
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | MICHELA MENGHI | -- | 1882 | 2023-03-03 17:37:40 | | | |
| 2 | Rita Xu | Meta information modification | 1882 | 2023-03-08 04:44:38 | | |
Video Upload Options
DiGeorge syndrome (DGS) is a rare genetic disease caused by microdeletions of the 22q11.2 region (DGS1). A haploinsufficiency at 10p level has been proposed also as a DGS cause (DGS2). Clinical manifestations are variable. The most frequent features are thymic hypoplasia or aplasia with consequent immune deficiency, cardiac malformations, hypoparathyroidism, facial and palatine abnormalities, variable degrees of cognitive impairment and psychiatric disorders. The specific aim of this descriptive report is to discuss the correlation between oxidative stress and neuroinflammation in DGS patients with microdeletions of the 22q11.2 region. The deleted chromosomic region maps various genes involved in mitochondrial metabolisms, such as DGCR8 and TXNRD2, that could lead to reactive oxygen species (ROS) increased production and antioxidant depletion.
1. Introduction
2. DiGeorge Syndrome
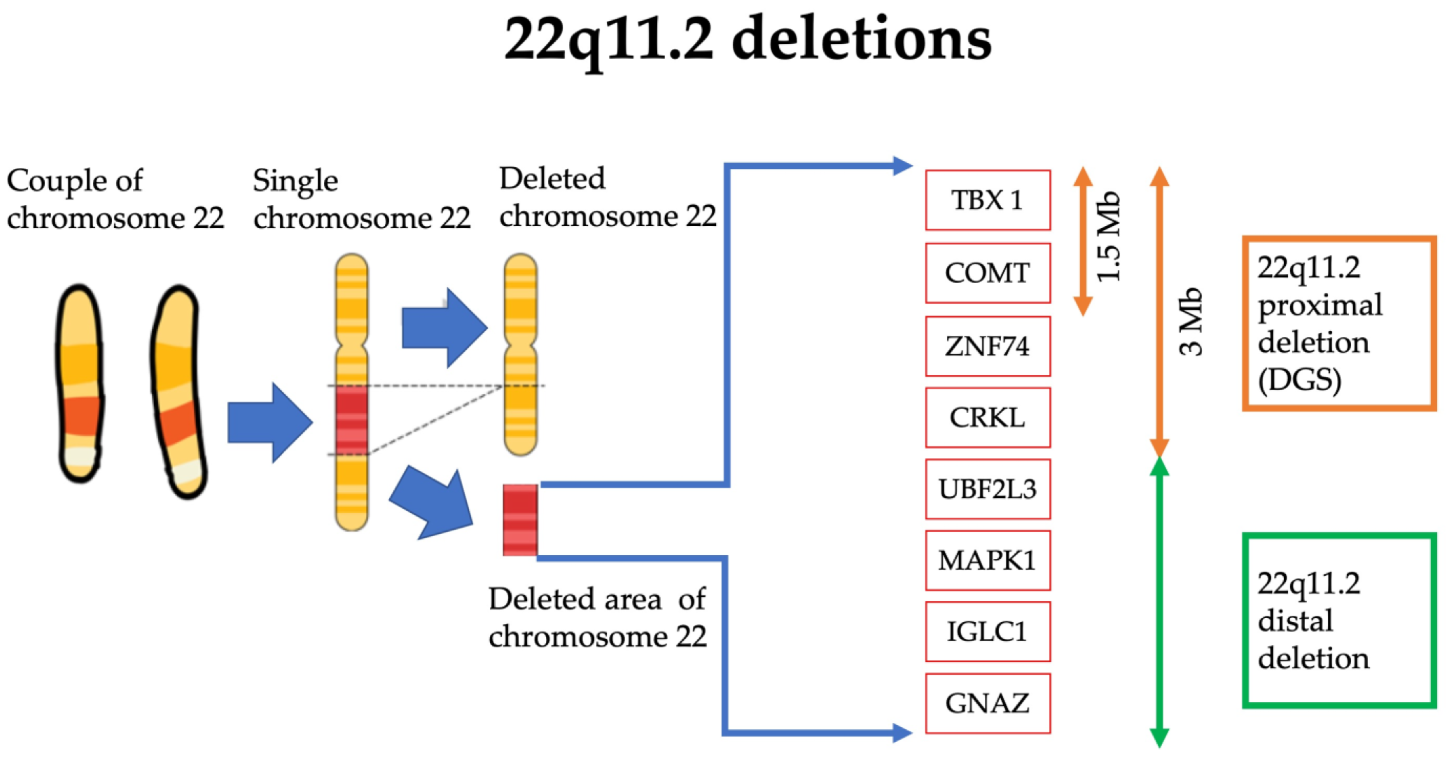
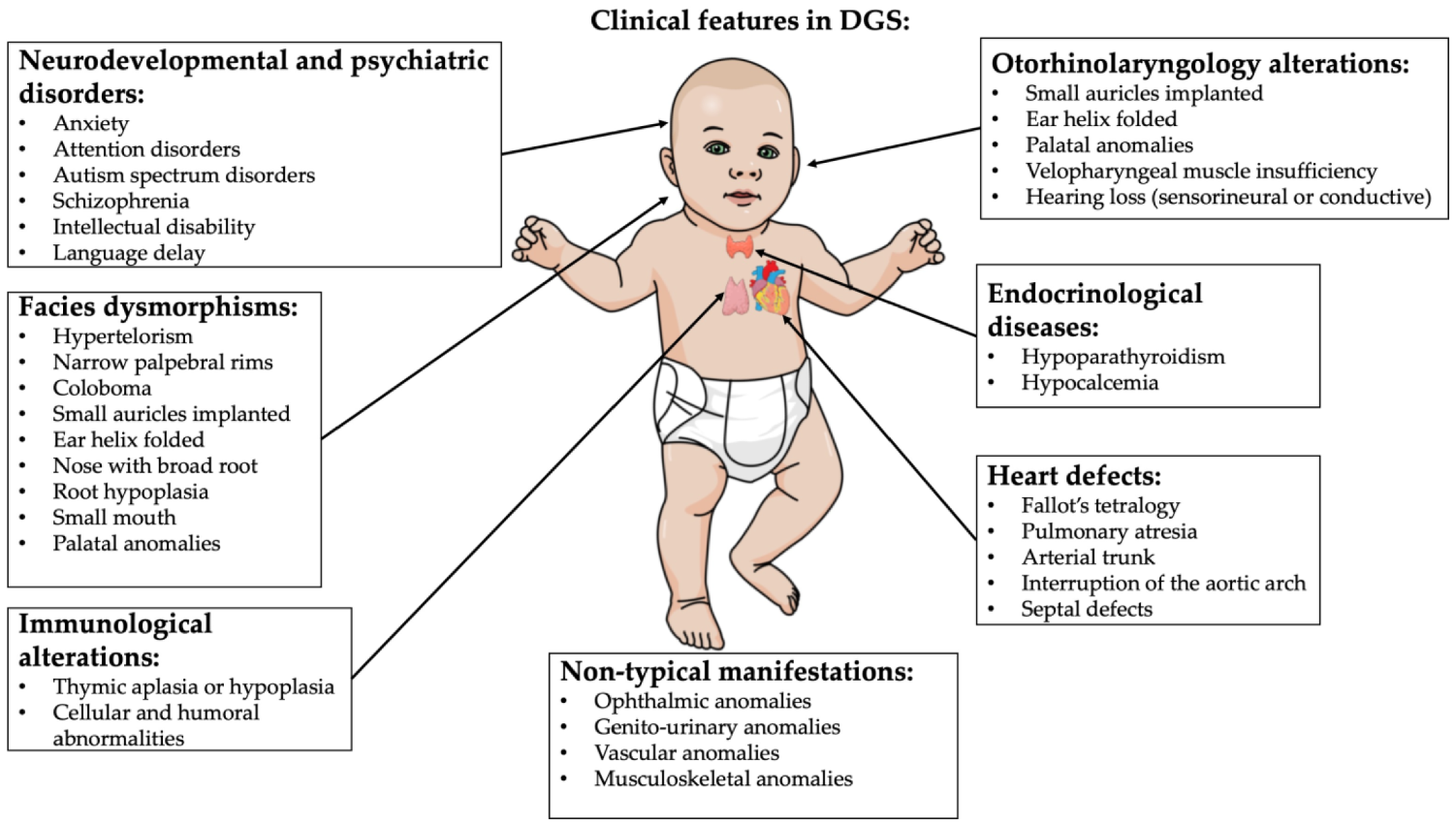
-
Cardiac abnormality (commonly interrupted aortic arch, truncus arteriosus and tetralogy of Fallot)
-
Abnormal facies
-
Thymic aplasia or hypoplasia
-
Cleft palate
-
Hypocalcemia/hypoparathyroidism early in life
- -
-
Palatal abnormalities (50%), particularly velopharyngeal incompetence, submucosal cleft palate and cleft palate; characteristic facial features (present in the majority of Caucasian individuals) including hypertelorism;
- -
-
Cyanosis (bluish skin due to poor circulation of oxygen-rich blood);
- -
-
Congenital heart disease (40% of individuals), particularly conotruncal malformations (interrupted aortic arch (50%), pulmonary atresia, persistent truncus arteriosus (34%), tetralogy of Fallot and ventricular septal defect);
- -
-
Hearing loss (both conductive and sensorineural) (hearing loss with craniofacial syndromes);
- -
-
Hypocalcemia (50%) (due to hypoparathyroidism);
- -
-
Significant feeding problems (30%);
- -
-
Learning difficulties (90%), including cognitive deficits and attention deficit disorders;
- -
-
Laryngo-tracheo-esophageal anomalies;
- -
-
Growth hormone deficiency;
- -
-
Renal anomalies (37%);
- -
-
Psychiatric disorders;
- -
-
Autoimmune disorders;
- -
-
Immunodeficiency present in about 75% of patients, related to thymic aplasia or hypoplasia determining alterations in both humoral and cell-mediated immune responses;
- -
-
Immune disorders due to reduced T cell numbers;
- -
-
Schizophrenia develops in 25–30% by adulthood;
- -
-
Seizures (with or without hypocalcemia);
- -
-
Skeletal abnormalities.
3. Oxidative Stress in DiGeorge Syndrome
| Oxidative Stress in DiGeorge Syndrome | |
|---|---|
| Genetic or Enzymatic Alterations: | Markers of Oxidative Stress: |
| Increase in mitochondria-associated oxidative stress in layer 2/3 projection neurons in mouse models | Disruption of layer 2/3 projection neurons related to association cortico-cortical connections [4] |
| Overproduction of modified proteins such as Met sulfoxide from Met, indole-3-lactate from Trp and aminomalonate Overproduction of hexoses such as 5-hydroxymethyl-2-furanoic acid and hexuronic acid in children |
Inhibition of Complex IV, Complex V and creatine kinase [8] |
| Loss of DGCR8 gene in animal models | Inhibition of superoxide dismutase 2 (SOD2) [29][30] Increase in ROS [29][30] Accelerated senescence [29][30] |
| Loss of TXNRD2 gene in children | Reduction in antioxidant defense [8][31] |
References
- McDonald-McGinn, D.M.; Sullivan, K.E.; Marino, B.; Philip, N.; Swillen, A.; Vorstman, J.A.S.; Zackai, E.H.; Emanuel, B.S.; Vermeesch, J.R.; Morrow, B.E.; et al. 22q11.2 deletion syndrome. Nat. Rev. Dis. Primers 2015, 1, 15071.
- Daw, S.C.M.; Taylor, C.; Kraman, M.; Call, K.; Mao, J.-I.; Schuffenhauer, S.; Meitinger, T.; Lipson, T.; Goodship, J.; Scambler, P. A common region of 10p deleted in DiGeorge and velocardiofacial syndromes. Nat. Genet. 1996, 13, 458–460.
- Schuffenhauer, S.; Lichtner, P.; Peykar-Derakhshandeh, P.; Murken, J.; Haas, O.A.; Back, E.; Wolff, G.; Zabel, B.; Barisic, I.; Rauch, A.; et al. Deletion mapping on chromosome 10p and definition of a critical region for the second DiGeorge syndrome locus (DGS2). Eur. J. Hum. Genet. 1998, 6, 213–225.
- Fernandez, A.; Meechan, D.W.; Karpinski, B.A.; Paronett, E.M.; Bryan, C.A.; Rutz, H.L.; Radin, E.A.; Lubin, N.; Bonner, E.R.; Popratiloff, A.; et al. Mitochondrial Dysfunction Leads to Cortical Under-Connectivity and Cognitive Impairment. Neuron 2019, 102, 1127–1142.
- Sullivan, K.E. Chromosome 22q11.2 deletion syndrome and DiGeorge syndrome. Immunol. Rev. 2019, 287, 186–201.
- Meechan, D.W.; Tucker, E.S.; Maynard, T.M.; LaMantia, A.S. Diminished dosage of 22q11 genes disrupts neurogenesis and cortical development in a mouse model of 22q11 deletion/DiGeorge syndrome. Proc. Natl. Acad. Sci. USA 2009, 106, 16434–16445.
- Conti, E.; Grifone, N.; Sarkozy, A.; Tandoi, C.; Marino, B.; Digilio, M.C.; Mingarelli, R.; Pizzuti, A.; Dallapiccola, B. DiGeorge subtypes of nonsyndromic conotruncal defects: Evidence against a major role of TBX1 gene. Eur. J. Hum. Genet. 2003, 11, 349–351.
- Napoli, E.; Tassone, F.; Wong, S.; Angkustsiri, K.; Simon, T.J.; Song, G.; Giulivi, C. Mitochondrial citrate transporter-dependent metabolic signature in the 22q11.2 deletion syndrome. J. Biol. Chem. 2015, 290, 23240–23253.
- Jonas, R.K.; Montojo, C.A.; Bearden, C.E. The 22q11.2 deletion syndrome as a window into complex neuropsychiatric disorders over the lifespan. Biol. Psychiatry 2014, 75, 351–360.
- Toritsuka, M.; Kimoto, S.; Muraki, K.; Landek-Salgado, M.A.; Yoshida, A.; Yamamoto, N.; Horiuchi, Y.; Hiyama, H.; Tajinda, K.; Keni, N.; et al. Deficits in microRNA-mediated Cxcr4/Cxcl12 signaling in neurodevelopmental deficits in a 22q11 deletion syndrome mouse model. Proc. Natl. Acad. Sci. USA 2013, 110, 17552–17557.
- Sergi, C.; Serpi, M.; Müller-Navia, J.; Schnabel, P.A.; Hagl, S.; Otto, H.F.; Ulmer, H.E. CATCH 22 syndrome: Report of 7 infants with follow-up data and review of the recent advancements in the genetic knowledge of the locus 22q11. Pathologica 1999, 91, 166–172.
- Demczuk, S.; Aurias, A. DiGeorge syndrome and related syndromes associated with 22q11.2 deletions. A review. Ann. Genet. 1995, 38, 59–76.
- Motahari, Z.; Moody, S.A.; Maynard, T.M.; LaMantia, A.-S. In the line-up: Deleted genes associated with DiGeorge/22q11.2 deletion syndrome: Are they all suspects? J. Neurodev. Disord. 2019, 11, 7.
- Fiksinski, A.M.; Hoftman, G.D.; Vorstman, J.A.S.; Bearden, C.E. A genetics-first approach to understanding autism and schizophrenia spectrum disorders: The 22q11.2 deletion syndrome. Mol. Psychiatry 2023, 28, 341–353.
- Sullivan, K.E. Chromosome 22q11.2 deletion syndrome: DiGeorge syndrome/velocardiofacial Syndrome. Immunol. Allergy Clin. N. Am. 2008, 28, 353–366.
- McLean-Tooke, A.; Spickett, G.P.; Gennery, A.R. Immunodeficiency and autoimmunity in 22q11.2 deletion syndrome. Scand. J. Immunol. 2007, 66, 1–7.
- Cirillo, A.; Lioncino, M.; Maratea, A.; Passariello, A.; Fusco, A.; Fratta, F.; Monda, E.; Caiazza, M.; Signore, G.; Esposito, A.; et al. Clinical Manifestations of 22q11.2 Deletion Syndrome. Heart Fail Clin. 2022, 18, 155–164.
- Kuo, C.Y.; Signer, R.; Saitta, S.C. Immune and Genetic Features of the Chromosome 22q11.2 Deletion (DiGeorge Syndrome). Curr. Allergy Asthma Rep. 2018, 18, 75.
- Bayat, M.; Bayat, A. Neurological manifestation of 22q11.2 deletion syndrome. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2022, 43, 1695–1700.
- Goldmuntz, E. 22q11.2 deletion syndrome and congenital heart disease. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 64–72.
- Burn, J. Closing time for CATCH22. J. Med. Genet. 1999, 36, 737–738.
- McDonald-Mcginn, D.M.; Sullivan, K.E. Chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome). Medicine (Baltimore) 2011, 90, 1–18.
- Kim, G.; Moon, E.; Park, J.M.; Lee, B.D.; Lee, Y.M.; Jeong, H.J.; Kim, S.Y.; Lee, K.; Suh, H. Various psychiatric manifestation in digeorge syndrome (22q11.2 deletion syndrome): A case report. Clin Psychopharmacol. Neurosci. 2020, 18, 458–462.
- Fomin, A.B.F.; Pastorino, A.C.; Kim, C.A.; Pereira, A.C.; Carneiro-Sampaio, M.; Abe Jacob, C.M. DiGeorge Syndrome: A not so rare disease. Clinics 2010, 65, 865–869.
- Muchova, J.; Zitnanova, I.; Durackova, Z. Oxidative stress and Down syndrome. Do antioxidants play a role in therapy? Physiol. Res. 2014, 63, 535–542.
- Jung, Y.-D.; Park, S.-K.; Kang, D.; Hwang, S.; Kang, M.-H.; Hong, S.-W.; Moon, J.-H.; Shin, J.-S.; Jin, D.-H.; You, D.; et al. Epigenetic regulation of miR-29a/miR-30c/DNMT3A axis controls SOD2 and mitochondrial oxidative stress in human mesenchymal stem cells. Redox Biol. 2020, 37, 101716.
- Crowe, D.A.; Goodwin, S.J.; Blackman, R.K.; Sakellaridi, S.; Sponheim, S.R.; MacDonald, A.W.; Chafee, M.V. Prefrontal neurons transmit signals to parietal neurons that reflect executive control of cognition. Nat. Neurosci. 2013, 16, 1484–1491.
- Just, M.A.; Cherkassky, V.L.; Keller, T.A.; Minshew, N.J. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain 2004, 127, 1811–1821.
- Cheng, T.-L.; Wang, Z.; Liao, Q.; Zhu, Y.; Zhou, W.-H.; Xu, W.; Qiu, Z. MeCP2 Suppresses Nuclear MicroRNA Processing and Dendritic Growth by Regulating the DGCR8/Drosha Complex. Dev. Cell. 2014, 28, 547–560.
- Earls, L.R.; Gaines Fricke, R.; Yu, J.; Berry, R.B.; Baldwin, L.T.; Zakharenko, S.S. Age-dependent microRNA control of synaptic plasticity in 22q11 deletion syndrome and schizophrenia. J. Neurosc. 2012, 32, 14132–14144.
- Maynard, T.; Meechan, D.; Dudevoir, M.; Gopalakrishna, D.; Peters, A.; Heindel, C.; Sugimoto, T.; Wu, Y.; Lieberman, J.; LaMantia, A.-S. Mitochondrial localization and function of a subset of 22q11 deletion syndrome candidate genes. Mol. Cell. Neurosci. 2008, 39, 439–451.
- Padula, M.C.; Schaer, M.; Scariati, E.; Schneider, M.; Van De Ville, D.; Debbané, M.; Eliez, S. Structural and functional connectivity in the default mode network in 22q11.2 deletion syndrome. J. Neurodev. Disord. 2015, 7, 23.
- Mukai, J.; Liu, H.; Burt, R.; Swor, D.E.; Lai, W.-S.; Karayiorgou, M.; Gogos, J.A. Evidence that the gene encoding ZDHHC8 contributes to the risk of schizophrenia. Nat. Genet. 2004, 36, 725–731.
- Prabakaran, S.; Swatton, J.E.; Ryan, M.M.; Huffaker, S.J.; Huang, J.T.-J.; Griffin, J.L.; Wayland, M.; Freeman, T.; Dudbridge, F.; Lilley, K.S.; et al. Mitochondrial dysfunction in schizophrenia: Evidence for compromised brain metabolism and oxidative stress. Mol. Psychiatry 2004, 9, 684–697.
- Hanschmann, E.-M.; Lönn, M.E.; Schütte, L.D.; Funke, M.; Godoy, J.R.; Eitner, S.; Hudemann, C.; Lillig, C.H. Both thioredoxin 2 and glutaredoxin 2 contribute to the reduction of the mitochondrial 2-Cys peroxiredoxin Prx3. J. Biol. Chem. 2010, 285, 40699–40705.
- Liu, H.; Abecasis, G.R.; Heath, S.C.; Knowles, A.; Demars, S.; Chen, Y.-J.; Roos, J.L.; Rapoport, J.L.; Gogos, J.A.; Karayiorgou, M. Genetic variation in the 22q11 locus and susceptibility to schizophrenia. Proc. Natl. Acad. Sci. USA 2002, 99, 16859–16864.
- Liu, H.; Heath, S.C.; Sobin, C.; Roos, J.L.; Galke, B.L.; Blundell, M.L.; Lenane, M.; Robertson, B.; Wijsman, E.M.; Rapoport, J.L.; et al. Genetic variation at the 22q11 PRODH2/DGCR6 locus presents an unusual pattern and increases susceptibility to schizophrenia. Proc. Natl. Acad. Sci. USA 2002, 99, 3717–3722.





