
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ines Dias Teixeira | -- | 2756 | 2023-03-02 10:15:23 | | | |
| 2 | Jessie Wu | Meta information modification | 2756 | 2023-03-03 01:55:46 | | | | |
| 3 | Jessie Wu | + 6 word(s) | 2762 | 2023-03-03 02:03:33 | | | | |
| 4 | Jessie Wu | + 2 word(s) | 2764 | 2023-03-03 02:04:54 | | | | |
| 5 | Jessie Wu | -1 word(s) | 2763 | 2023-03-03 02:06:27 | | |
Video Upload Options
Diabetic foot ulcers (DFU) are one of the most serious and devastating complications of diabetes and account for a significant decrease in quality of life and costly healthcare expenses worldwide. This condition affects around 15% of diabetic patients and is one of the leading causes of lower limb amputations. DFUs generally present poor clinical outcomes, mainly due to the impaired healing process and the elevated risk of microbial infections which leads to tissue damage. Antimicrobial resistance poses a rising threat to global health, thus hampering DFU treatment and care. Faced with this reality, it is pivotal to find greener and less environmentally impactful alternatives for fighting these resistant microbes. Antimicrobial peptides are small molecules that play a crucial role in the innate immune system of the host and can be found in nature. Some of these molecules have shown broad-spectrum antimicrobial properties and wound-healing activity, making them good potential therapeutic compounds to treat DFUs.
1. Diabetic Foot Ulcers
2. Microbiota in Diabetic Foot Ulcers
3. Antimicrobial Peptides
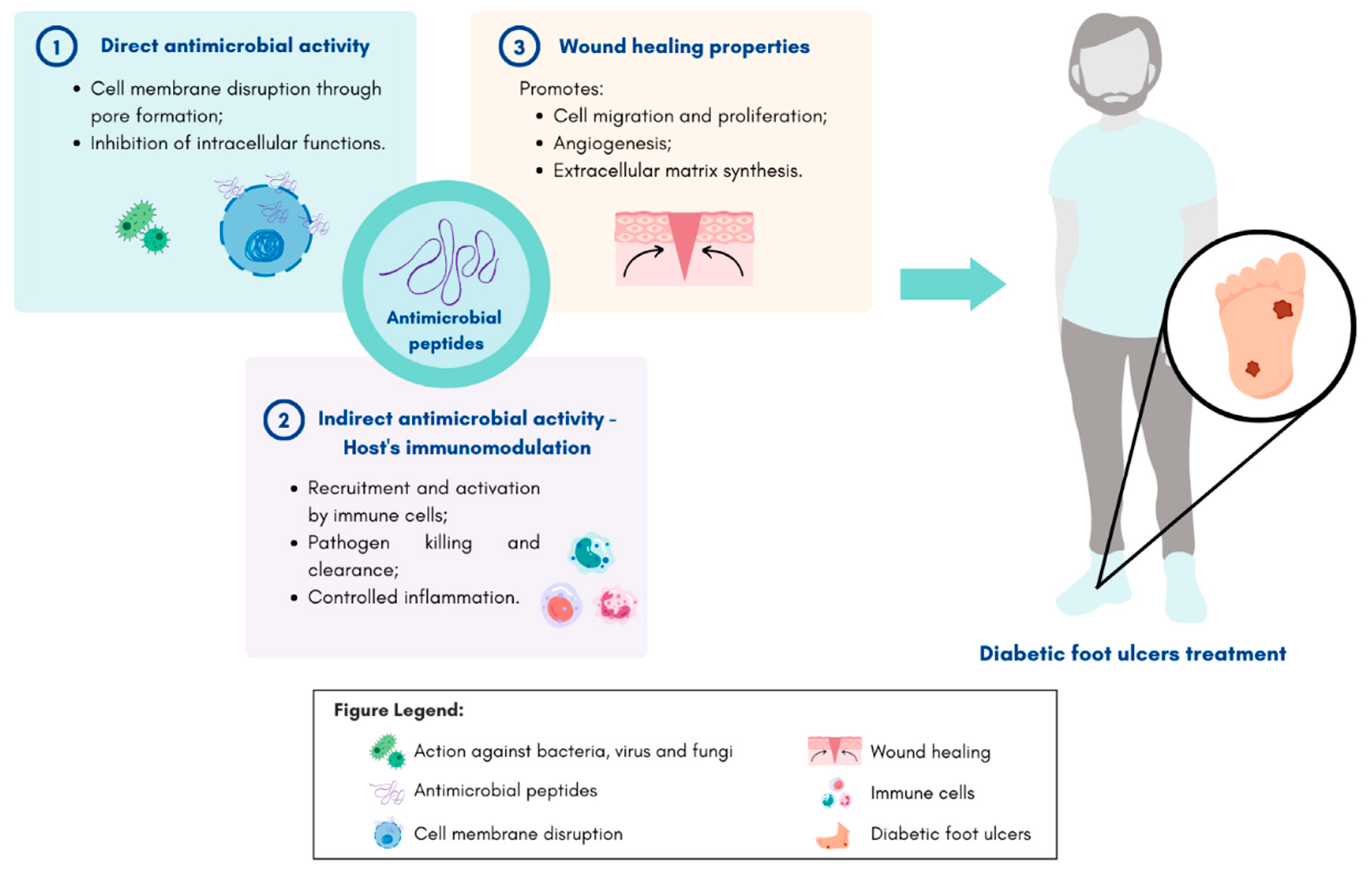
4. Therapeutic Use of Green Antimicrobial Peptides in Diabetic Foot Ulcer
| Source | AMPs/Sequences | Susceptible Species | Other Effects | Ref. |
|---|---|---|---|---|
| Plants | ||||
| Phaseolus vulgaris seeds | PvD1 | Candida albicans Candida parapsilosis Candida guilliermondii Candida tropicalis Saccharomyces cerevisiae |
Activity against tumor cells | [35][45] |
| Ziziphus jujuba fruits | Snakin-Z | Staphylococcus aureus Escherichia coli Bacillus subtili Klebsiella pneumoniae |
Antioxidant activity | [43][46] |
| Viola odorata | Cycloviolacin O2 | S. enterica serovar Typhimurium LT2 Escherichia coli Klebsiella pneumoniae Pseudomonas aeruginosa |
Activity against tumor cells | [36][47] |
| Cocos nucifera L. | Cn-AMP1 Cn-AMP2 Cn-AMP3 |
Escherichia coli Bacillus subtilis Pseudomonas aeruginosa Staphylococcus aureus |
Activity against tumor cells Immunostimulatory activity |
[23] |
| Cottonseed defatted protein powder | CHQQEQRP DENFRKF EWPEEGQRR KPPIMPIGKG KDFPGRR LGLRSGIILCNV PRNFQQQLR QNLNALQPK SQEATSPR |
Staphylococcus aureus (ATCC27068) Escherichia coli (ATCC25922) Steptococcus (CMCC35668) Salmonella (CMCC50013) |
- | [48] |
| Nicotiana tabacum | LFchimera | Escherichia coli | - | [49] |
| Nicotiana tabacum | Colicin M | Escherichia coli Klebsiella pneumoniae |
- | [50] |
| Nicotiana tabacum | Protegrin-1 | Klebsiella pneumoniae Staphylococcus aureus Escherichia coli Mycobacterium bovis Candida albicans |
- | [51] |
| Microalgae | ||||
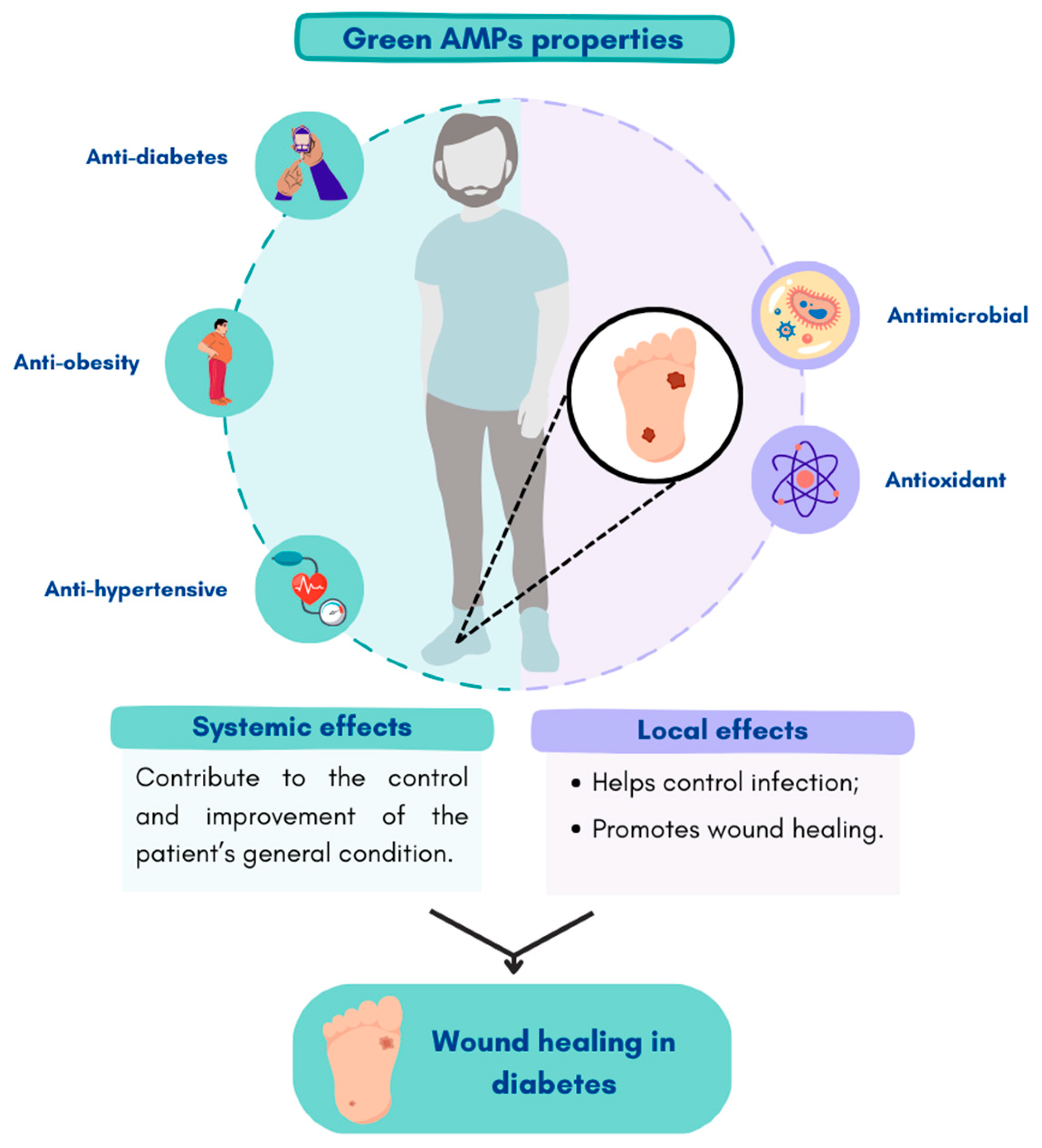
| Spirulina platensis | SP-1 | Escherichia coli Staphylococcus aureus |
Antioxidant, antihypertensive, anti-diabetes, and anti-obesity |
[31][32] |
| Limnospira maxima | KLENCNYAVELGK | Escherichia coli Staphylococcus aureus |
- | [30] |
| Lyngbya sp. | Lyngbyazothrins mixture C/D | Bacillus subtilis Escherichia coli Pseudomonas aeruginosa Serratia marcesens |
- | [40] |
| Lyngbya majuscula | Pitipeptolides C-F | Mycobacterium tuberculosis | Activity against tumor cells | [37][52] |
| Microcystis aeruginosa (NIES-88) |
Kawaguchipeptin B | Staphylococcus aureus | - | [44] |
| Hawaii and Caribbean collection of cyanobacteria | Laxaphycin A | Listeria monocytogenes Bacillus cereus Staphylococcus aureus |
Activity against tumor cells | [37][41] |
| Laxaphycin B | Listeria monocytogenes Bacillus cereus Staphylococcus aureus |
|||
| Laxaphycin B3 | Bacillus cereus | |||
| Tetraselmis suecica | AQ-1766 AQ-3001 AQ-3002 AQ3369 AQ-3370 AQ-3371 AQ-3372 |
Escherichia coli Salmonella typhimurium Pseudomonas aeruginosa Bacillus cereus Methicillin-resistant S. aureus (MRSA) Listeria monocytogenes |
- | [42] |
References
- Petkovic, M.; Sørensen, A.E.; Leal, E.C.; Carvalho, E.; Dalgaard, L.T. Mechanistic actions of microRNAs in diabetic wound healing. Cells 2020, 9, 2228.
- Da Silva, J.; Leal, E.C.; Carvalho, E. Bioactive antimicrobial peptides as therapeutic agents for infected diabetic foot ulcers. Biomolecules 2021, 11, 1894.
- Guo, S.A.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229.
- Singer, A.J.; Clark, R.A.F. Cutaneous Wound Healing. New Engl. J. Med. 1999, 341, 738–746.
- Ramirez-Acuña, J.M.; Cardenas-Cadena, S.A.; Marquez-Salas, P.A.; Garza-Veloz, I.; Perez-Favila, A.; Cid-Baez, M.A.; Flores-Morales, V.; Martinez-Fierro, M.L. Diabetic foot ulcers: Current advances in antimicrobial therapies and emerging treatments. Antibiotics 2019, 8, 193.
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.; Black, D.S.; Walsh, W.R.; Kumar, N. A new era of antibiotics: The clinical potential of antimicrobial peptides. Int. J. Mol. Sci. 2020, 21, 7047.
- Uivaraseanu, B.; Bungau, S.; Tit, D.M.; Fratila, O.; Rus, M.; Maghiar, T.A.; Pantis, C.; Vesa, C.M.; Zaha, D.C. Clinical, pathological and microbiological evaluation of diabetic foot syndrome. Medicina 2020, 56, 380.
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253.
- Rademacher, F.; Gläser, R.; Harder, J. Antimicrobial peptides and proteins: Interaction with the skin microbiota. Exp. Dermatol. 2021, 30, 1496–1508.
- Kalan, L.R.; Meisel, J.S.; Loesche, M.A.; Horwinski, J.; Soaita, I.; Chen, X.; Uberoi, A.; Gardner, S.E.; Grice, E.A. Strain-and species-level variation in the microbiome of diabetic wounds is associated with clinical outcomes and therapeutic efficacy. Cell Host Microbe 2019, 25, 641–655.
- Kalan, L.R.; Brennan, M.B. The role of the microbiome in nonhealing diabetic wounds. Ann. N. Y. Acad. Sci. 2019, 1435, 79–92.
- Kareliya, H.; Bichile, L.; Bal, A.; Varaiya, A.; Bhalekar, P. Fungal Infection in Diabetic Foot a Clinicomicrobiological Study. Acta Sci. Microbiol. 2019, 2, 49–55.
- Kalan, L.; Loesche, M.; Hodkinson, B.P.; Heilmann, K.; Ruthel, G.; Gardner, S.E.; Grice, E.A. Redefining the Chronic-Wound Microbiome: Fungal Communities Are Prevalent, Dynamic, and Associated with Delayed Healing. MBio 2016, 7, e01058-16.
- Pouget, C.; Dunyach-Remy, C.; Pantel, A.; Schuldiner, S.; Sotto, A.; Lavigne, J.P. Biofilms in diabetic foot ulcers: Significance and clinical relevance. Microorganisms 2020, 8, 1580.
- Banu, A.; Noorul Hassan, M.M.; Rajkumar, J.; Srinivasa, S. Spectrum of bacteria associated with diabetic foot ulcer and biofilm formation: A prospective study. Australas. Med. J. 2015, 8, 280–285.
- Fry, D.E. Antimicrobial peptides. Surg. Infect. 2018, 19, 804–811.
- Depta, J.; Małkowska, P.; Wysokińska, M.; Todorska, K.; Sierawska, O.; Hrynkiewicz, R.; Bebnowska, D.; Niedźwiedzka-Rystwej, P. Therapeutic Role of Antimicrobial Peptides in Diabetes Mellitus. Biologics 2022, 2, 92–106.
- Gaiser, R.A. Antimicrobial Peptides and the Interplay between Microbes and Host: Towards Preventing Porcine Infections with Streptococcus Suis. Ph.D. Thesis, Wageningen University and Research, Wageningen, The Netherlands, 2016.
- Gallo, R.L.; Nizet, V. Endogenous production of antimicrobial peptides in innate immunity and human disease. Curr. Allergy Asthma Rep. 2003, 3, 402–409.
- Zhang, L.J.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14–R19.
- Gonzalez-Curiel, I.; Trujillo, V.; Montoya-Rosales, A.; Rincon, K.; Rivas-Calderon, B.; De Haro-Acosta, J.; Marin-Luevano, P.; Lozano-Lopez, D.; Enciso-Moreno, J.A.; Rivas-Santiago, B. 1,25-dihydroxyvitamin D3 induces LL-37 and HBD-2 production in keratinocytes from diabetic foot ulcers promoting wound healing: An in vitro model. PLoS ONE 2014, 9, e111355.
- Al-Shibly, I.K.; Alhamdany, M.H.; Al-Kaif, R.A.I.; Al-Kaif, L.A. Immunological Base Behind the Increased Susceptibility of Diabetic Patients for Infections. Indian J. Public Health 2019, 10, 3047–3051.
- Mandal, S.M.; Dey, S.; Mandal, M.; Sarkar, S.; Maria-Neto, S.; Franco, O.L. Identification and structural insights of three novel antimicrobial peptides isolated from green coconut water. Peptides 2009, 30, 633–637.
- Lima, P.G.; Oliveira, J.T.; Amaral, J.L.; Freitas, C.D.; Souza, P.F. Synthetic antimicrobial peptides: Characteristics, design, and potential as alternative molecules to overcome microbial resistance. Life Sci. 2021, 278, 119647.
- Silva, O.N.; Porto, W.F.; Migliolo, L.; Mandal, S.M.; Gomes, D.G.; Holanda, H.H.; Silva, R.S.P.; Dias, S.C.; Costa, M.P.; Costa, C.R.; et al. Cn-AMP1: A new promiscuous peptide with potential for microbial infections treatment. Pept. Sci. 2012, 98, 322–331.
- Santana, M.J.; de Oliveira, A.L.; Queiroz Júnior, L.H.; Mandal, S.M.; Matos, C.O.; Dias, R.D.O.; Franco, O.L.; Lião, L.M. Structural insights into Cn-AMP1, a short disulfide-free multifunctional peptide from green coconut water. FEBS Lett. 2015, 589, 639–644.
- Sathya, R.; MubarakAli, D.; MohamedSaalis, J.; Kim, J.W. A systemic review on microalgal peptides: Bioprocess and sustainable applications. Sustainability 2021, 13, 3262.
- Leal, E.C.; Emanuelli, T.; Santos, D.; Moura, J.; Fonseca, A.C.R.; Burgeiro, A.; Carvalho, E. Dysregulation of endoplasmic reticulum stress response in skin wounds in a streptozotocin-induced diabetes mouse model. J. Mol. Endocrinol. 2023.
- Leal, E.C.; Carvalho, E.; Tellechea, A.; Kafanas, A.; Tecilazich, F.; Kearney, C.; Kuchibhotla, S.; Auster, M.E.; Kokkotou, E.; Mooney, D.J.; et al. Substance P promotes wound healing in diabetes by modulating inflammation and macrophage phenotype. Am. J. Pathol. 2015, 185, 1638–1648.
- Sathya, R.; MubarakAli, D.; Mehboob Nousheen, M.G.; Vasimalai, N.; Thajuddin, N.; Kim, J.-W. An Investigation of Pepsin Hydrolysate of Short Antibacterial Peptides Derived from Limnospira Sp. Appl. Biochem. Biotechnol. 2022, 194, 5580–5593.
- Sun, Y.; Chang, R.; Li, Q.; Li, B. Isolation and characterization of an antibacterial peptide from protein hydrolysates of Spirulina platensis. Eur. Food Res. Technol. 2016, 242, 685–692.
- Yücetepe, A.; Özçelik, B. Bioactive peptides isolated from microalgae Spirulina platensis and their biofunctional activities. Akad. Gıda 2016, 14, 412–417.
- Figueiredo, A.; Leal, E.C.; Carvalho, E. Protein tyrosine phosphatase 1B inhibition as a potential therapeutic target for chronic wounds in diabetes. Pharmacol. Res. 2020, 159, 104977.
- Leal, E.C.; Carvalho, E. Heme Oxygenase-1 as Therapeutic Target for Diabetic Foot Ulcers. Int. J. Mol. Sci. 2022, 23, 12043.
- Figueira, T.N.; Oliveira, F.D.; Almeida, I.; Mello, É.O.; Gomes, V.M.; Castanho, M.A.; Gaspar, D. Challenging metastatic breast cancer with the natural defensin PvD1. Nanoscale 2017, 9, 16887–16899.
- Gerlach, S.L.; Rathinakumar, R.; Chakravarty, G.; Göransson, U.; Wimley, W.C.; Darwin, S.P.; Mondal, D. Anticancer and chemosensitizing abilities of cycloviolacin O2 from Viola odorata and psyle cyclotides from Psychotria leptothyrsa. Pept. Sci. 2010, 94, 617–625.
- Qamar, H.; Hussain, K.; Soni, A.; Khan, A.; Hussain, T.; Chénais, B. Cyanobacteria as Natural Therapeutics and Pharmaceutical Potential: Role in Antitumor Activity and as Nanovectors. Molecules 2021, 26, 247.
- Kim, D.S.; Scherer, D.S.P.E. Obesity, Diabetes, and Increased Cancer Progression. Diabetes Metab. J. 2021, 45, 799–812.
- Wang, M.; Yang, Y.; Liao, Z. Diabetes and cancer: Epidemiological and biological links. World J. Diabetes 2020, 11, 227–238.
- Zainuddin, E.N.; Jansen, R.; Nimtz, M.; Wray, V.; Preisitsch, M.; Lalk, M.; Mundt, S. LyngbyazothrinsA−D, Antimicrobial CycliUndecapeptides from the Cultured Cyanobacterium Lyngbya sp. J. Nat. Prod. 2009, 72, 2080.
- Dussault, D.; Vu, K.D.; Vansach, T.; Horgen, F.D.; Lacroix, M. Antimicrobial effects of marine algal extracts and cyanobacterial pure compounds against five foodborne pathogens. Food Chem. 2016, 199, 114–118.
- Guzmán, F.; Wong, G.; Román, T.; Cárdenas, C.; Alvárez, C.; Schmitt, P.; Albericio, F.; Rojas, V. Identification of antimicrobial peptides from the microalgae Tetraselmis suecica (Kylin) Butcher and bactericidal activity improvement. Mar. Drugs 2019, 17, 453.
- Zare-Zardini, H.; Tolueinia, B.; Hashemi, A.; Ebrahimi, L.; Fesahat, F. Antioxidant and cholinesterase inhibitory activity of a new peptide from Ziziphus jujuba fruits. Am. J. Alzheimer’s Dis. Other Dement.® 2013, 28, 702–709.
- Ishida, K.; Matsuda, H.; Murakami, M.; Yamaguchi, K. Kawaguchipeptin B, an antibacterial cyclic undecapeptide from the cyanobacterium Microcystis aeruginosa. J. Nat. Prod. 1997, 60, 724–726.
- Games, P.D.; Dos Santos, I.S.; Mello, É.O.; Diz, M.S.; Carvalho, A.O.; de Souza-Filho, G.A.; Cunha, M.; Vasconcelos, I.M.; Ferreira, B.D.S.; Gomes, V.M. Isolation, characterization and cloning of a cDNA encoding a new antifungal defensin from Phaseolus vulgaris L. seeds. Peptides 2008, 29, 2090–2100.
- Daneshmand, F.; Zare-Zardini, H.; Ebrahimi, L. Investigation of the antimicrobial activities of Snakin-Z, a new cationic peptide derived from Zizyphus jujuba fruits. Nat. Prod. Res. 2013, 27, 2292–2296.
- Pränting, M.; Lööv, C.; Burman, R.; Göransson, U.L.F.; Andersson, D.I. The cyclotide cycloviolacin O2 from Viola odorata has potent bactericidal activity against Gram-negative bacteria. J. Antimicrob. Chemother. 2010, 65, 1964–1971.
- Song, W.; Kong, X.; Hua, Y.; Chen, Y.; Zhang, C.; Chen, Y. Identification of antibacterial peptides generated from enzymatic hydrolysis of cottonseed proteins. LWT 2020, 125, 109199.
- Chahardoli, M.; Fazeli, A.; Ghabooli, M. Recombinant production of bovine Lactoferrin-derived antimicrobial peptide in tobacco hairy roots expression system. Plant Physiol. Biochem. 2018, 123, 414–421.
- Łojewska, E.; Sakowicz, T.; Kowalczyk, A.; Konieczka, M.; Grzegorczyk, J.; Sitarek, P.; Skała, E.; Czarny, P.; Sliwinski, T.; Kowalczyk, T. Production of recombinant colicin M in Nicotiana tabacum plants and its antimicrobial activity. Plant Biotechnol. Rep. 2020, 14, 33–43.
- Patiño-Rodríguez, O.; Ortega-Berlanga, B.; Llamas-González, Y.Y.; Flores-Valdez, M.A.; Herrera-Díaz, A.; Montes-de-Oca-Luna, R.; Korban, S.S.; Alpuche-Solís, A.G. Transient expression and characterization of the antimicrobial peptide protegrin-1 in Nicotiana tabacum for control of bacterial and fungal mammalian pathogens. Plant Cell Tissue Organ Cult. PCTOC 2013, 115, 99–106.
- Montaser, R.; Paul, V.J.; Luesch, H. Pitipeptolides C–F, antimycobacterial cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula from Guam. Phytochemistry 2011, 72, 2068–2074.




