Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mário P. Marques | -- | 5487 | 2023-02-28 13:38:23 | | | |
| 2 | Catherine Yang | Meta information modification | 5487 | 2023-03-01 01:38:57 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Marques, M.P.; Mendonça, L.; Neves, B.G.; Varela, C.; Oliveira, P.; Cabral, C. Pharmacological Wound Healing Evidence of Iberian Lamiaceae. Encyclopedia. Available online: https://encyclopedia.pub/entry/41740 (accessed on 14 January 2026).
Marques MP, Mendonça L, Neves BG, Varela C, Oliveira P, Cabral C. Pharmacological Wound Healing Evidence of Iberian Lamiaceae. Encyclopedia. Available at: https://encyclopedia.pub/entry/41740. Accessed January 14, 2026.
Marques, Mário P., Laura Mendonça, Beatriz G. Neves, Carla Varela, Paulo Oliveira, Célia Cabral. "Pharmacological Wound Healing Evidence of Iberian Lamiaceae" Encyclopedia, https://encyclopedia.pub/entry/41740 (accessed January 14, 2026).
Marques, M.P., Mendonça, L., Neves, B.G., Varela, C., Oliveira, P., & Cabral, C. (2023, February 28). Pharmacological Wound Healing Evidence of Iberian Lamiaceae. In Encyclopedia. https://encyclopedia.pub/entry/41740
Marques, Mário P., et al. "Pharmacological Wound Healing Evidence of Iberian Lamiaceae." Encyclopedia. Web. 28 February, 2023.
Copy Citation
The traditional use of the family Lamiaceae has been highlighted in several ethnobotanical assays carried out within the Iberian Peninsula. Usually, Lamiaceae species are rich essential oil-bearing plants with a great diversity of phenolic compounds, polyphenols, iridoids, diterpenoids, triterpenoids, saponins and, in some restricted cases, pyridine and pyrrolidine alkaloids.
Iberian Peninsula
Lamiaceae
skin
wound healing
scientific validation
1. Lavandula stoechas L.
1.1. Ethnobotanical Uses and General Considerations
L. stoechas (Figure 1A) has a circum-Mediterranean distribution, and it is widely known for its applications in cosmetic, food, perfumery, and pharmaceutical industries [1]. This lavender is traditionally used as a carminative, antispasmodic, expectorant, anticonvulsant, analgesic, sedative, and diuretic [2]. It is also widely referred in ethnobotanical literature for the treatment of skin injuries and burns, either as antiseptic or healing/vulnerary of wounds [2][3].

Figure 1. Lamiaceae species with scientific evidence for their wound healing use in the Iberian Peninsula. Images of the plants (A)—Lavandula stoechas L., (B)—Marrubium vulgare L., (C)—Origanum vulgare L., (D)—Prunella vulgaris L., (E)—Salvia officinalis L., (F)—Salvia rosmarinus Schleid (Syn: Rosmarinus officinalis L.), (G)—Salvia verbenaca L. and (H)—Thymus vulgaris L. were obtained from the website Plants of the World Online (http://www.plantsoftheworldonline.org/ accessed on 4th February 2023).
1.2. Phytochemical Background
Flavonoids, tannins, sterols, coumarins, mucilages, and triterpenoids such as oleanolic and ursolic acids, have been identified as major classes of compounds in its hydroethanolic extract [4]. Recently, Baali et al. [2] reported that the prominent compound found in L. stoechas’ methanolic extract is rosmarinic acid, besides luteolin-7-O-glucoside, luteolin-7-O-glucuronide, salvianolic acid B, and quercetin-3-O-galactoside (Figure 2) [2]. On the other hand, and similarly to other aromatic plants of the Lamiaceae family, L. stoechas’ essential oils may present certain chemical variability. Even though the essential oils from several locations around the Mediterranean basin are mainly characterized by the presence of monoterpene ketones, such as fenchone and camphor, or by the oxygenated monoterpene 1,8-cineole, besides other volatiles such as linalool, linalyl acetate, terpineol, terpinen-4-ol, α-pinene, viridiflorol, camphene, among others [4][5].
1.3. Pharmacological Evidence
A clinical trial by Vakilian et al. [6] evaluated the efficacy of L. stoechas’ essential oil as a wound healing agent in episiotomy, a common perineal incision in obstetric and midwifery. This study included 120 randomized primiparous women divided in two main groups: patients treated with a sitz bath containing five to seven drops of the essential oil, and a control group of patients treated with povidone-iodine®. On the 10th day postpartum, the incision wounds were evaluated, and 25 patients treated with the sitz bath did not manifest any pain (p = 0.06), similarly to those treated only with povidone-iodine®, suggesting the efficacy of L. stoechas essential oil in pain relief. Furthermore, it reduced redness in the episiotomy area when compared to the control group (p < 0.001). Following this investigation, subsequent studies have been made to evaluate the utility of other lavender species essential oils on episiotomy healing [7][8][9].
Regarding the treatment of burn wounds, another clinical trial was performed for 14 days with 111 randomized patients with second-degree burns. This trial suggested that the incorporation of the essential oils of L. stoechas and Pelargonium roseum Ehrh. In a cream containing Aloe vera L. significantly reduced pain in patients with superficial second-degree skin burns, from day 0 to the 7th (p = 0.014) and the 14th (p = 0.05), when compared to the control group comprising patients treated with the standard drug silver sulfadiazine® (SSD) 1% cream [10].
The wound healing effects of L. stoechas using in vivo excision wound models have also been evaluated [2][5]. From this perspective, using Wistar albino rats, the methanolic extracts of L. stoechas and Mentha pulegium L. were incorporated in ointments at two distinct concentrations (5 and 10%) [2]. During 18 consecutive days the extract-containing ointments were topically applied (0.5 g per rat) once a day until the complete re-epithelization of the excision wounds. A wound contraction of 93.1 ± 2.88% and 97.19 ± 1.06% was observed on the 18th day in rats treated with ointments containing the L. stoechas extract at 5 and 10%, respectively. Besides that, wound contraction with the lavender-containing ointment (10%) group was statistically higher (p < 0.001) in comparison with the positive control group, an allantoin-based pharmaceutical formulation. The herbal ointment containing L. stoechas extract was promising for re-epithelization and cell migration, a critical step in the wound healing proliferative phase. Furthermore, appreciable granulation tissue and higher collagen quantity, without inflammatory signs such as oedema or erythema, were other observed characteristics. The authors further hypothesize that phytochemical compounds such as hydroxycinnamic acids, flavanols, flavones, and flavonols may explain the wound healing activity observed [2]. In another recent study, a cream prepared with the essential oil of L. stoechas at 0.5% showed the highest effect on excision wound models compared to the reference Madecassol®, a registered therapeutic cream. On the 4th, 11th, and 16th day, wound contractions were, respectively, 26.4%, 78%, and 96.3% for the group of rats treated with this herbal formulation, compared to 8.5%, 64.1%, and 86.1% for the control group. The authors concluded that the L. stoechas cream induced a significant decrease in the epithelization period, wound area and scar thickness, along with a significant increase in wound contraction. Moreover, this treatment also resulted in decreased inflammatory parameters and a great rate of tissue perfusion and proliferation, as well as remodeling and re-epithelization [5].
The capacity of L. stoechas to induce fibroblast proliferation and consequent in vitro wound healing was also explored in another study using an aqueous extract. This revealed that the growth and migration of fibroblasts was promoted at 24, 48, and 72 h transducing in a wound closure of 21.3%, 27.4%, and 29.2%, respectively [11].
Before the scientific evidence around L. stoechas, new drug delivery systems have been designed. Therefore, Mahmoudi et al. [12] synthesized silver nanoparticles (AgNPs) with a reductant methanolic extract of L. stoechas [12]. AgNPs presented antioxidant properties and antibacterial potential against S. aureus and P. aeruginosa which are common wound infecting bacteria. As these nanoparticles exhibited biocompatibility at an effective and non-toxic concentration (62.5 μg/mL), authors suggest their application for wound healing [12]. It is worth mentioning that other investigations have been carried out to develop innovative strategies for drug delivery of essential oils from other Lavandula species [13].
2. Marrubium vulgare L.
2.1. Ethnobotanical Uses and General Considerations
In traditional medicine, M. vulgare (Figure 1B) is mostly used to treat gastrointestinal and respiratory diseases [14], and also for several skin conditions, either in the Iberian Peninsula [15] or in other countries of the Mediterranean region [16][17]. Interestingly, the scientific-based wound healing activity of this Lamiaceae has received some attention in the Mediterranean basin [16][18][19].
2.2. Phytochemical Background
M. vulgare is a poor essential oil-bearing plant with low extraction yields ranging between 0.03% and 0.06%. However, several monoterpenes have been identified in its essential oil, such as camphene, p-cymol, fenchone, limonene, α-pinene, sabinene, and α-terpinolene (Figure 2) [14]. Considering terpenoids, M. vulgare is enriched in several diterpenes such as the labdane-type diterpene marrubin. This is responsible for the plant’s bitter characteristic and it is also a chemotaxonomic marker for the genus Marrubium [14][18]. The triterpenoids lupeol and oleanolic acid, as well as phytosterols such as β-sitosterol, have been reported for this Lamiaceae species [14]. On the other hand, the flavonoid family in M. vulgare is mainly represented by flavones such as luteolin, ladanein, and apigenin, flavone derivatives such as apigenin-7-O-glucoside and luteolin-7-O-glucoside, and the flavonol derivatives quercetin-3-O-galactoside and rutin. For a long time, and since this plant is part of the Lamioideae subfamily, rosmarinic acid was thought to be absent. However, a few reports have shown its presence in M. vulgare extracts and caffeic, ferulic, and chlorogenic acids [14].
2.3. Pharmacological Evidence
Recently, Mssilou et al. [16] assessed the activity of the hydroethanolic extract of M. vulgare to heal skin burns induced on the dorsal part of rats during a period of 21 days. The hydroethanolic extract of Dittrichia viscosa (L.) Greuter and the ointment-based combination with M. vulgare extract was equally investigated. According to the observations, the topical application of the ointment with M. vulgare extract, also in combination with D. viscosa, recorded remarkable and progressive wound closure at the 21st day, when compared to the controls that were unable to induce complete wound healing in the same period of time. Moreover, equal promising results were observed for inflammation and pain, other key parameters of the wound healing process [16]. Indeed, de Souza et al. [20] demonstrated the analgesic properties of an hydroalcoholic extract of M. vulgare regarding different models of pain in mice. In another study, Yahiaoui et al. [19] showed that the acetonic extract of M. vulgare improved the quality of the scar tissue and wound contraction by around 93.79% after 14 days, compared to control rats treated with Madecassol® with a wound closure of around 96.55%.
Amri et al. [18] conducted an in vitro cell-based investigation with a methanolic extract. Authors found that in a non-toxic concentration (5 μg/mL), the extract showed to promote migration and proliferation of dermal fibroblasts (NHDF cell line), reaching complete confluence after 48 h of extract application. Besides that, the aqueous extract of M. vulgare demonstrated not only antioxidant potential, but also interesting hemostatic activity. The latter is suggested to arise from the presence of condensed tannins, which are well-known for their astringency activity. In fact, tannins are important hemostatic agents working positively in wound and burn healing. Moreover, they observed that wound healing is independent from the presence of the diterpene marrubiin [21].
3. Origanum vulgare L.
3.1. Ethnobotanical Uses and General Considerations
O. vulgare (Figure 1C), commonly known as oregano, is a widespread herbaceous Lamiaceae found in Europe, North Africa, America, and Asia. A wide range of activities have been scientifically evidenced such as antimicrobial, antioxidant, anti-inflammatory, antitumor, antihyperglycemic, and anti-Alzheimer activities and in skin disorders [22]. Its ethnopharmacological uses are mainly related to respiratory conditions, such as cold symptoms, including cough, as well as digestive disorders and dermatological affections [1][22][23]. In the Catalonian region of Ripollès district, Spain, there are reports of using O. vulgare’s flowers in embrocation for their vulnerary properties [24].
3.2. Phytochemical Background
O. vulgare is a rich essential oil-bearing plant and for this reason, the most relevant family of phytochemicals are the volatiles found in its essential oil. According to the analysis of several oregano essential oils, they are chemically polymorphic, existing with several chemotypes based on major compounds. Monoterpene phenolics such as carvacrol and thymol are prominent in oregano essential oils, along with linalool, γ-terpinene, p-cymene, and the sesquiterpenes β-caryophyllene and germacrene D. Besides essential oils, O. vulgare is also a rich source of flavonoids, tannins, and phenolic glycosides. From this point of view, luteolin-O-glucuronide and luteolin-7-O-glucoside have been pointed out as main flavonoid derivatives found in hydroalcoholic extracts, decoctions, and infusions. Smaller molecules such as caffeic, protocatechuic, vanillic, and o-coumaric acids have been equally identified, with rosmarinic acid as the major phenolic acid (Figure 2) [22].
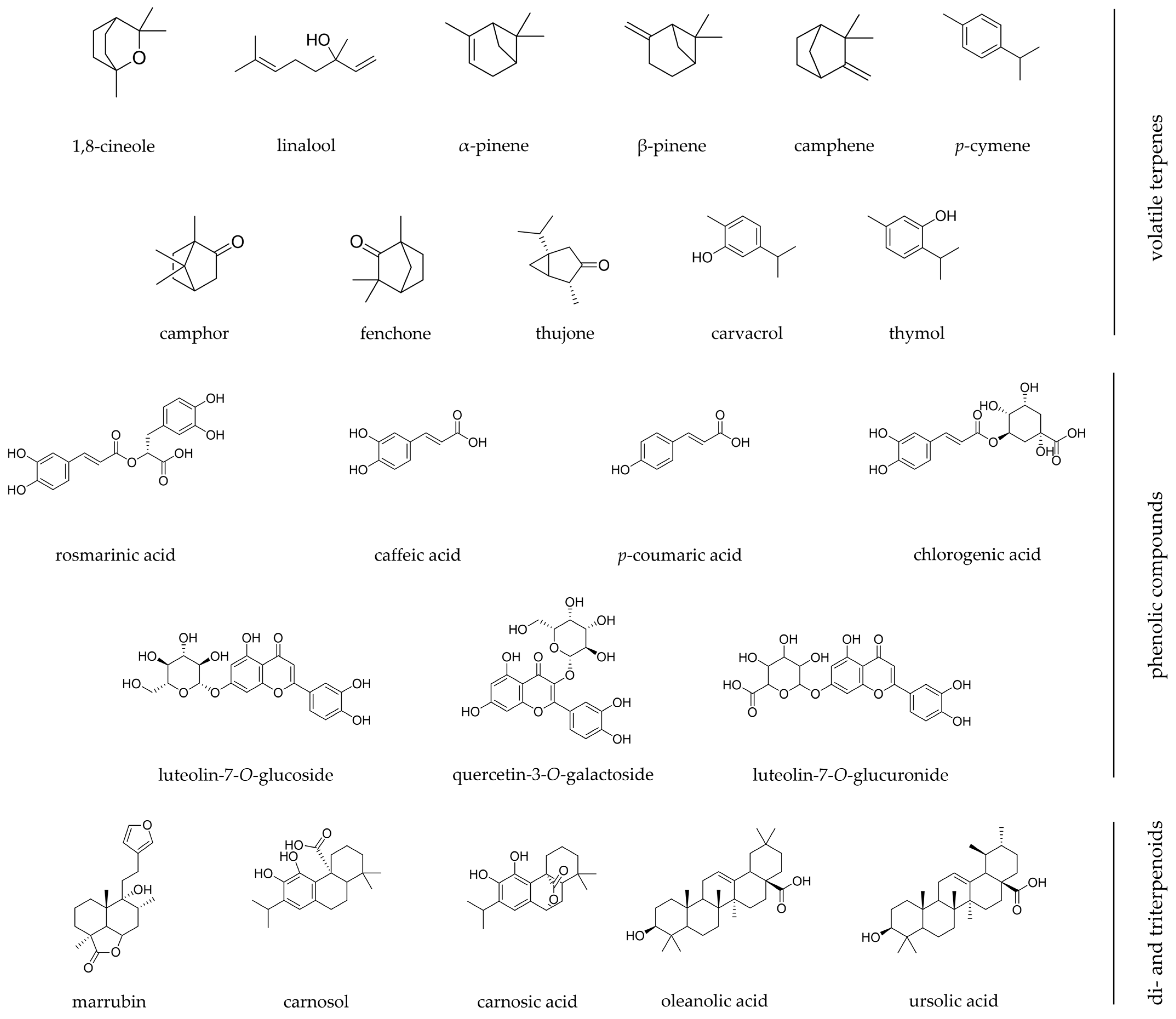
Figure 2. Chemical structures of the most relevant phytochemicals found among Iberian Lamiaceae species with wound healing scientific validation. Chemical structures were designed using ChemDraw software.
3.3. Pharmacological Evidence
In a randomized pilot petrolatum-controlled clinical trial, an ointment containing an O. vulgare aqueous extract was tested by topical application, twice a day, on patients with excision wounds. The group treated with O. vulgare ointment presented a significant improvement in comparison to the petrolatum-treated group regarding skin pigmentation, vascularization, thickness, relief, and pliability. It is worth mentioning that the ointment proved to be a good antimicrobial product on post-surgical excision injuries, namely against S. aureus [25].
The design of innovative drug delivery systems has been pursued by some authors [26][27][28]. In this sense, considering in vivo excision wound models, a study on synthesized titanium dioxide nanoparticles (TiO2.NPs) loaded with an oregano aqueous extract was made. Rats treated with TiO2.NPs presented a wound closure of 94% on the 12th day of evaluation while the control group remained at 86%. In addition, animals under this treatment showed an increased collagen content and degree of re-epithelization, as well better fibroblasts and macrophages aggregation [27]. Recently, a biocompatible pharmaceutical formulation with potential wound dressing was designed to overcome the instability and skin irritancy that may arise from the direct application of essential oils. In this study, the essential oil of O. vulgare was encapsulated in a poly (L-lactide-co-caprolactone) (PLCL)/silk fibroin (SF) nanofiber membrane through electrospinning. Results from the in vivo assays showed that the designed therapeutic system improved re-epithelialization and the formation of granulation tissue, and it also stimulated angiogenesis and collagen accumulation [26]. Afterwards, in vivo diabetic wound models were studied by the same team where the co-delivery nanofibrous membranes loaded with two bioactives, the essential oil of O. vulgare and zinc oxide, were tested. The bioactive multifunctional nanofibrous wound dressing system was revealed to promote tissue regeneration, re-epithelialization and collagen accumulation. Besides that, and according to the observed expression of VEGF, the angiogenic response was highly stimulated as well. The observations highlighted that the typical inflammatory process in diabetic wounds was also inhibited [28].
On the other hand, according to an in vitro scratch-based assay, the application of O. vulgare essential oil (25 μg/mL) promoted cell migration after 72 h [29]. Additionally, investigators stimulated keratinocytes with IFN-γ and histamine to induce ROS generation. They found that ROS levels were significantly reduced, along with the levels of inflammation and of the matrix metalloproteinase biomarkers [29]. Similarly, in another work, pre-inflamed human dermal fibroblasts were treated with O. vulgare essential oil, showing to inhibit both inflammatory and tissue remodeling biomarkers. In the end, authors suggested that the tested carvacrol-rich essential oil is a potential ingredient for skin-related products with anti-inflammatory activity [30]. Interestingly, carvacrol and thymol, phenolic monoterpenes abundant in the essential oils of plants from Origanum sp. and Thymus sp. genera, have demonstrated beneficial wound healing properties. According to Costa et al. [31], these monoterpenes act in the three distinct phases of the wound healing process: reducing inflammation, excessive ROS production, and infection, followed by the enhancement of angiogenesis, re-epithelialization, and tissue remodeling with final collagen synthesis along with the proliferation of dermal fibroblasts and epidermal keratinocytes.
4. Prunella vulgaris L.
4.1. Ethnobotanical Uses and General Considerations
According to its ethnopharmacological uses, P. vulgaris (Figure 1D) is used around the world to treat several skin conditions [32]. In the Iberian Peninsula, its traditional topical application is mostly associated with its antiseptic activity, with flowers used in infusions or decoctions for washes and baths [24][33][34]. Vulnerary and healing properties are equally attributed to this plant species, being topically applied as a cataplasm or in baths for wounds healing [24][35].
4.2. Phytochemical Background
Triterpenoids is the major and most important phytochemical group in P. vulgaris. They are skeletons of 30 atoms of carbon divided in three main types, oleanane, ursane, and lupane, and they may be present in the free, ester, or glycosylated form. In P. vulgaris, oleanolic acid and ursolic acid are pointed as the main triterpenoids (Figure 2) [36][37]. Steroids are also present and are mainly represented by phytosterols and their derived saponins, such as sitosterol and stigmasterol [37]. Other sterols have also been identified, such as daucosterol and α-spinasterol [36]. Besides triterpenes and sterols, a wide range of flavonoids have been equally identified, such as homoorientin, wogonin, quercetin-3-O-β-D-rhamnoside, kaempferol-3-O-β-D-glucoside, hesperidin, and acacetin-7-O-β-D-glucopyranoside [36]. Considering phenolic-derived compounds, P. vulgaris is rich in coumarins such as umbelliferone, scopoletin, and esculetin, and in phenolic acids such as rosmarinic, caffeic, and ellagic acids, the most important given the several associated pharmacological activities [36][37][38].
4.3. Pharmacological Evidence
Recently, the wound healing activity of the P. vulgaris was assessed in an in vivo bioactivity-guided fractionation assay from its methanolic extract. Extracts at 1% were incorporated in ointment formulations and topically applied on wound models (incision and circular excision). Findings showed that, in the incisional wound, the ethyl acetate extract increased 39.3% of the tensile strength of the wound, while in the excisional wound a wound contraction of around 86.3% was observed after 12 days. Six bioactive compounds (ethyl rosmarinate, methyl arjunolate, ursolic acid, chlorogenic acid, rosmarinic acid, and methyl-3-epimaslinate) were identified in the ethyl acetate sub-extract proving to be the most effective for wound healing. Furthermore, ursolic, chlorogenic, and rosmarinic acids were shown to positively influence the anti-inflammatory and wound healing effects of P. vulgaris [32].
The thermal-induced wound healing properties of P. vulgaris were investigated in aqueous extracts. The in vivo study was performed for 14 days, and the plant-derived extract (10%) was incorporated in a cream, showing a better healing capacity for burn wounds when compared to the standard SSD® cream. According to these findings, the topical application of P. vulgaris stimulated collagen production and antioxidant efficiency by lipid peroxidation suppression, a decrease in inflammation, along with an increase in the proliferation of keratinocytes, leading ultimately to wound contraction and re-epithelialization by the 14th day [38].
5. Salvia officinalis L.
5.1. Ethnobotanical Uses and General Considerations
S. officinalis (Figure 1E), known as common sage or garden sage, is widely used as a seasoning and flavoring condiment in culinary arts. In traditional medicine, it is used for different ailments [39]. This species has, in fact, been the focus of careful scientific validation. Hence, numerous pharmacological activities have been reported such as anticancer, anti-inflammatory, antinociceptive, antioxidant, antimicrobial, antimutagenic, antidementia, hypoglycemic, and hypolipidemic [40]. The aerial parts have been used for wounds in the Iberian Peninsula, specifically through infusion used for baths and washes, to clean and enhance wound healing [41][42][43].
5.2. Phytochemical Background
More than 120 different compounds have been identified in sage’s essential oils. Furthermore, this plant species presents chemical variations according to the parts used for extraction. Linalool is the main compound in stems, α-pinene and 1,8-cineole are predominant in the flowers, while bornyl acetate, camphene, camphor, humulene, limonene, and thujone are predominant in essential oils extracted from leaves (Figure 2). Besides, alcoholic and aqueous extracts have mainly flavonoids, such as luteolin-7-glucoside, while methanolic extracts present appreciable amounts of phenolic acids, such as caffeic and 3-caffeoylquinic acids. On the other hand, the infusion of S. officinalis has rosmarinic and ellagic acids as major bioactive compounds [44].
5.3. Pharmacological Evidence
Karimbazeh et al. [45] evaluated the potential of the hydroethanolic leaf extract of S. officinalis. Firstly, a circular excision full-thickness wound was inflicted on the anterior-dorsal side of each rat to study the wound contraction ratio, period of re-epithelization, and histopathological change. Then, an incision wound was made through the skin and cutaneous muscle in the right side of depilated back. Ointments consisting of Eucerin® (25%) and Vaseline® (75%) with hydroethanolic extract of S. officinalis at 1, 3, and 5% were prepared and then topically applied once a day for 9 days. On the 10th day there was no sign of acute skin irritation in all tested animals. The highest tested concentration (5%) allowed an increase of the wound contraction and breaking strength ratio, and it also reduced the period of re-epithelialization. An increase in hydroxyproline content in dead space wounds was observed when compared to the control group, as it also promoted the formation of granulation tissue. Furthermore, S. officinalis proved to up-regulate macrophage and fibroblast distribution, increasing collagen deposition and promoting the proliferative stage of wound healing.
On the other hand, a study by Farahpour et al. [46] assessed the effect of S. officinalis’ essential oil on infected wounds. After inoculation with P. aeruginosa and S. aureus and the infliction of circular full-thickness wounds, ointments containing 2 and 4% of S. officinalis’ essential oil were applied once a day for 14 days. It showed a shortening of the inflammatory phase of the healing process once pro-inflammatory cytokines expression was reduced. Furthermore, cellular proliferation was stimulated via the upregulation of cyclin-D1 and Bcl-2 expression. By regulating FGF-2 and VEGF expressions, S. officinalis promotes neovascularization and tissue antioxidant status. In another study, Eshani et al. [47] used S. officinalis aqueous extract to synthesize ZnO/magnetite-based nanocomposites (ZnO/Mgt-NCs) that were further tested in both in vitro and in vivo experiments using infected wound models. Firstly, the in vitro assay demonstrated strong antibacterial properties when tested against Streptococcus pyogenes and P. aeruginosa. As for the in vivo assay, it revealed an improvement of the histological parameters and a decrease of the bacterial population growth on wounds treated with ZnO/Mgt-NCs. Additionally, granulation tissue, collagen density, and epithelization were improved. In turn, the development of a novel polyvinyl alcohol-based (PVA) nanofiber mat loaded with bioactive compounds from Hypericum perforatum L., S. officinalis, and T. vulgaris also revealed interesting antioxidant and antimicrobial activities, proving the potential of the innovative formulation [48].
6. Salvia rosmarinus Schleid. (Syn: Rosmarinus officinalis L.)
6.1. Ethnobotanical Uses and General Considerations
According to the most recent phylogenetic criteria, the former scientific name Rosmarinus officinalis L. is considered a synonym of the currently accepted Salvia rosmarinus Schleid. (Figure 1F), since the genus Rosmarinus was merged into the genus Salvia [49]. Several ethnobotanical surveys report the healing effect of rosemary, either for wounds or burns, in the Portuguese traditional medicine [34][42][50][51]. Likewise, in Spain, Benítez et al. [3] reports the application of its essential oil to heal skin injuries, and Aceituno [52] states the vulnerary effect of a cataplasm made with leaves, while in Navarra this plant is traditionally used for the treatment of wounds, boils, and furuncles [41].
6.2. Phytochemical Background
Since several chemotypes have been reported, most of the volatile compounds found in rosemary essential oil are monoterpenes, the most common ones being α-pinene, 1,8-cineole, borneol, limonene, and the ketones camphor and verbenone. Similarly, the sesquiterpene β-caryophyllene has been also identified [49][53][54]. Diterpenes such as rosmarol, carnosol, and carnosic acid, as well triterpenes such as ursolic and oleanolic acids have been found in appreciable amounts (Figure 2). The anti-inflammatory activity of rosemary is actually related to their synergic activity [49]. Phenolic acids such as rosmarinic and caffeic acids, and the flavonoids class (eriocitrin, luteolin 3′-O-β-D-glucuronide, hesperidin, diosmin, isoscutellarein 7-O-glucoside, hispidulin 7-O-glucoside, and genkwanin), comprise other important bioactive compounds found in the extracts of S. rosmarinus [49][54].
6.3. Pharmacological Evidence
Once it is one of the topmost validated Lamiaceae species, a monograph about this medicinal plant can be found in the European Medicines Agency [55] and two other monographs are comprised in the European Pharmacopoeia [56]. S. rosmarinus finds important applications, namely in the food industry as a food additive and preservative [53], or as an active ingredient in cosmetic formulations [49].
Regarding the upcoming scientific evidence on the wound healing effect of S. rosmarinus, a recent clinical trial was conducted with 80 primiparous women to evaluate the healing effect of a rosemary cream on episiotomy. Results of this study indicated that on the 10th day postpartum, the group of women treated twice a day with rosemary cream had a statistically higher (p < 0.001) rate of episiotomy healing when compared to the placebo group [57].
The effects of S. rosmarinus on treating diabetic wounds have also been investigated. Abu-Al-Basal [58] studied its effect in alloxan-induced diabetic mice. After the infliction of full-thickness excision wounds, a group of mice was treated with S. rosmarinus essential oil (25 μL) twice a day for 3 days, while another group was submitted to an intraperitoneal injection of aqueous rosemary extract (10%) (0.2 mL), following an evaluation for 15 days. Statistically significant positive differences (p < 0.01) were found between rosemary-derived treatments and control groups. Furthermore, the essential oil treatment was more effective than the aqueous extract exhibiting an evident amelioration of several parameters related to the wound healing process. Similarly, Umasankar et al. [59] also confirmed the positive role of the essential oil of S. rosmarinus for wound closure of both streptozotocin-induced diabetic rats and non-diabetic rats. It is worth mentioning that a rosemary extract (5%) was also tested on full-thickness wounds inflicted on rabbits and the effect compared with povidone-iodine® and isotonic saline solution [60].
In another interesting study, the wound healing effect of the essential oil of S. rosmarinus and Melaleuca alternifolia Cheel, in combination or separated, in chitosan-loaded formulations, were evaluated in vivo on excision wounds inflicted on rats. It was observed that the most successful chitosan-derived formulation was the one loaded with the combination of both essential oils, presenting a full re-epithelization with activated hair follicles, comparable to the positive control [61]. For instance, Khezri et al. [62] focused on encapsulated essential oil into nanostructured lipid carriers (EO-NLCs). Accordingly, gels with EO-NLCs and with only essential oil were prepared and applied to heal full-thickness wounds infected with S. aureus and P. aeruginosa. Results showed that EO-NLCs had antibacterial activity while promoting in vivo wound closure, angiogenesis, fibroblast infiltration, re-epithelialization, and collagen production. Moreover, an increase in key wound healing cytokines reduced inflammation and edema, while an increase in serum levels of VEGF may explain the observed positive angiogenic response [62]. Recently, Gavan et al. [63] aimed to develop carbomer-based hydrogel wound dressings containing ethanolic extracts of S. rosmarinus and two other plants (Achillea millefolium L. and Calendula officinalis L.). The S. rosmarinus loaded hydrogel was a promising formulation for wound healing therapy, similar to the hydrogel loaded with the mixture of the three studied extracts [63].
The healing effect of rosemary on burn wounds has also been tested by other authors [64][65][66][67]. Regarding the in vivo thermal-induced wounds, the activity of two different ointments prepared with S. rosmarinus and Populus alba L. essential oils was studied. Results at the end of 25 days of study showed S. rosmarinus’ ointment to have a bigger wound contraction of 4.44 ± 0.07 cm2 compared to only 1.06 ± 0.44 cm2 for the Madecassol® control group. Rosemary essential oil did not show acute toxicity and this report also suggests a significant healing activity during the proliferative phase of the wound healing process [65]. Recently, Khalil et al. [66] revealed the potential of an acetonic extract of S. rosmarinus against multidrug resistant pathogens that frequently infect burn wounds.
An Eucerin®-based cream containing rosemary’s essential oil at 2 and 4% was tested on excision wounds infected by Candida albicans. The 4% preparation yielded the best results compared to the other groups with a remarkable decrease in the infection and inflammation parameters. It also improved fibroblast proliferation, leading to complete wound contraction on the 16th day of the experiment [68]. The effect of Eucerin®-based formulations with rosemary extracts at different concentrations (15, 10 and 5%) was also evaluated on excision wounds of rats [69], as well as the effect of rosemary’s essential oil regarding the healing of skin lesions of mice [70]. In turn, one study evaluated in Wistar albino rats after plastic surgery reported the potential of rosemary’s essential oil to increase skin flaps survival, encompassing the avoidance of necrosis [71].
7. Salvia verbenaca L.
7.1. Ethnobotanical Uses and General Considerations
S. verbenaca (Figure 1G) is a widespread Salvia species occurring in the Mediterranean region but also around Europe and in Asia [72]. This species exhibits various bioactive properties such as antibacterial, anticancer, antioxidant, antileishmanial, antidiabetic, immunomodulatory, and wound healing [72].
In Morocco, S. verbenaca is topically applied for burns healing and abscesses [73]. Regarding the Iberian traditional medicine, in Sierra Norte de Madrid (Spain), the leaves of S. verbenaca are macerated and prepared in olive oil as a vulnerary treatment for burns and wounds [52]. Benítez et al. [3] reported that in Western Granada (Spain), a decoction of the whole plant is used to treat skin injuries. Meanwhile, in the Ripollès district, Catalonia (Spain), the leaves are externally applied as an ointment to relieve pain and stimulate burns healing, thus functioning as an antipyrotic agent [24].
7.2. Phytochemical Background
The chemical constitution of S. verbenaca has been analyzed from several plant organs, in both wild and cultivated plants, revealing mainly flavonoids, terpenoids, phenolic acids, alkaloids [72], and fatty acids [73]. The terpenoids identified in S. verbenaca’s essential oil are mostly α-pinene, β-pinene, sabinene, 1,8-cineole, β-phellandrene, linalool, p-cymene, linalyl acetate, (E)-β-ocimene, (Z)-β-ocimene, tricyclene, camphor, among many others. For instance, the phenolic acids identified in methanolic extracts were p-hydroxybenzoic acid, vanillic acid, rosmarinic acid, p-coumaric acid, caffeic acid, and phenolic diterpenes. Similarly, carnosol, carnosic acid, and methyl carnosate have also been identified (Figure 2) [72]. As for the main unsaturated fatty acids, linolenic and linoleic acids were assigned [73].
7.3. Pharmacological Evidence
Guaoguao et al. [74] explored the effect of S. verbenaca extracts for the healing of second-degree burn injuries, in an SSD® controlled study. It revealed that on the 9th day of the experiment, the skin injury areas were 29.17% for the cream base treatment, 44.34%, 47.55%, and 49.16% for the hexane, ethyl acetate, and n-butanol extracts, respectively, and 41.09% for the positive control SSD®. Results showed that the healing process was accelerated in rats submitted to the application of extract loaded creams. Noteworthy, the n-butanol extract was even more efficient than the standard SSD® treatment. Following these experiments, Guaoguao et al. [75] assessed the safety of a hydroalcoholic extract obtained from the aerial parts of S. verbenaca that was successively fractionated with hexane, ethyl acetate, and n-butanol. Crude residues were massaged in the shaved rat’s healthy skin. After 14 days, animals were healthy and had no skin injuries, thus proving the absence of dermal toxicity or inflammation after the topical application of the extract.
In another interesting study, Righi et al. [76] evaluated the in vivo antiphlogistic potential of an hydromethanolic extract obtained from the aerial parts of this same Salvia species using a xylene-induced edema model. Mice treated with S. verbenaca extract reduced the weight increase caused by xylene, especially at 600 mg/kg of body weight that afforded 50% of edema inhibition. This concentration was as effective as the reference anti-inflammatory agents, such as indomethacin and dexamethasone, non-steroidal and steroidal anti-inflammatory drugs, respectively.
8. Thymus vulgaris L.
8.1. Ethnobotanical Uses and General Considerations
T. vulgaris (Figure 1H), commonly known as thyme or garden thyme, is known for having an extensive array of pharmacological properties [1], also with important applications in culinary, perfumery, and cosmetics fields [77]. From this perspective, several pharmacological activities are reported, namely antibacterial, antioxidant, anti-inflammatory, antiviral, and anti-cancerous [77].
From the Iberian ethnomedicinal perspective of this thyme species, several reports have mentioned its potential application for wound healing. Firstly, a decoction of the aerial parts is referred to be used in the region of High River Ter Valley, Catalonia [78], as a vulnerary for the treatment of wounds. In Alt Empordà, Catalonia, Parada et al. [33] also documented the topical application of the aerial part of this species for the treatment of wounds. For instance, Cavero et al. [41] reported that in Navarra, the decoction of the aerial parts, is used to clean wounds and prepare ointments with wax, honey, and olive oil. The infusion of the aerial parts at the flowering stage is also reported to be used in the form of baths for wound healing [24][41].
8.2. Phytochemical Background
In T. vulgaris, different classes of compounds can be found such as phenolics, terpenoids, flavonoids, steroids, alkaloids, tannins, and saponins. Phenolic compounds are the ones with more pharmacological significance, and among them, rosmarinic, caffeic, p-coumaric, geranic, p-hydroxybenzoic, gentisic, syringic, and ferulic acids have been highlighted. Thyme’s essential oil is mostly rich in thymol and carvacrol, but it also has geraniol, linalool, α- and β-pinene, p-cymene, and γ-terpinene (Figure 2). Apigenin, luteolin, cirsimaritin, genkwanin, and xanthomicrol are some of the identified flavonoids [77].
8.3. Pharmacological Evidence
Regarding Mekkaoui et al. [64], T. vulgaris honey was mixed separately with the essential oils of O. vulgare, S. rosmarinus, and T. vulgaris, each at 0.5%. Interestingly, the honey containing T. vulgaris’ essential oil provided the best outcomes in this study with wound closure rates for thermal and chemical-inflected burns of 85.21% and 82.14%, respectively. Furthermore, this treatment provided the shortest healing period of 14 days with a great potential to treat burn wounds compared to Madecassol® or honey-alone treated animals. Notwithstanding, in a previous in vivo study, nitric oxide (NO), which was shown to be overproduced in thermal-induced wounds, progressively decreased during healing. However, in this investigation, thyme’s essential oil was shown to enhance the reduction of NO levels, presenting comparable results to conventional drugs used in burns management, such as SSD®. Rats treated with thyme’s essential oil also showed better results regarding the formation of new tissue [79].
In the Panah et al. [80] study, circular wounds were inflected on rats and after 21 days, results showed that the animals treated with ointments bearing thyme’s essential oil had a statistically significant better distribution of fibroblasts and macrophages, besides promoting angiogenesis and collagen deposition, when compared to the control group treated with Eucerin® (25%) and Vaseline® (75%). Similarly, the daily application of an ointment containing an ethanolic extract of T. vulgaris also afforded potent wound closure [81].
Given the substantial evidence showing the beneficial effects of T. vulgaris as a promising wound healing natural product, numerous studies have focused on developing innovative wound dressing systems. As an example, thyme’s essential oil was encapsulated in sodium caseinate (Na CAS) nanomicelles resulting in a gelatin nanocomposite hydrogel formulation. Interestingly, this study proved that this delivering system not only promotes wound closure, but also reduces the inflammatory factor IL-6, while encompassing an increase of VEGF and transforming growth factor-β1. Furthermore, an appreciable antibacterial activity was equally found with this hydrogel disrupting bacterial membranes followed by alkaline phosphatase leakage [82]. Another relevant study has led to the creation of an electrospun zein/thyme essential oil (TEO) nanofibrous membrane aiming to overcome some drawbacks of the conventional electrospun fibrous wound dressings, such as the lack of adjustment when topically applied on irregular wounds [83].
Chitosan films loaded with T. vulgaris’ essential oil at 1.2% also showed suitable antioxidant activity given the high amounts of carvacrol, and antimicrobial activity against some bacterial strains such as E. coli, Klebsiella pneumoniae, P. aeruginosa, and gram positive S. aureus [84]. An antimicrobial wound dressing system of chitosan/Poly(ethylene oxide) nanofiber loaded with T. vulgaris’ extract, synthetized through electrospinning, was equally afforded [85]. Similarly, the extract of T. vulgaris loaded into chitosan, eggshell membrane, and soluble eggshell membrane film were also attempted [86], and Poly(vinyl alcohol)-based nanofiber mats were also loaded with T. vulgaris hydroalcoholic extract [48].
References
- Mssillou, I.; Bakour, M.; Slighoua, M.; Laaroussi, H.; Saghrouchni, H.; Ez-Zahra Amrati, F.; Lyoussi, B.; Derwich, E. Investigation on Wound Healing Effect of Mediterranean Medicinal Plants and Some Related Phenolic Compounds: A Review. J. Ethnopharmacol. 2022, 298, 115663.
- Baali, F.; Boumerfeg, S.; Boudjelal, A.; Denaro, M.; Ginestra, G.; Baghiani, A.; Righi, N.; Deghima, A.; Benbacha, F.; Smeriglio, A.; et al. Wound-Healing Activity of Algerian Lavandula stoechas and Mentha pulegium Extracts: From Traditional Use to Scientific Validation. Plant Biosyst. 2022, 156, 427–439.
- Benítez, G.; González-Tejero, M.R.; Molero-Mesa, J. Pharmaceutical Ethnobotany in the Western Part of Granada Province (Southern Spain): Ethnopharmacological Synthesis. J. Ethnopharmacol. 2010, 129, 87–105.
- Ez zoubi, Y.; Bousta, D.; Farah, A. A Phytopharmacological Review of a Mediterranean Plant: Lavandula stoechas L. Clin. Phytosci. 2020, 6, 9.
- Boukhatem, M.N.; Chader, H.; Houche, A.; Oudjida, F.; Benkebaili, F.; Hakim, Y. Topical Emulsion Containing Lavandula stoechas Essential Oil as a Therapeutic Agent for Cutaneous Wound Healing. J 2021, 4, 288–307.
- Vakilian, K.; Atarha, M.; Bekhradi, R.; Chaman, R. Healing Advantages of Lavender Essential Oil during Episiotomy Recovery: A Clinical Trial. Complement. Ther. Clin. Pract. 2011, 17, 50–53.
- Sheikhan, F.; Jahdi, F.; Khoei, E.M.; Shamsalizadeh, N.; Sheikhan, M.; Haghani, H. Episiotomy Pain Relief: Use of Lavender Oil Essence in Primiparous Iranian Women. Complement. Ther. Clin. Pract. 2012, 18, 66–70.
- Marzouk, T.; Barakat, R.; Ragab, A.; Badria, F.; Badawy, A. Lavender-Thymol as a New Topical Aromatherapy Preparation for Episiotomy: A Randomised Clinical Trial. J. Obstet. Gynaecol. 2015, 35, 472–475.
- Abedian, S.; Abedi, P.; Jahanfar, S.; Iravani, M.; Zahedian, M. The Effect of Lavender on Pain and Healing of Episiotomy: A Systematic Review. Complement. Ther. Med. 2020, 53, 102510.
- Panahi, Y.; Beiraghdar, F.; Akbari, H.; Bekhradi, H.; Taghizadeh, M.; Sahebkar, A. A Herbal Cream Consisting of Aloe vera, Lavandula stoechas, and Pelargonium roseum as an Alternative for Silver Sulfadiazine in Burn Management. Asian Biomed. 2012, 6, 273–278.
- Addis, R.; Cruciani, S.; Santaniello, S.; Bellu, E.; Sarais, G.; Ventura, C.; Maioli, M.; Pintore, G. Fibroblast Proliferation and Migration in Wound Healing by Phytochemicals: Evidence for a Novel Synergic Outcome. Int. J. Med. Sci. 2020, 17, 1030–1042.
- Mahmoudi, R.; Aghaei, S.; Salehpour, Z.; Mousavizadeh, A.; Khoramrooz, S.S.; Taheripour Sisakht, M.; Christiansen, G.; Baneshi, M.; Karimi, B.; Bardania, H. Antibacterial and Antioxidant Properties of Phyto-Synthesized Silver Nanoparticles Using Lavandula stoechas Extract. Appl. Organomet. Chem. 2020, 34, 1–9.
- Sequeira, R.S.; Miguel, S.P.; Cabral, C.S.D.; Moreira, A.F.; Ferreira, P.; Correia, I.J. Development of a Poly(Vinyl Alcohol)/Lysine Electrospun Membrane-Based Drug Delivery System for Improved Skin Regeneration. Int. J. Pharm. 2019, 570, 118640.
- Aćimović, M.; Jeremić, K.; Salaj, N.; Gavarić, N.; Kiprovski, B.; Sikora, V.; Zeremski, T. Marrubium vulgare L.: A Phytochemical and Pharmacological Overview. Molecules 2020, 25, 2898.
- González, J.A.; García-Barriuso, M.; Amich, F. Ethnobotanical Study of Medicinal Plants Traditionally Used in the Arribes Del Duero, Western Spain. J. Ethnopharmacol. 2010, 131, 343–355.
- Mssillou, I.; Agour, A.; Slighoua, M.; Chebaibi, M.; Amrati, F.E.Z.; Alshawwa, S.Z.; Al Kamaly, O.; El Moussaoui, A.; Lyoussi, B.; Derwich, E. Ointment-Based Combination of Dittrichia viscosa L. and Marrubium vulgare L. Accelerate Burn Wound Healing. Pharmaceuticals 2022, 15, 289.
- Ajjoun, M.; Kharchoufa, L.; Alami Merrouni, I.; Elachouri, M. Moroccan Medicinal Plants Traditionally Used for the Treatment of Skin Diseases: From Ethnobotany to Clinical Trials. J. Ethnopharmacol. 2022, 297, 115532.
- Amri, B.; Martino, E.; Vitulo, F.; Corana, F.; Ben-Kaâb, L.B.; Rui, M.; Rossi, D.; Mori, M.; Rossi, S.; Collina, S. Marrubium vulgare L. Leave Extract: Phytochemical Composition, Antioxidant and Wound Healing Properties. Molecules 2017, 22, 1851.
- Yahiaoui, F.; Zaouani, M.; Kardjadj, M.; Laghouati, A.; Kadour, R.; Bouzid, N.B.; Mahdi, M.H. Antibacterial, Antioxidant and Wound Healing Activities of Marrubium vulgare and Cytisus triflorus Extracts Native to Algeria. Phytoth’erapie 2018, 16, S32–S39.
- De Souza, M.M.; De Jesus, R.A.P.; Cechinel-Filho, V.; Schlemper, V. Analgesic Profile of Hydroalcoholic Extract Obtained from Marrubium vulgare. Phytomedicine 1998, 5, 103–107.
- Ghedadba, N.; Hambaba, L.; Fercha, N.; Houas, B.; Abdessemed, S.; Mokhtar, S.M.O. Assessment of Hemostatic Activity of the Aqueous Extract of Leaves of Marrubium vulgare L., a Mediterranean Lamiaceae Algeria. J. Health Sci. 2016, 2, 253–258.
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.M.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653.
- Lordani, T.V.A.; De Lara, C.E.; Ferreira, F.B.P.; De Souza Terron Monich, M.; Da Silva, C.M.; Lordani, C.R.F.; Bueno, F.G.; Teixeira, J.J.V.; Lonardoni, M.V.C. Therapeutic Effects of Medicinal Plants on Cutaneous Wound Healing in Humans: A Systematic Review. Mediat. Inflamm. 2018, 2018, 7354250.
- Rigat, M.; Vallès, J.; Dambrosio, U.; Gras, A.; Iglésias, J.; Garnatje, T. Plants with Topical Uses in the Ripollès District (Pyrenees, Catalonia, Iberian Peninsula): Ethnobotanical Survey and Pharmacological Validation in the Literature. J. Ethnopharmacol. 2015, 164, 162–179.
- Jennifer Ragi, M.D.; Amy Pappert, M.D.; Babar Rao, M.D. Oregano Extract Ointment for Wound Healing: A Randomized, Double-Blind, Petrolatum-Controlled Study Evaluating Efficacy. J. Drugs Dermatol. 2011, 10, 1168–1172.
- Khan, A.u.R.; Huang, K.; Jinzhong, Z.; Zhu, T.; Morsi, Y.; Aldalbahi, A.; El-Newehy, M.; Yan, X.; Mo, X. PLCL/Silk Fibroin Based Antibacterial Nano Wound Dressing Encapsulating Oregano Essential Oil: Fabrication, Characterization and Biological Evaluation. Colloids Surfaces B Biointerfaces 2020, 196, 111352.
- Sankar, R.; Dhivya, R.; Shivashangari, K.S.; Ravikumar, V. Wound Healing Activity of Origanum vulgare Engineered Titanium Dioxide Nanoparticles in Wistar Albino Rats. J. Mater. Sci. Mater. Med. 2014, 25, 1701–1708.
- Khan, A.U.R.; Huang, K.; Khalaji, M.S.; Yu, F.; Xie, X.; Zhu, T.; Morsi, Y.; Jinzhong, Z.; Mo, X. Multifunctional Bioactive Core-Shell Electrospun Membrane Capable to Terminate Inflammatory Cycle and Promote Angiogenesis in Diabetic Wound. Bioact. Mater. 2021, 6, 2783–2800.
- Avola, R.; Granata, G.; Geraci, C.; Napoli, E.; Graziano, A.C.E.; Cardile, V. Oregano (Origanum vulgare L.) Essential Oil Provides Anti-Inflammatory Activity and Facilitates Wound Healing in a Human Keratinocytes Cell Model. Food Chem. Toxicol. 2020, 144, 111586.
- Han, X.; Parker, T.L. Anti-Inflammatory, Tissue Remodeling, Immunomodulatory, and Anticancer Activities of Oregano (Origanum vulgare) Essential Oil in a Human Skin Disease Model. Biochim. Open 2017, 4, 73–77.
- Costa, M.F.; Durço, A.O.; Rabelo, T.K.; Barreto, R.d.S.S.; Guimarães, A.G. Effects of Carvacrol, Thymol and Essential Oils Containing Such Monoterpenes on Wound Healing: A Systematic Review. J. Pharm. Pharmacol. 2019, 71, 141–155.
- Küpeli Akkol, E.; Renda, G.; İlhan, M.; Bektaş, N.Y. Wound Healing Acceleration and Anti-Inflammatory Potential of Prunella vulgaris L.: From Conventional Use to Preclinical Scientific Verification. J. Ethnopharmacol. 2022, 295, 115411.
- Parada, M.; Carrió, E.; Bonet, M.À.; Vallès, J. Ethnobotany of the Alt Empordà Region (Catalonia, Iberian Peninsula). Plants Used in Human Traditional Medicine. J. Ethnopharmacol. 2009, 124, 609–618.
- Vinagre, C.; Vinagre, S.; Carrilho, E. The Use of Medicinal Plants by the Population from the Protected Landscape of “Serra de Montejunto”, Portugal. J. Ethnobiol. Ethnomed. 2019, 15, 1–30.
- Rodrigues, J. Contributo Para o Estudo Etnobotânico Das Plantas Medicinais e Aromáticas Na Area Protegida Da Serra Do Açor. Appsa, Icn. 2002. Available online: http://www.etnobotanica.uevora.pt/2002%20Joana%20CRodrigues%20PAM%20Serra%20do%20Acor%20APPSA.pdf (accessed on 17 July 2022).
- Bai, Y.; Xia, B.; Xie, W.; Zhou, Y.; Xie, J.; Li, H.; Liao, D.; Lin, L.; Li, C. Phytochemistry and Pharmacological Activities of the Genus Prunella. Food Chem. 2016, 204, 483–496.
- Pan, J.; Wang, H.; Chen, Y. Prunella vulgaris L.—A Review of Its Ethnopharmacology, Phytochemistry, Quality Control and Pharmacological Effects. Front. Pharmacol. 2022, 13, 903171.
- Li, C.C.; Meng, Q.G.; Liang, X.Q.; Xiao, J.Z. The Enhanced Epithelialization Potential of the Aqueous Extract of Prunella vulgaris in Thermally Injured Rats. Arch. Biol. Sci. 2016, 68, 225–235.
- Khiya, Z.; Hayani, M.; Gamar, A.; Kharchouf, S.; Amine, S.; Berrekhis, F.; Bouzoubae, A.; Zair, T.; El Hilali, F. Valorization of the Salvia officinalis L. of the Morocco Bioactive Extracts: Phytochemistry, Antioxidant Activity and Corrosion Inhibition. J. King Saud Univ. -Sci. 2019, 31, 322–335.
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological Properties of Salvia officinalis and Its Components. J. Tradit. Complement. Med. 2017, 7, 433–440.
- Cavero, R.Y.; Akerreta, S.; Calvo, M.I. Medicinal Plants Used for Dermatological Affections in Navarra and Their Pharmacological Validation. J. Ethnopharmacol. 2013, 149, 533–542.
- Mendonça de Carvalho, L.M. Estudos de Etnobotânica e Botânica Económica No Alentejo. 2006. Available online: https://estudogeral.uc.pt/handle/10316/2078 (accessed on 12 July 2022).
- Calvo, M.I.; Akerreta, S.; Cavero, R.Y. Pharmaceutical Ethnobotany in the Riverside of Navarra (Iberian Peninsula). J. Ethnopharmacol. 2011, 135, 22–33.
- Vitale, S.; Colanero, S.; Placidi, M.; Emidio, G.D.; Tatone, C.; Amicarelli, F.; Alessandro, A.M.D. Phytochemistry and Biological Activity of Medicinal Plants in Wound Healing: An Overview of Current Research. Molecules 2022, 27, 3566.
- Karimzadeh, S.; Farahpour, M.R. Topical Application of Salvia officinalis Hydroethanolic Leaf Extract Improves Wound Healing Process. Indian J. Exp. Biol. 2017, 55, 98–106.
- Farahpour, M.R.; Pirkhezr, E.; Ashrafian, A.; Sonboli, A. Accelerated Healing by Topical Administration of Salvia officinalis Essential Oil on Pseudomonas aeruginosa and Staphylococcus aureus Infected Wound Model. Biomed. Pharmacother. 2020, 128, 110120.
- Ehsani, P.; Farahpour, M.R.; Mohammadi, M.; Mahmazi, S.; Jafarirad, S. Green Fabrication of ZnO/Magnetite-Based Nanocomposite—Using Salvia officinalis Extract with Antibacterial Properties Enhanced Infected Full-Thickness Wound. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 628, 127362.
- Serbezeanu, D.; Bargan, A.; Homocianu, M.; Aflori, M.; Rîmbu, C.M.; Enache, A.A.; Vlad-Bubulac, T. Electrospun Polyvinyl Alcohol Loaded with Phytotherapeutic Agents for Wound Healing Applications. Nanomaterials 2021, 11, 3336.
- de Macedo, L.M.; Santos É, M.D.; Militão, L.; Tundisi, L.L.; Ataide, J.A.; Souto, E.B.; Mazzola, P.G. Rosemary (Rosmarinus officinalis L., Syn Salvia rosmarinus Spenn.) and its topical applications: A review. Plants 2020, 9, 651.
- Neves, J.M.; Matos, C.; Moutinho, C.; Queiroz, G.; Gomes, L.R. Ethnopharmacological Notes about Ancient Uses of Medicinal Plants in Trás-Os-Montes (Northern of Portugal). J. Ethnopharmacol. 2009, 124, 270–283.
- Carvalho, A.M.P. Etnobotánica Del Parque Natural de Montesinho. Plantas, Tradición y Saber Popular En Un Territorio Del Nordeste de Portugal; Instituto Politecnico de Braganca: Bragança, Portugal, 2005.
- Aceituno, L.M. Estudio Etnobotánico y Agroecológico de La Sierra Norte de Madrid; Universidad Autónoma de Madrid: Madrid, Spain, 2010.
- Ribeiro-Santos, R.; Carvalho-Costa, D.; Cavaleiro, C.; Costa, H.S.; Albuquerque, T.G.; Castilho, M.C.; Ramos, F.; Melo, N.R.; Sanches-Silva, A. A Novel Insight on an Ancient Aromatic Plant: The Rosemary (Rosmarinus officinalis L.). Trends Food Sci. Technol. 2015, 45, 355–368.
- González-Minero, F.J.; Bravo-Díaz, L.; Ayala-Gómez, A. Rosmarinus officinalis L. (Rosemary): An Ancient Plant with Uses in Personal Healthcare and Cosmetic. Cosmetics 2020, 7, 77.
- European Medicines Agency Community Herbal Monograph on Rosmarinus officinalis L., Aetheroleum. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal__Community_herbal_monograph/2011/01/WC500101493.pdf (accessed on 5 November 2022).
- European Directorate for the Quality of Medicines. European Pharmacopeia, 5th ed.; Council of Europe: Strasbourg, France, 2005.
- Hadizadeh-Talasaz, F.; Mardani, F.; Bahri, N.; Rakhshandeh, H.; Khajavian, N.; Taghieh, M. Effect of Rosemary Cream on Episiotomy Wound Healing in Primiparous Women: A Randomized Clinical Trial. BMC Complement. Med. Ther. 2022, 22, 1–10.
- Abu-Al-Basal, M.A. Healing Potential of Rosmarinus Officinalis L. on Full-Thickness Excision Cutaneous Wounds in Alloxan-Induced-Diabetic BALB/c Mice. J. Ethnopharmacol. 2010, 131, 443–450.
- Umasankar, K.; Nambikkairaj, B.; Manley Backyavathy, D. Effect of Topical Treatment of Rosmarinus officinalis Essential Oil on Wound Healing in Streptozotocin Induced Diabetic Rats. Nat. Environ. Pollut. Technol. 2012, 11, 607–611.
- Yilmaz, R.; Özyildiz, Z.; Temmaoğullari, F.; Hayat, A. The Effects of Rosemary (Rosemarinus officinalis) Extract On Wound Healing in Rabbits. Fırat Üniversitesi Sağ Bil Vet Dergisi 2012, 26, 105–109.
- Labib, R.M.; Ayoub, I.M.; Michel, H.E.; Mehanny, M.; Kamil, V.; Hany, M.; Magdy, M.; Moataz, A.; Maged, B.; Mohamed, A. Appraisal on the Wound Healing Potential of Melaleuca alternifolia and Rosmarinus officinalis L. Essential Oil-Loaded Chitosan Topical Preparations. PLoS ONE 2019, 14, e0219561.
- Khezri, K.; Farahpour, M.R.; Mounesi Rad, S. Accelerated Infected Wound Healing by Topical Application of Encapsulated Rosemary Essential Oil into Nanostructured Lipid Carriers. Artif. Cells Nanomed. Biotechnol. 2019, 47, 980–988.
- Gavan, A.; Colobatiu, L.; Hanganu, D.; Bogdan, C.; Olah, N.K.; Achim, M.; Mirel, S. Development and Evaluation of Hydrogel Wound Dressings Loaded with Herbal Extracts. Processes 2022, 10, 242.
- Mekkaoui, M.; Assaggaf, H.; Qasem, A.; El-Shemi, A.; Abdallah, E.M.; Bouidida, E.H.; Mrabti, H.N.; Cherrah, Y.; Alaoui, K. Ethnopharmacological Survey and Comparative Study of the Healing Activity of Moroccan Thyme Honey and Its Mixture with Selected Essential Oils on Two Types of Wounds on Albino Rabbits. Foods 2022, 11, 28.
- Belkhodja, H.; Boumediene, M.; Aicha, M.T.T.; Djilali, B.; Asmaa, B. Wound Healing Activity of the Essential Oils of Rosmarinus officinalis and Populus alba in a Burn Wound Model in Rats. SAR J. Pathol. Microbiol. 2020, 1, 1–9.
- Khalil, M.A.; Abd El-Zaher, E.H.F.; El-Salam, O.A.; Ali, S.S. Exploring the Therapeutic Potential of Acetonic Plant Extracts in the Healing of Skin Wounds Infected with Multidrug Resistant Pathogens. J. Appl. Biomed. 2022, 20, 45–55.
- Farhan, A.; Alsuwayt, B.; Alanazi, F.; Yaseen, A.; Ashour, M.A. Evaluation and HPLC Characterisation of a New Herbal Ointment for the Treatment of Full-Thickness Burns in Rats. J. Taibah Univ. Med. Sci. 2021, 16, 152–161.
- Nejati, H.; Farahpour, M.R.; Nagadehi, M.N. Topical Rosemary officinalis Essential Oil Improves Wound Healing against Disseminated Candida Albicans Infection in Rat Model. Comp. Clin. Path. 2015, 24, 1377–1383.
- Alizargar, J.; Kuchaki, E.; Shaabani, A.; Namazi, M. Properties of Wound Healing Activities of Rosemary Extract. J. Biol. Act. Prod. Nat. 2012, 2, 218–224.
- de Araújo, J.T.; de Oliveira, P.F.; Sá, P.S.F.; Távora, N.P.L.; Pinheiro, A.V.; Trindade, T.B.M.; Pinto, V.H.F.; Lima, C.S.; Barcessat, A.R.P.; Pinheiro, M.T. Effect of Essential Oil of Rosmarinus officinalis L. (Rosemary) on the Healing of Cutaneous Lesions in Mice. J. Chem. Pharm. Res. 2017, 9, 381–386.
- Ince, B.; Yildirim, A.M.; Okur, M.I.; Dadaci, M.; Yoruk, E. Effects of Rosmarinus officinalis on the Survivability of Random-Patterned Skin Flaps: An Experimental Study. J. Plast. Surg. Hand Surg. 2015, 49, 83–87.
- Mrabti, H.N.; El Menyiy, N.; Charfi, S.; Saber, M.; Bakrim, S.; Alyamani, R.A.; Rauf, A.; Ali, A.M.H.; Abdallah, E.M.; El Omari, N.; et al. Phytochemistry and Biological Properties of Salvia verbenaca L.: A Comprehensive Review. BioMed Res. Int. 2022, 2022, 3787818.
- Khouchlaa, A.; Et-Touys, A.; Lakhdar, F.; Laasri, F.E.; El Idrissi, A.E.Y.; Zaakour, F. Ethnomedicinal Use, Phytochemistry, Pharmacology, and Toxicology of Salvia verbenaca L.: A Review. Biointerface Res. Appl. Chem. 2022, 12, 1437–1469.
- Guaouguaou, F.-E.; Taghzouti, K.; Oukabli, M.; Es-Safi, N.E. The Effect of Salvia verbenaca Extracts for Healing of Second-Degree Burn Wounds in Rats. Curr. Bioact. Compd. 2018, 14, 419–427.
- Guaouguaou, F.E.; Taghzouti, K.; Oukabli, M.; Masrar, A.; Chabraoui, L.; Bouabdellah, M.; Es-Safi, N.E. Acute and Subchronic Oral and Dermal Toxicological Studies of Salvia verbenaca Extracts in Mice and Rats. J. Herbs Spices Med. Plants 2019, 25, 33–42.
- Righi, N.; Boumerfeg, S.; Deghima, A.; Fernandes, P.A.R.; Coelho, E.; Baali, F.; Cardoso, S.M.; Coimbra, M.A.; Baghiani, A. Phenolic Profile, Safety Assessment, and Anti-Inflammatory Activity of Salvia verbenaca L. J. Ethnopharmacol. 2021, 272, 113940.
- Patil, S.M.; Ramu, R.; Shirahatti, P.S.; Shivamallu, C.; Amachawadi, R.G. A Systematic Review on Ethnopharmacology, Phytochemistry and Pharmacological Aspects of Thymus vulgaris Linn. Heliyon 2021, 7, e07054.
- Rigat, M.; Bonet, M.À.; Garcia, S.; Garnatje, T.; Vallès, J. Studies on Pharmaceutical Ethnobotany in the High River Ter Valley (Pyrenees, Catalonia, Iberian Peninsula). J. Ethnopharmacol. 2007, 113, 267–277.
- Dursun, N.; Liman, N.; Özyazgan, I.; Güneş, I.; Saraymen, R. Role of Thymus Oil in Burn Wound Healing. J. Burn Care Rehabil. 2003, 24, 395–399.
- Panah, K.G.; Hesaraki, S.; Farahpour, M.R. Histopathological Evaluation of Thymus vulgaris on Wound Healing. Indian J. Fundam. Appl. Life Sci. 2014, 4, 3538–3544.
- Shaban, N.S.; Tohamy, M.A.; El-Banna, H.A.; Abeer, M.R.; El-Gendy, A.A.; Asmaa, O. Phytochemical and Pharmacological Studies of Ethanolic Extract of Thymus vulgaris. World J. Pharm. Pharm. Sci. 2015, 4, 1988–2001.
- Alsakhawy, S.A.; Baghdadi, H.H.; El-Shenawy, M.A.; Sabra, S.A.; El-Hosseiny, L.S. Encapsulation of Thymus vulgaris Essential Oil in Caseinate/Gelatin Nanocomposite Hydrogel: In Vitro Antibacterial Activity and in Vivo Wound Healing Potential. Int. J. Pharm. 2022, 628, 122280.
- Liu, J.X.; Dong, W.H.; Mou, X.J.; Liu, G.S.; Huang, X.W.; Yan, X.; Zhou, C.F.; Jiang, S.; Long, Y.Z. In Situ Electrospun Zein/Thyme Essential Oil-Based Membranes as an Effective Antibacterial Wound Dressing. ACS Appl. Bio Mater. 2020, 3, 302–307.
- Altiok, D.; Altiok, E.; Tihminlioglu, F. Physical, Antibacterial and Antioxidant Properties of Chitosan Films Incorporated with Thyme Oil for Potential Wound Healing Applications. J. Mater. Sci. Mater. Med. 2010, 21, 2227–2236.
- Sadri, M.; Karimi-Nazari, E.; Hosseini, H.; Emamgholi, A. New Chitosan/Poly(Ethylene Oxide)/Thyme Nanofiber Prepared by Electrospinning Method for Antimicrobial Wound Dressing. J. Nanostruct. 2016, 6, 322–328.
- Webb, B.C.W.; Rafferty, S.; Vreugdenhil, A.J. Preparation and Characterization of Antibacterial Films with Eggshell-Membrane Biopolymers Incorporated with Chitosan and Plant Extracts. Polymers 2022, 14, 383.
More
Information
Subjects:
Dermatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.4K
Revisions:
2 times
(View History)
Update Date:
01 Mar 2023
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




