
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Szymon Graczyk | -- | 1455 | 2023-02-26 12:27:00 | | | |
| 2 | Jessie Wu | Meta information modification | 1455 | 2023-02-27 02:45:25 | | |
Video Upload Options
Stents are tubular ducts made of non-invasive materials designed to maintain the continuous flow of air through the airway lumen, or various types of fluid in the case of the urinary and circulatory systems. Stenting in veterinary medicine has been a rapidly growing method of interventional surgery. This procedure is usually performed in the respiratory and urinary tracts, but there are cases of stenting of blood vessels or gastrointestinal structures. It is based on maintaining the permeability of a given tubular structure, thus allowing the passage of gas or liquid. This procedure is often performed as a first-line treatment in situations where pharmacological agents do not work and as an alternative method, often cheaper than the classically performed ones. There are also cases where stenting is used as a palliative treatment, e.g., to enable defecation in colonic obstruction due to tumour infiltration of the colon wall. Stenting is often a life-saving or comfort-improving procedure for animals, but one should also be aware of possible postoperative complications and be prepared for any adversity.
1. Introduction
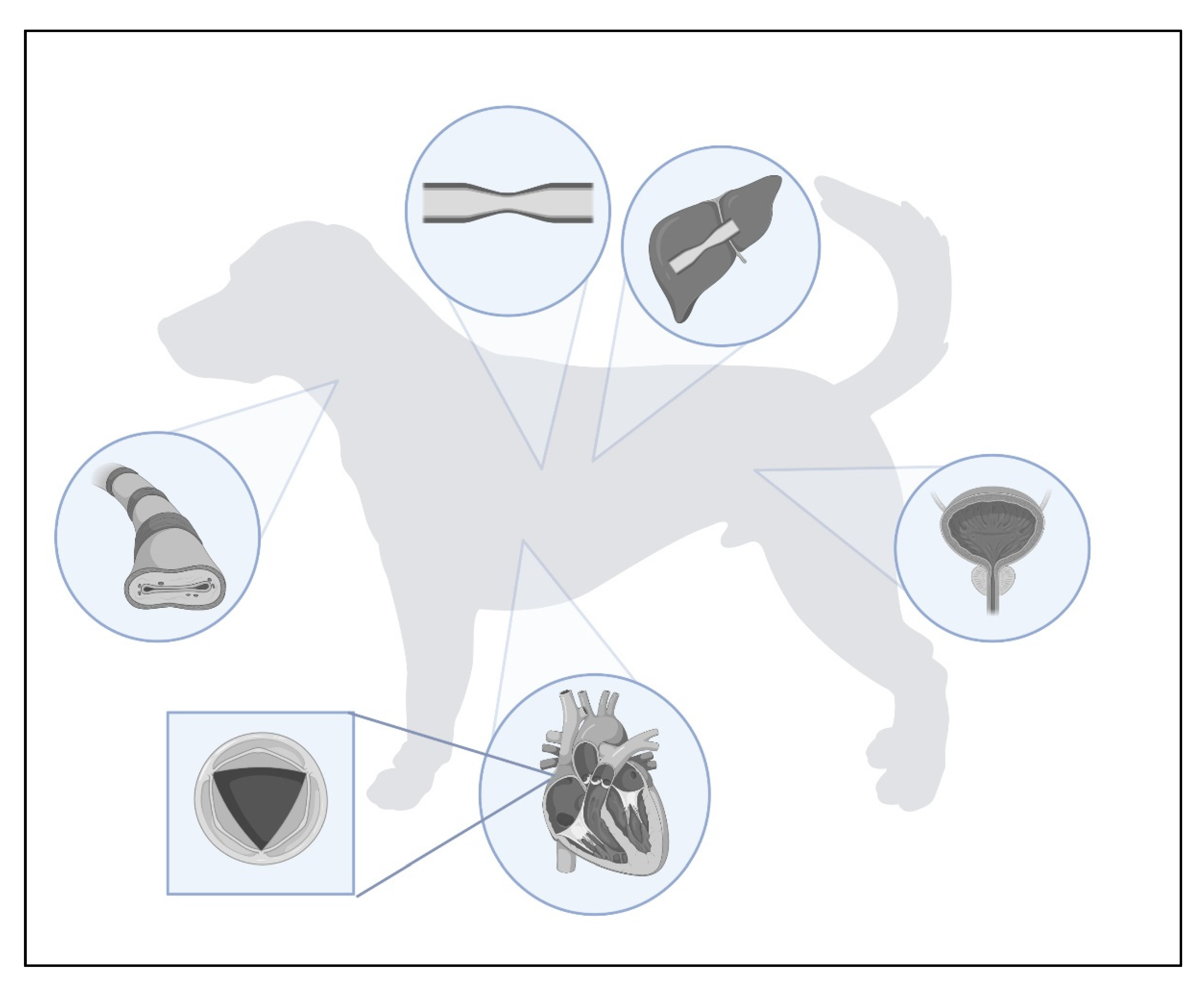
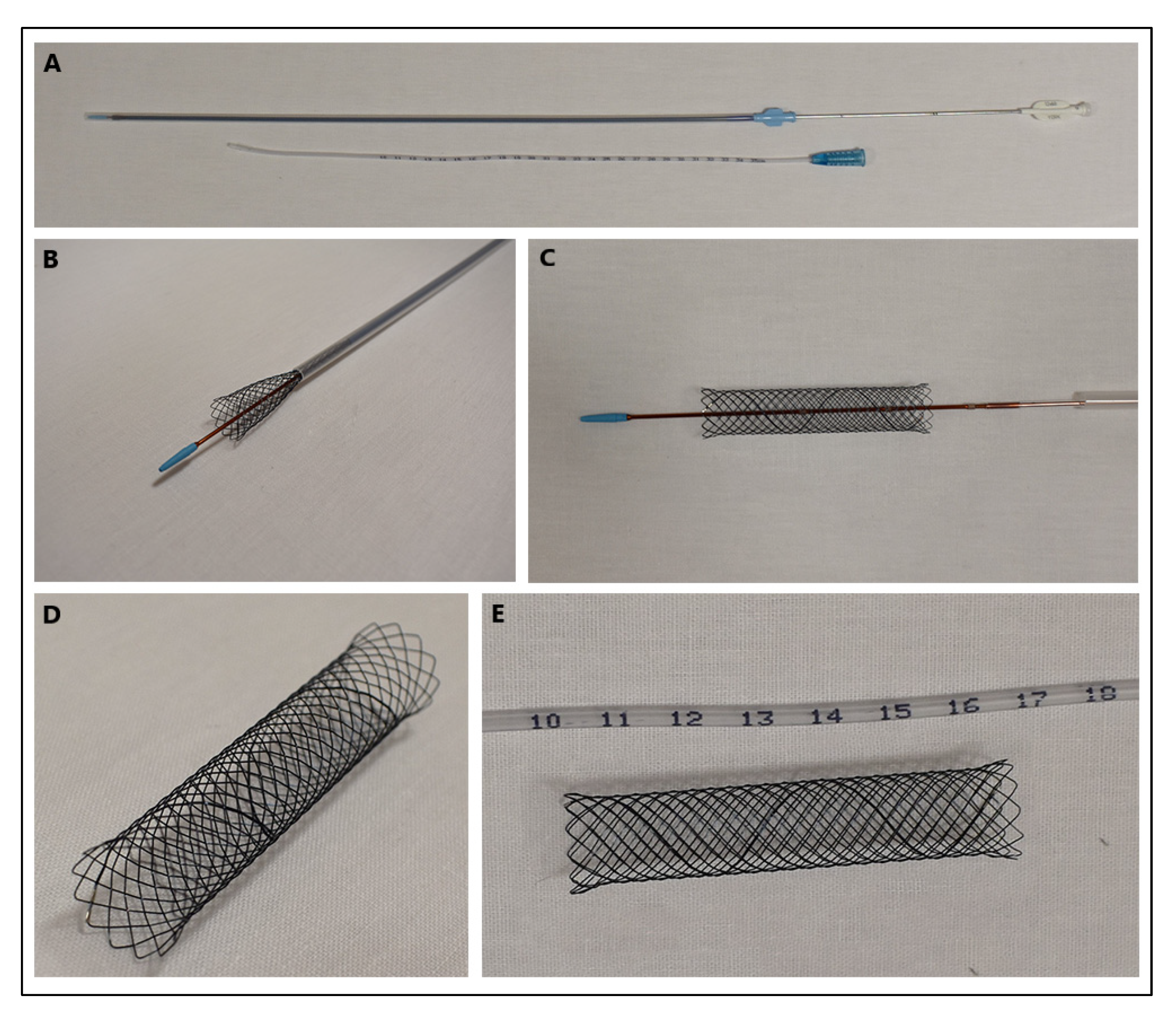
2. Types of Stents
| Type of Stent | Stent Material | Stent Placement Site | Animal | Author |
|---|---|---|---|---|
| Metallic | Nitinol | Trachea | Dog | [32] [33] [34] [35] [25] [36] [27] [37] [38] |
| Urethra | Dog | [39] [40] [41] [42] |
||
| Colon | Dog | [8] | ||
| Azygos vein | Dog | [43] | ||
| Hepatic vein | Dog | [44] | ||
| Caudal Vena Cava | Dog | [45] | ||
| Elgiloy | Trachea | Dog | [27] | |
| Biodegradable | Polylactide | Bile ducts | Dog | [13] |
| Polydioxanone | Bile ducts | Porcine | [14] | |
| Polycaprolactone | Trachea | Rabbit | [15] | |
| Polymeric | Polyethylene | Ureter | Cat | [46] [47] [48] [49] |
| Ureter | Dog | [50] [51] [6] [52] |
||
| Silicone | Medical-grade silicone | Larynx | Dog | [16] |
| Drug eluting | Paclitaxel | Coronary artery Iliac artery |
Porcine Rabbit |
[12] |
| Bile ducts | Dog | [13] | ||
| Bile ducts | Porcine | [14] | ||
| Zotarolimus | Coronary artery Iliac artery |
Porcine Rabbit |
[12] | |
| Biolimus/sirolimus | Coronary artery | Porcine | [11] | |
| Iliac artery | Rabbit | [12] | ||
| Femoral artery | Dog | [17] | ||
| Cefotaxime | Bile ducts | Dog | [19] | |
| Arsenic trioxide | Iliac artery | Rabbit | [10] | |
| Triclosan | Ureter | Rabbit | [18] |
References
- Sigwart, U.; Puel, J.; Mirkovitch, V.; Joffre, F.; Kappenberger, L. Intravascular Stents to Prevent Occlusion and Re-Stenosis after Transluminal Angioplasty. N. Eng. J. Med. 1987, 316, 701–706.
- Stone, E.A.; Withrow, S.J.; Page, R.L.; Schwarz, P.D.; Wheeler, S.L.; Iii, H.B.S. Ureterocolonlc Anastomosis in Ten Dogs with Transitional Cell Carcinoma. Vet. Surg. 1988, 17, 147–153.
- Peterson, S.L.; Smith, M.M.; Senders, C.W. Evaluation of a stented laryngoplasty for correction of cranial glottic stenosis in four dogs. J. Am. Vet. Med. Assoc. 1987, 191, 1582–1584.
- Raske, M.; Weisse, C.; Berent, A.C.; McDougall, R.; Lamb, K. Immediate, Short-, and Long-Term Changes in Tracheal Stent Diameter, Length, and Positioning after Placement in Dogs with Tracheal Collapse Syndrome. J. Vet. Intern. Med. 2018, 32, 782–791.
- Lulich, J.P. Evaluation of Temporary Urethral Stents in the Management of Malignant and Nonmalignant Urethral Diseases in Dogs. Vet. Sci. 2022, 9, 63.
- Kuntz, J.A.; Berent, A.C.; Weisse, C.W.; Bagley, D.H. Double Pigtail Ureteral Stenting and Renal Pelvic Lavage for Renal-Sparing Treatment of Obstructive Pyonephrosis in Dogs: 13 Cases (2008–2012). J. Am. Vet. Med. Assoc. 2015, 246, 216–225.
- Patata, V.; Scalise, F.; Sorropago, G.; Marchesotti, F.; Nicoli, S.; Auriemma, E.; Rondelli, V.; Pesaresi, M.; Glaus, T.M.; Baron Toaldo, M.; et al. Closure of an Unusual Morphology Patent Ductus Arteriosus with a Covered Stent in a Dog. J. Vet. Cardiol. 2020, 32, 7–15.
- Culp, W.T.N.; MacPhail, C.M.; Perry, J.A.; Jensen, T.D. Use of a Nitinol Stent to Palliate a Colorectal Neoplastic Obstruction in a Dog. J. Am. Vet. Med. Assoc. 2011, 239, 222–227.
- Balsa, I.M.; Culp, W.T.; Palm, C.A.; Hopper, K.; Hardy, B.T.; Ben-Aderet, D.G.; Mayhew, P.D.; Drobatz, K.J. Factors associated with postobstructive diuresis following decompressive surgery with placement of ureteral stents or subcutaneous ureteral bypass systems for treatment of ureteral obstruction in cats: 37 cases (2010–2014). J. Am. Vet. Med. Assoc. 2019, 254, 944–952.
- Yang, W.; Ge, J.; Liu, H.; Zhao, K.; Liu, X.; Qu, X.; Li, W.; Huang, Y.; Sun, A.; Zou, Y. Arsenic trioxide eluting stent reduces neointima formation in a rabbit iliac artery injury model. Cardiovasc. Res. 2006, 72, 483–493.
- Hagiwara, H.; Hiraishi, Y.; Terao, H.; Hirai, T.; Sakaoka, A.; Sasaki, M.; Murota, S.; Inoue, K.; Kimura, J. Vascular responses to a biodegradable polymer (polylactic acid) based biolimus A9-eluting stent in porcine models. EuroIntervention 2012, 8, 743–751.
- Nakazawa, G.; Finn, A.V.; John, M.C.; Kolodgie, F.D.; Virmani, R. The significance of preclinical evaluation of sirolimus-, paclitaxel-, and zotarolimus-eluting stents. Am. J. Cardiol. 2007, 100, 36–44.
- Shi, J.; Lv, Y.; Yu, L.; Zhang, B.; Zhang, X.; Fan, C.; Geng, Z. Interest of a New Biodegradable Stent Coated with Paclitaxel on Anastomotic Wound Healing after Biliary Reconstruction. Eur. J. Gastroenterol. Hepatol. 2013, 25, 1415–1423.
- Grolich, T.; Crha, M.; Novotný, L.; Kala, Z.; Hep, A.; Nečas, A.; Hlavsa, J.; Mitáš, L.; Misík, J. Self-Expandable Biodegradable Biliary Stents in Porcine Model. J. Surg. Res. 2015, 193, 606–612.
- Liu, K.S.; Liu, Y.H.; Peng, Y.J.; Liu, S.J. Experimental Absorbable Stent Permits Airway Remodeling. J. Thorac. Cardiovasc. Surg. 2011, 141, 463–468.
- Théron, M.L.; Lahuerta-Smith, T. Laryngeal Silicone Stent as a Treatment Option for Laryngeal Paralysis in Dogs: A Preliminary Study of 6 Cases. J. Vet. Sci. 2022, 23, e58.
- Hou, R.; Wu, L.; Wang, J.; Yang, Z.; Tu, Q.; Zhang, X.; Huang, N. Surface-Degradable Drug-Eluting Stent with Anticoagulation, Antiproliferation, and Endothelialization Functions. Biomolecules 2019, 9, 69.
- Cadieux, P.A.; Chew, B.H.; Knudsen, B.E.; DeJong, K.; Rowe, E.; Reid, G.; Denstedt, J.D. Triclosan Loaded Ureteral Stents Decrease Proteus Mirabilis 296 Infection in a Rabbit Urinary Tract Infection Model. J. Urol. 2006, 175, 2331–2335.
- Gwon, D.; Lee, S.S.; Kim, E.Y. Cefotaxime-Eluting Covered Self-Expandable Stents in a Canine Biliary Model: Scanning Electron Microscopic Study of Biofilm Formation. Acta Radiol. 2012, 53, 1127–1132.
- Stoeckel, D.; Pelton, A.; Duerig, T. Self-Expanding Nitinol Stents: Material and Design Considerations. Eur. Radiol. 2004, 14, 292–301.
- Wever, D.J.; Veldhuizen, A.G.; De Vries, J.; Busscher, H.J.; Uges, D.R.A.; Van Horn, J.R. Electrochemical and surface characterization of a nickel–titanium alloy. Biomaterials 1998, 19, 761–769.
- Iserson, K.V.J.-F.-B. Charrière: The Man behind the “French” Gauge. J. Emerg. Med. 1987, 5, 545–548.
- Stöckel, D. Nitinol-A material with unusual properties. Endovasc. Upd. 1998, 1, 1–8.
- Maleckis, K.; Anttila, E.; Aylward, P.; Poulson, W.; Desyatova, A.; MacTaggart, J.; Kamenskiy, A. Nitinol Stents in the Femoropopliteal Artery: A Mechanical Perspective on Material, Design, and Performance. Ann. Biomed. Eng. 2018, 46, 684–704.
- Woo, H.M.; Kim, M.J.; Lee, S.G.; Nam, H.S.; Kwak, H.H.; Lee, J.S.; Park, I.C.; Hyun, C. Intraluminal Tracheal Stent Fracture in a Yorkshire Terrier. Can. Vet. J. 2007, 48, 1063–1066.
- Lopez-Minguez, S.; Serrano-Casorran, C.; Guirola, J.A.; Rodriguez-Zapater, S.; Bonastre, C.; De Gregorio, M.A. New Tracheal Stainless Steel Stent Pilot Study: Twelve Month Follow-up in a Rabbit Model. PeerJ 2019, 7, e7797.
- Violette, N.P.; Weisse, C.; Berent, A.C.; Lamb, K.E. Correlations among Tracheal Dimensions, Tracheal Stent Dimensions, and Major Complications after Endoluminal Stenting of Tracheal Collapse Syndrome in Dogs. J. Vet. Intern. Med. 2019, 33, 2209–2216.
- Liatsikos, E.N.; Kallidonis, P.; Stolzenburg, J.U.; Karnabatidis, D. Ureteral Stents: Past, Present and Future. Expert Rev. Med. Devices 2009, 6, 313–324.
- Baek, J.H.; Kim, B.M.; Heo, J.H.; Kim, D.J.; Nam, H.S.; Kim, Y.D. Outcomes of Endovascular Treatment for Acute Intracranial Atherosclerosis-Related Large Vessel Occlusion. Stroke 2018, 49, 2699–2705.
- Taylor, S.; Rozanski, E.; Sato, A.F.; Rush, J.E. Vascular Stent Placement for Palliation of Mass-Associated Chylothorax in Two Dogs. J. Am. Vet. Med. Assoc. 2017, 251, 696–701.
- Lange, D.; Bidnur, S.; Hoag, N.; Chew, B.H. Ureteral stent-associated complications—Where we are and where we are going. Nat. Rev. Urol. 2015, 12, 17–25.
- Mittleman, E.; Weisse, C.; Mehler, S.J.; Lee, J.A. Fracture of an Endoluminal Nitinol Stent Used in the Treatment of Tracheal Collapse in a Dog. J. Am. Vet. Med. Assoc. 2004, 225, 1217–1221.
- Tinga, S.; Thieman Mankin, K.M.; Peycke, L.E.; Cohen, N.D. Comparison of Outcome After Use of Extra-Luminal Rings and Intra-Luminal Stents for Treatment of Tracheal Collapse in Dogs. Vet. Surg. 2015, 44, 858–865.
- Sura, P.A.; Krahwinkel, D.J. Self-Expanding Nitinol Stents for the Treatment of Tracheal Collapse in Dogs: 12 Cases (2001–2004). J. Am. Vet. Med. Assoc. 2008, 232, 228–236.
- Durant, A.M.; Sura, P.; Rohrbach, B.; Bohling, M.W. Use of Nitinol Stents for End-Stage Tracheal Collapse in Dogs. Vet. Surg. 2012, 41, 807–817.
- Yoon, H.Y.; Choi, J.W.; Kim, J.H.; Kim, J.H. Use of a Double-Wire Woven Uncovered Nitinol Stent for the Treatment of Refractory Tracheal Collapse in a Dog: A Case Report. Case Rep. Vet. Med. 2017, 62, 98–104.
- Serrano-Casorran, C.; Lopez-Minguez, S.; Rodriguez-Zapater, S.; Bonastre, C.; Guirola, J.A.; de Gregorio, M.A.A. New Airway Spiral Stent Designed to Maintain Airway Architecture with an Atraumatic Removal after Full Epithelization—Research of Feasibility and Viability in Canine Patients with Tracheomalacia. Pediatr. Pulmonol. 2020, 55, 1757–1764.
- Uemura, A.; Ozai, Y.; Hamabe, L.; Yoshida, T.; Tanaka, R. Surgical Outcomes in Dogs with Tracheal Collapse Treated with a Novel Cross-and-Hook Braided Endoluminal Stent. J. Vet. Sci. 2022, 23, e46.
- Blackburn, A.L.; Berent, A.C.; Weisse, C.W.; Brown, D.C. Evaluation of Outcome Following Urethral Stent Placement for the Treatment of Obstructive Carcinoma of the Urethra in Dogs: 42 Cases (2004–2008). J. Am. Vet. Med. Assoc. 2013, 242, 59–68.
- Radhakrishnan, A. Urethral Stenting for Obstructive Uropathy Utilizing Digital Radiography for Guidance: Feasibility and Clinical Outcome in 26 Dogs. J. Vet. Intern. Med. 2017, 31, 427–433.
- Arnold, S.; Weber, U. Urethral sphincter mechanism incompetence in male dogs. In Kirks Current Veterinary Therapy, 15th ed.; Bonagura, J.D., Twedt, D.C., Eds.; Elsevier Saunders: Philadephia, PA, USA, 2014; Volume 9, pp. 896–898.
- Weisse, C.; Berent, A.; Todd, K.; Clifford, C.; Solomon, J. Evaluation of Palliative Stenting for Management of Malignant Urethral Obstructions in Dogs. J. Am. Vet. Med. Assoc. 2006, 229, 226–234.
- Schlicksup, M.D.; Weisse, C.W.; Berent, A.C.; Solomon, J.A. Use of Endovascular Stents in Three Dogs with Budd-Chiari Syndrome. J. Am. Vet. Med. Assoc. 2009, 235, 544–550.
- Hoehne, S.N.; Milovancev, M.; Hyde, A.J.; demorais, H.A.; Scollan, K.F.; Nemanic, S. Placement of a Caudal Vena Cava Stent for Treatment of Budd-Chiari–like Syndrome in a 4-Month-Old Ragdoll Cat. J. Am. Vet. Med. Assoc. 2014, 245, 414–418.
- Monnet, E. Biliary Stenting. In Gastrointestinal Surgical Techniques in Small Animals, 1st ed.; Monnet, E., Smeak, D.D., Eds.; John Willey & Sons: Hoboken, NJ, USA, 2020; Volume 39, pp. 293–296.
- Wormser, C.; Clarke, D.L.; Aronson, L.R. Outcomes of Ureteral Surgery and Ureteral Stenting in Cats: 117 Cases (2006–2014). J. Am. Vet. Med. Assoc. 2016, 248, 518–525.
- Berent, A.C.; Weisse, C.; Beal, M.W.; Brown, D.C.; Todd, K.; Bagley, D. Use of Indwelling, Double-Pigtail Stents for Treatment of Malignant Ureteral Obstruction in Dogs: 12 Cases (2006–2009). J. Am. Vet. Med. Assoc. 2011, 238, 1017–1025.
- Beal, M.W. Interventional Management of Urethral Obstructions. Vet. Clin. N. Am. Small Anim. 2018, 48, 863–874.
- Wormser, C.; Clarke, D.L.; Aronson, L.R. End-to-End Ureteral Anastomosis and Double-Pigtail Ureteral Stent Placement for Treatment of Iatrogenic Ureteral Trauma in Two Dogs. J. Am. Vet. Med. Assoc. 2015, 247, 92–97.
- Defarges, A.; Berent, A.; Dunn, M. New alternatives for minimally invasive management of uroliths: Ureteroliths. Compend. Contin. Educ. Vet. 2013, 35, E4.
- Berent, A.C. Ureteral obstructions in dogs and cats: A review of traditional and new interventional diagnostic and therapeutic options. J. Vet. Emerg. Crit. Care 2011, 21, 86–103.
- Pavia, P.R.; Berent, A.C.; Weisse, C.W.; Neiman, D.; Lamb, K.; Bagley, D. Outcome of Ureteral Stent Placement for Treatment of Benign Ureteral Obstruction in Dogs: 44 Cases (2010–2013). J. Am. Vet. Med. Assoc. 2018, 252, 721–731.




