How Much Natural Mountain Regions Are Affected by Heavy Tourism. Sci. Total Environ. 2022, 806, 151355.
- Baquero, F.; Martínez, J.-L.; Cantón, R. Antibiotics and Antibiotic Resistance in Water Environments. Curr. Opin. Biotechnol. 2008, 19, 260–265.
- Khan, G.A.; Berglund, B.; Khan, K.M.; Lindgren, P.-E.; Fick, J. Occurrence and Abundance of Antibiotics and Resistance Genes in Rivers, Canal and near Drug Formulation Facilities—A Study in Pakistan. PLoS ONE 2013, 8, e62712.
- Fram, M.S.; Belitz, K. Occurrence and Concentrations of Pharmaceutical Compounds in Groundwater Used for Public Drinking-Water Supply in California. Sci. Total Environ. 2011, 409, 3409–3417.
- Jiang, L.; Hu, X.; Xu, T.; Zhang, H.; Sheng, D.; Yin, D. Prevalence of Antibiotic Resistance Genes and Their Relationship with Antibiotics in the Huangpu River and the Drinking Water Sources, Shanghai, China. Sci. Total Environ. 2013, 458–460, 267–272.
- Gullberg, E.; Cao, S.; Berg, O.G.; Ilbäck, C.; Sandegren, L.; Hughes, D.; Andersson, D.I. Selection of Resistant Bacteria at Very Low Antibiotic Concentrations. PLoS Pathog. 2011, 7, e1002158.
- Grenni, P.; Ancona, V.; Barra Caracciolo, A. Ecological Effects of Antibiotics on Natural Ecosystems: A Review. Microchem. J. 2018, 136, 25–39.
- Felis, E.; Kalka, J.; Sochacki, A.; Kowalska, K.; Bajkacz, S.; Harnisz, M.; Korzeniewska, E. Antimicrobial Pharmaceuticals in the Aquatic Environment—Occurrence and Environmental Implications. Eur. J. Pharmacol. 2020, 866, 172813.
- Cairns, J.; Ruokolainen, L.; Hultman, J.; Tamminen, M.; Virta, M.; Hiltunen, T. Ecology Determines How Low Antibiotic Concentration Impacts Community Composition and Horizontal Transfer of Resistance Genes. Commun. Biol. 2018, 1, 35.
- Ghoshdastidar, A.J.; Fox, S.; Tong, A.Z. The Presence of the Top Prescribed Pharmaceuticals in Treated Sewage Effluents and Receiving Waters in Southwest Nova Scotia, Canada. Environ. Sci. Pollut. Res. 2015, 22, 689–700.
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban Wastewater Treatment Plants as Hotspots for the Release of Antibiotics in the Environment: A Review. Water Res. 2013, 47, 957–995.
- Le-Clech, P. Membrane Bioreactors and Their Uses in Wastewater Treatments. Appl. Microbiol. Biotechnol. 2010, 88, 1253–1260.
- Yang, W.; Cicek, N. Treatment of Swine Wastewater by Submerged Membrane Bioreactors with Consideration of Estrogenic Activity Removal. Desalination 2008, 231, 200–208.
- Marti, E.; Monclús, H.; Jofre, J.; Rodriguez-Roda, I.; Comas, J.; Balcázar, J.L. Removal of Microbial Indicators from Municipal Wastewater by a Membrane Bioreactor (MBR). Bioresour. Technol. 2011, 102, 5004–5009.
- Kovalova, L.; Siegrist, H.; Singer, H.; Wittmer, A.; McArdell, C.S. Hospital Wastewater Treatment by Membrane Bioreactor: Performance and Efficiency for Organic Micropollutant Elimination. Environ. Sci. Technol. 2012, 46, 1536–1545.
- Xiao, Y.; Yaohari, H.; De Araujo, C.; Sze, C.C.; Stuckey, D.C. Removal of Selected Pharmaceuticals in an Anaerobic Membrane Bioreactor (AnMBR) with/without Powdered Activated Carbon (PAC). Chem. Eng. J. 2017, 321, 335–345.
- Xia, S.; Jia, R.; Feng, F.; Xie, K.; Li, H.; Jing, D.; Xu, X. Effect of Solids Retention Time on Antibiotics Removal Performance and Microbial Communities in an A/O-MBR Process. Bioresour. Technol. 2012, 106, 36–43.
- Li, B.; Zhang, T. Mass Flows and Removal of Antibiotics in Two Municipal Wastewater Treatment Plants. Chemosphere 2011, 83, 1284–1289.
- Liu, D.; Song, K.; Xie, G.; Li, L. MBR-UV/Cl2 System in Treating Polluted Surface Water with Typical PPCP Contamination. Sci. Rep. 2020, 10, 8835.
- Dong, X.; Li, Y.; Li, D.; Liao, D.; Quin, T.; Prakash, O.; Kumar, A.; Liu, J. A New 3D 8-Connected Cd(Ii) MOF as a Potent Photocatalyst for Oxytetracycline Antibiotic Degradation. CrystEngComm 2022, 24, 6933–6943.
- Naseer, M.N.; Jaafar, J.; Junoh, H.; Zaidi, A.A.; Kumar, M.; Alqahtany, A.; Jamil, R.; Alyami, S.H.; Aldossary, N.A. Metal-Organic Frameworks for Wastewater Decontamination: Discovering Intellectual Structure and Research Trends. Materials 2022, 15, 5053.
- Saddique, Z.; Imran, M.; Javaid, A.; Rizvi, N.B.; Akhtar, M.N.; Iqbal, H.M.N.; Bilal, M. Enzyme-Linked Metal Organic Frameworks for Biocatalytic Degradation of Antibiotics. Catal. Lett. 2023.
- Nosakhare Amenaghawon, A.; Lewis Anyalewechi, C.; Uyi Osazuwa, O.; Agbovhimen Elimian, E.; Oshiokhai Eshiemogie, S.; Kayode Oyefolu, P.; Septya Kusuma, H. A Comprehensive Review of Recent Advances in the Synthesis and Application of Metal-Organic Frameworks (MOFs) for the Adsorptive Sequestration of Pollutants from Wastewater. Sep. Purif. Technol. 2023, 311, 123246.
- Liu, X.; Shan, Y.; Zhang, S.; Kong, Q.; Pang, H. Application of Metal Organic Framework in Wastewater Treatment. Green Energy Environ. 2022, in press.
- Li, J.; Wang, H.; Yuan, X.; Zhang, J.; Chew, J.W. Metal-Organic Framework Membranes for Wastewater Treatment and Water Regeneration. Coord. Chem. Rev. 2020, 404, 213116.
- Zhou, Y.; Yang, Q.; Zhang, D.; Gan, N.; Li, Q.; Cuan, J. Detection and Removal of Antibiotic Tetracycline in Water with a Highly Stable Luminescent MOF. Sens. Actuators B Chem. 2018, 262, 137–143.
- Grela, A.; Kuc, J.; Bajda, T. A Review on the Application of Zeolites and Mesoporous Silica Materials in the Removal of Non-Steroidal Anti-Inflammatory Drugs and Antibiotics from Water. Materials 2021, 14, 4994.
- Blasioli, S.; Martucci, A.; Paul, G.; Gigli, L.; Cossi, M.; Johnston, C.T.; Marchese, L.; Braschi, I. Removal of Sulfamethoxazole Sulfonamide Antibiotic from Water by High Silica Zeolites: A Study of the Involved Host–Guest Interactions by a Combined Structural, Spectroscopic, and Computational Approach. J. Colloid Interface Sci. 2014, 419, 148–159.
- Zuo, X.; Qian, C.; Ma, S.; Xiong, J.; He, J.; Chen, Z. Removal of Sulfonamide Antibiotics from Water by High-Silica ZSM-5. Water Sci. Technol. 2019, 80, 507–516.
- Hacıosmanoğlu, G.G.; Mejías, C.; Bueno, J.M.; Santos, J.L.; Aparicio, I.; Alonso, E. Antibiotic Adsorption by Natural and Modified Clay Minerals as Designer Adsorbents for Wastewater Treatment: A Comprehensive Review. J. Environ. Manag. 2022, 317, 115397.




