
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Fenghua Wang | -- | 2121 | 2023-03-02 18:41:24 | | | |
| 2 | Dean Liu | Meta information modification | 2121 | 2023-03-10 02:38:34 | | |
Video Upload Options
Brassica vegetables are very important to human beings. Self-incompatibility (SI) is a common phenomenon in Brassica. Breeding by SI lines is an important way to utilize heterosis of Brassica vegetables. It is believed that the SI inheritance in Brassica species is controlled by three linkage genes on the S-locus, including SRK (S-locus receptor kinase), SCR (S-locus cystine-rich protein)/SP11 (S-locus protein 11), and SLG (S-locus glycoprotein). SRK is the female determinant and SCR/SP11 is the pollen S gene. The expression of SLG is necessary for SRK, and it enhances the SRK-mediated SI reaction. In addition to these three S-locus genes, some other functional molecules also have significant regulatory effects on SI, such as ARC1 (arm repeat containing 1), MLPK (M-locus protein kinase), Exo70A1 (exocyst compounds), THLl/THL2 (thioredoxin H-like), MOD (aquaporin), SLR (S-locus-related glycoprotein), BPCI (pollen calcium-binding protein I), etc. SI is also associated with the dominant/recessive relationship between S alleles.
1. Introduction
2. S-Locus Transducers of SSI
2.1. SRK
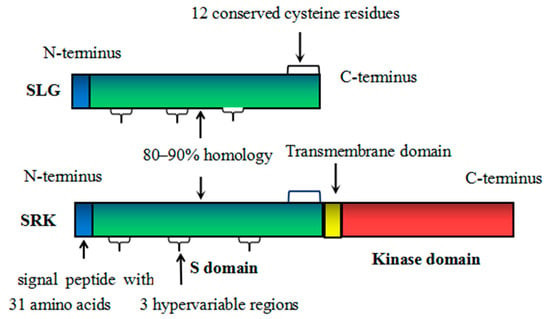
2.2. SCR/SP11
2.3. SLG
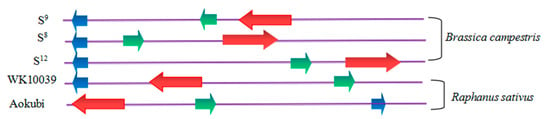
2.4. Genomic Organization of S-Locus Genes
2.5. The Relationship of S-Locus Genes in SI Reaction
References
- Wang, Q.; Zheng, P.; Zhang, L. Identification and classification of S haplotypes in radish (Raphanus sativus). Plant Breed. 2019, 138, 121–130.
- Kitashiba, H.; Nasrallah, J.B. Self-incompatibility in Brassicaceae crops: Lessons for interspecific incompatibility. Breed. Sci. 2014, 64, 23–37.
- Kachroo, A.; Nasrallah, M.E.; Nasrallah, J.B. Self-incompatibility in the Brassicaceae: Receptor-ligand signaling and cell-to-cell communication. Plant Cell 2002, 14, S227–S238.
- Watanabe, M.; Suwabe, K.; Suzuki, G. Molecular genetics, physiology, and biology of self-incompatibility in Brassicaceae. Proc. Jpn. Acad. Ser. B 2012, 88, 519–535.
- Stein, J.C.; Howlett, B.; Boyes, D.C. Molecular cloning of a putative receptor protein kinase gene encoded at the self-incompatibility locus of Brassica oleracea. Proc. Natl. Acad. Sci. USA 1991, 88, 8816–8820.
- Yamamoto, M.; Tantikanjana, T.; Nishio, T.; Nasrallah, M.E.; Nasrallah, J.B. Site-specific N-glycosylation of the S-locus receptor kinase and its role in the self-incompatibility response of the Brassicaceae. Plant Cell 2014, 26, 4749–4762.
- Glivan, T.L.; Goning, D.R.; Scharer, U.; Rothstein, S.J. Features of the extracellular domain of the S-locus receptor kinase from Brassica. Mol. Gen. Genet. 1994, 244, 630–637.
- Takasaki, T.; Hatakeyama, K.; Suzuki, G.; Watanabem, M.; Isogai, A.; Hinata, K. The S receptor kinase determines self-incompatibility in Brassica stigma. Nature 2000, 403, 913–916.
- Silva, N.F.; Stone, S.L.; Christie, L.N.; Sulaman, W.; Nazarian, K.A.P.; Burnett, L.A.; Arnoldo, M.A.; Rothstein, S.J.; Goring, D.R. Expression of the S receptor kinase in self-compatible Brassica napus cv. Westar leads to the allele-specific rejection of self-incompatible Brassica napus pollen. Mol. Genet. Genom. 2001, 265, 552–559.
- Takayama, S.; Shiba, H.; Iwano, M.; Shimosato, H.; Che, F.S.; Kai, N.; Watanabe, M.; Suzuki, G.; Hinata, K.; Isoga, A. The pollen determinant of self-incompatibility in Brassica campestris. Proc. Natl. Acad. Sci. USA 2000, 97, 1920–1925.
- Schopfer, C.R.; Nasrallah, M.E.; Nasrallah, J.B. The male determinant of self-incompatibility in Brassica. Science 1999, 286, 1697–1700.
- Franklin-Tong, V.E.; Franklin, F.C.H. Self-incompatibility in Brassica: The elusive pollen S gene is identified. Plant Cell 2000, 12, 305–308.
- Kusaba, M.; Dwyer, K.; Hendershot, J.; Vrebalov, J.; Nasrallah, N. Self-incompatibility in the genus Arabidopsis: Characterization of the S locus in the outcrossing A. lyrata and its autogamous relative A. thaliana. Plant Cell 2001, 13, 627–643.
- Shiba, H.; Iwano, M.; Entani, T.; Ishimoto, K.; Shimosato, H.; Che, F.S.; Satta, Y.; Ito, A.; Takada, Y.; Watanabe, M.; et al. The dominance of alleles controlling self-incompatibility in Brassica pollen is regulated at the RNA level. Plant Cell 2002, 14, 491–504.
- Murase, K.; Moriwaki, Y.; Mori, T.; Liu, X.; Masaka, C.; Takada, Y.; Maesaki, R.; Mishima, M.; Fujii, S.; Hirano, Y.; et al. Mechanism of self/nonself-discrimination in Brassica self-incompatibility. Nat. Commun. 2020, 11, 4916.
- Haseyama, Y.; Kitashiba, H.; Okamoto, S.; Tonouchi, E.; Sakamoto, K.; Nishio, T. Nucleotide sequence analysis of S-locus genes to unify S haplotype nomenclature in radish. Mol. Breed. 2018, 38, 116.
- Nasrallah, J.B.; Kao, T.H.; Goldberg, M.; Nasrallah, M.E. A cDNA clone encoding an S-locus-specific glycoprotein from Brassica oleracea. Nature 1985, 318, 263–267.
- Shiba, H.; Kimura, N.; Takayama, S.; Hinata, K.; Suzuki, A.; Isogai, A. Alteration of the self-incompatibility phenotype in Brassica by transformation of the antisense SLG gene. Biosci. Biotech. Bioch. 2000, 64, 1016–1024.
- Nasrallah, J.B.; Yu, S.M.; Nasrallah, M.E. Self-incompatibility genes of Brassica oleracea expression, isolation and structure. Proc. Natl. Acad. Sci. USA 1988, 85, 5551–5555.
- Nasrallah, J.B.; Nasrallah, M.E. The molecular genetics of self-incompatibility in Brassica. Annu. Rev. Genet. 1989, 23, 121–139.
- Nasrallah, J.B.; Kao, T.H.; Chen, C.H.; Goldberg, M.L.; Nasrallah, M.E. Amino-acid sequence of glycoproteins encoded by three alleles of the S-locus of Brassica oleracea L. Nature 1987, 326, 617–619.
- Nishio, T.; Hinata, K. Comparative studies on S-glycoproteins purified from different S-genotypes in self-incompatible Brassica species.1. Purification and chemical properties. Genetics 1982, 100, 641–647.
- Luu, D.T.; Marty-Mazars, D.; Trick, M.; Heizmann, D.P. Pollen-stigma adhesion in Brassica spp involves SLG and SLR1 glycoproteins. Plant Cell 1999, 11, 251–262.
- Kim, D.S.; Kim, S. Identifification of the S locus core sequences determining self-incompatibility and S multigene family from draft genome sequences of radish (Raphanus sativus L.). Euphytica 2018, 214, 16.
- Casselman, A.L.; Vrebalov, J.; Conner, J.A.; Singhal, A.; Giovannoni, J.; Nasrallah, M.E.; Nasrallah, J.B. Determining the physical limits of the Brassica S-locus by recombinational analysis. Plant Cell 2000, 12, 23–33.
- Tantikanjana, T.; Nasrallah, M.E.; Stein, J.C.; Chen, C.H.; Nasrallah, J.B. An alternative transcript of the S-locus glycoprotein gene in a classⅡ pollen-recessive self-incompatibility haplotype of Brassica oleracea encodes a membrane-anchored protein. Plant Cell 1993, 5, 657–666.
- Kao, T.H.; McCubbin, A.G. Plant biology-a social stigma. Nature 2000, 403, 840–841.
- Dixit, R.; Nasrallah, M.E.; Nasrallah, J.B. Post-transcriptional maturation of the S receptor kinase of Brassica correlates with co-expression of the S-locus glycoprotein in the stigmas of two Brassica strains and in transgenic tobacco plants. Plant Physiol. 2000, 124, 297–311.




